Abstract
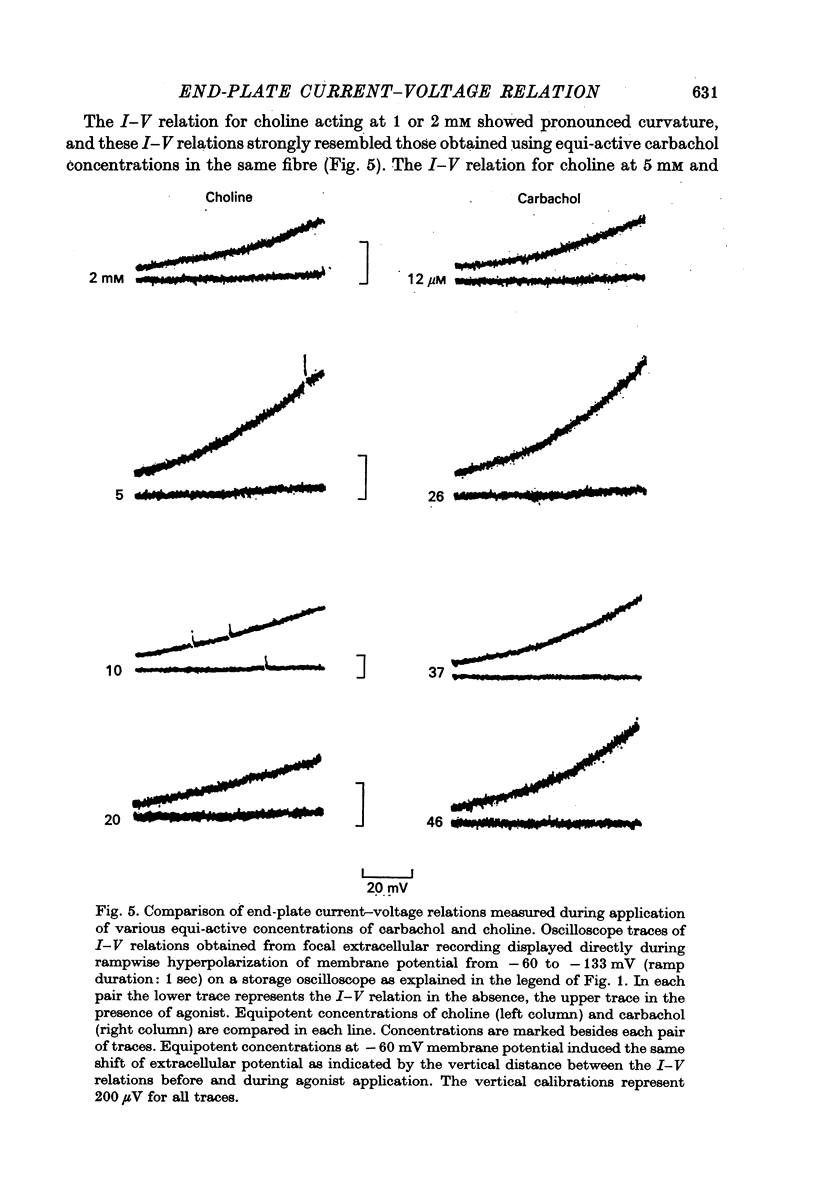
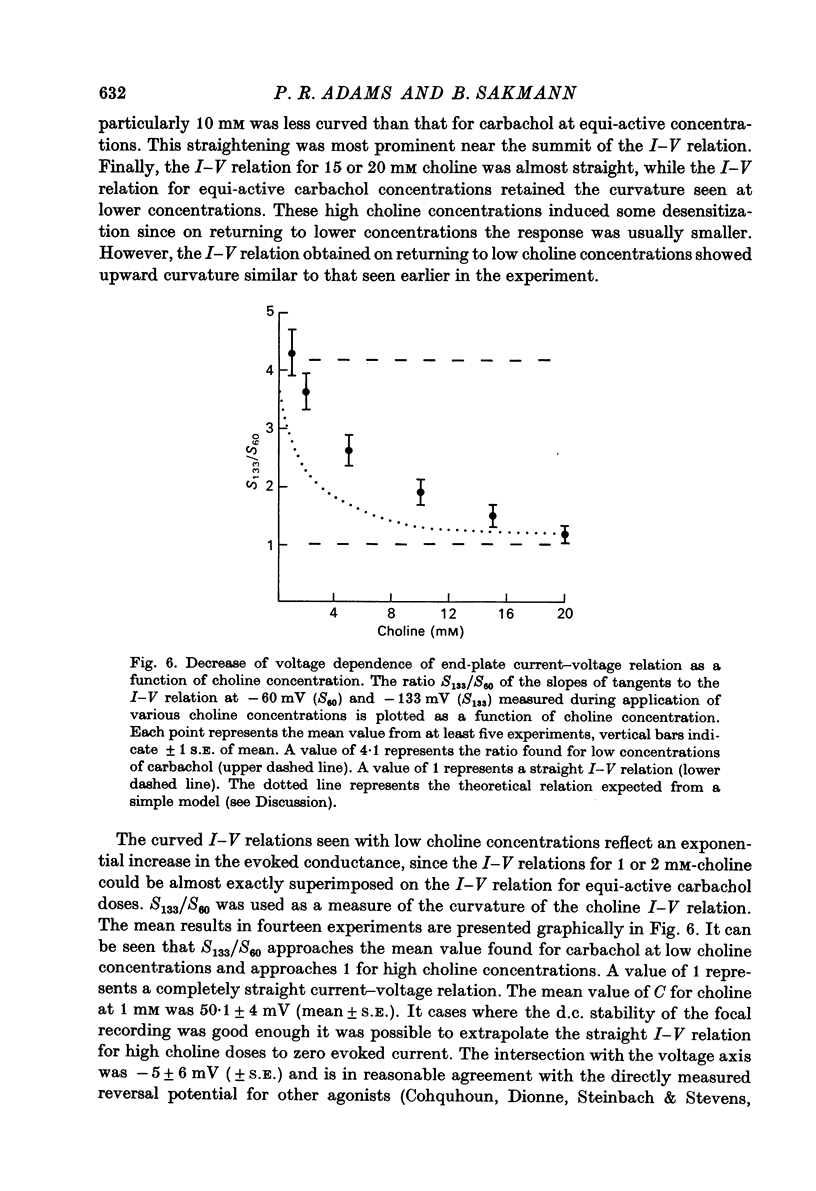
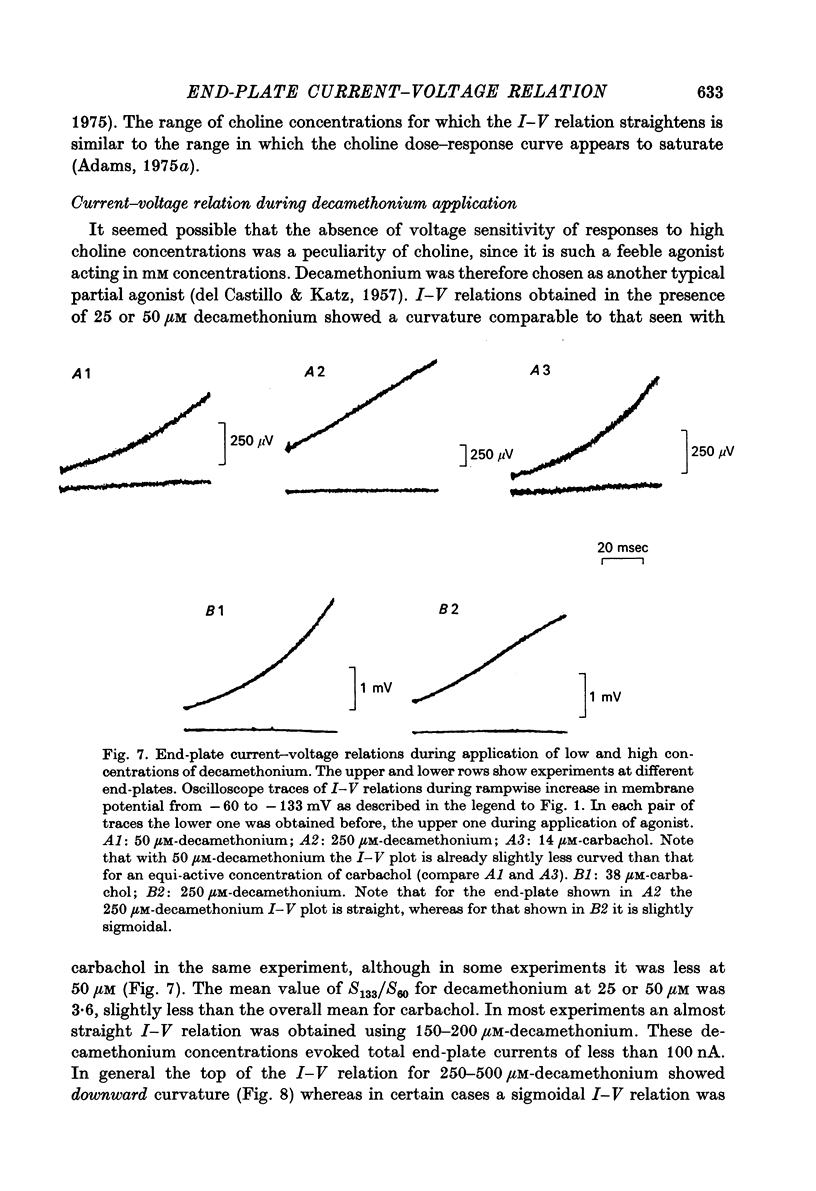
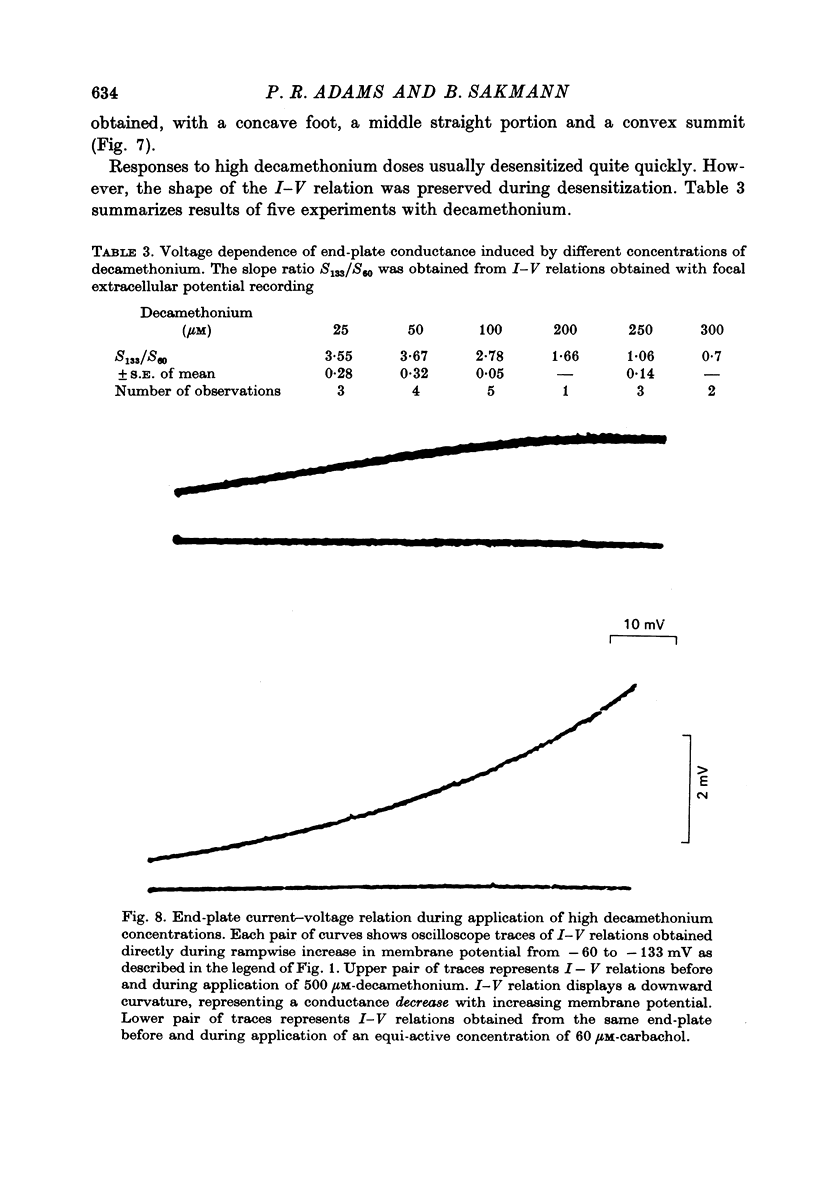
1. Local conductance changes produced by various bath-applied agonists at frog end-plate membrane were measured using focal recording of extracellular potential in voltage-clamped muscle fibres. The potential difference between a focal micropipette placed on the nerve terminal and another micro-pipette placed on or near inactive membrane was taken as proportional to the agonist-induced current through a small patch of an end-plate membrane. 2. The current-voltage (I--V) relation of active membrane was obtained directly by increasing the membrane potential in a ramp fashion. The change in membrane potential was slow enough for post-synaptic gating processes to reach equilibrium during the ramp. 3. During application of sufficiently low concentrations of full agonists (carbachol, (ACh) and partial agonists (choline and decamethonium) the I--V relation of end-plate membrane showed strong curvature in the range of -60 to -130 mV. The slope of I--V relations increased exponentially with membrane hyperpolarization, an e-fold change in conductance occurring for about 50 mV potential shift. 4. The curvature of the I--V relation of end-plate-membrane activated by the partial agonists choline and decamethonium became less as the agonist concentration was increased, and with high concentrations (choline 15 mM; decamethonium 250 micrometer) the I--V relation became almost straight. 5. When end-plate currents produced by high concentrations of partial agonists were matched by application of equi-active concentrations of carbachol, the carbachol-activated membrane still showed as much curvature in its I--V relation as when low concentrations of carbachol were used. 6. Choline and decamethonium concentrations for which the I--V relation was straight produced much greater depression of miniature end-plate currents than did carbachol concentrations which produced the same membrane current at the holding potential. 7. I--V relations for full agonists at high concentrations were obtained after alpha-bungarotoxin pre-treatment. During application of carbachol (400--500 micrometer) and ACh (30--40 micrometer; after complete inhibition of acetylcholinesterase activity) the I--V relation of end-plate membrane is much less curved than during application of low concentrations. 8. It is concluded that either the voltage sensitivity of agonist-induced end-plate conductance reflects voltage sensitivity of agonist binding, or the partial agonists used can exert a voltage-dependent 'local anaesthetic' action in addition to their agonist activity.
Full text
PDF



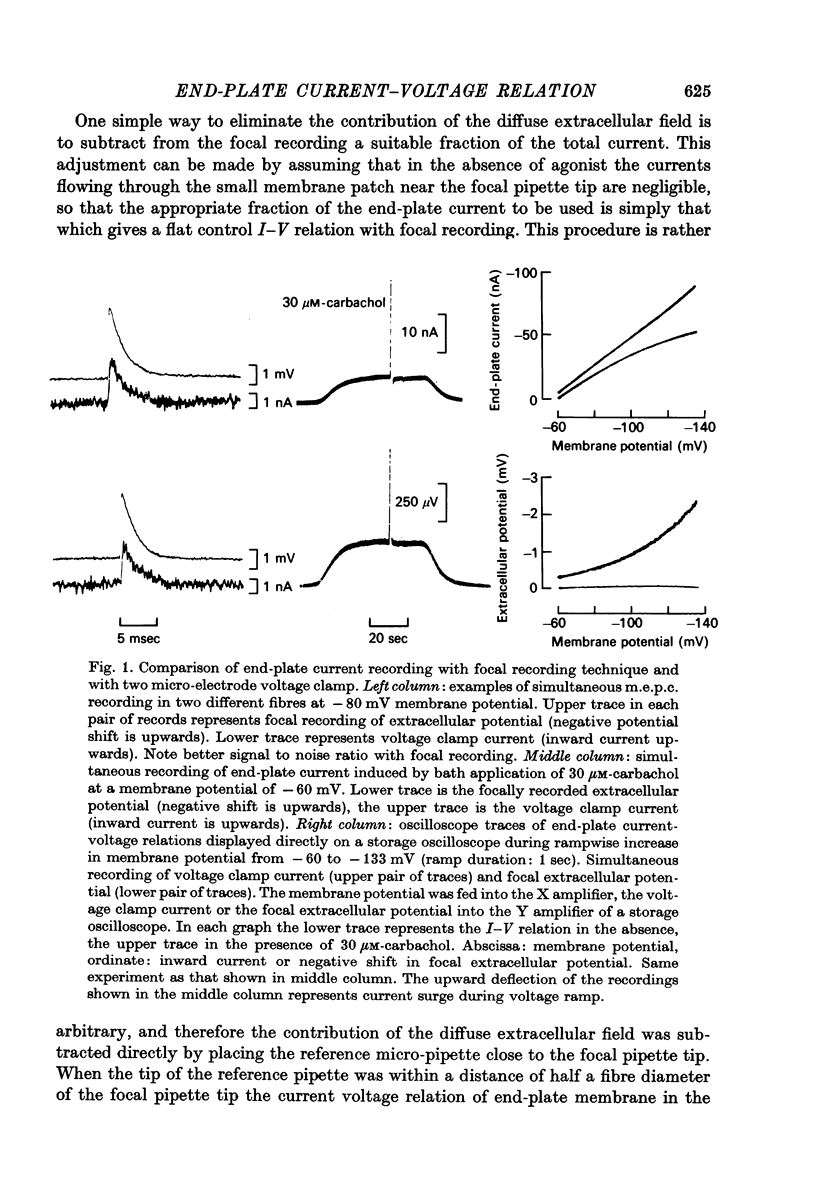


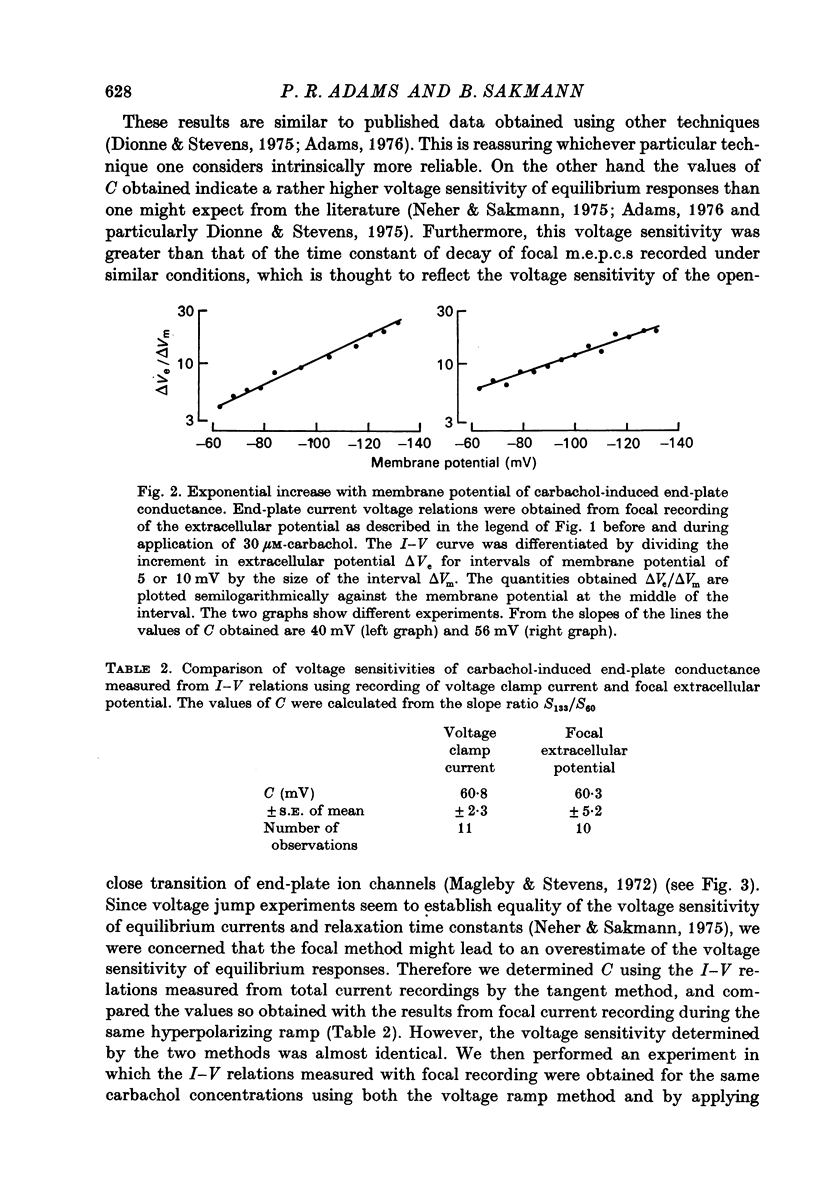
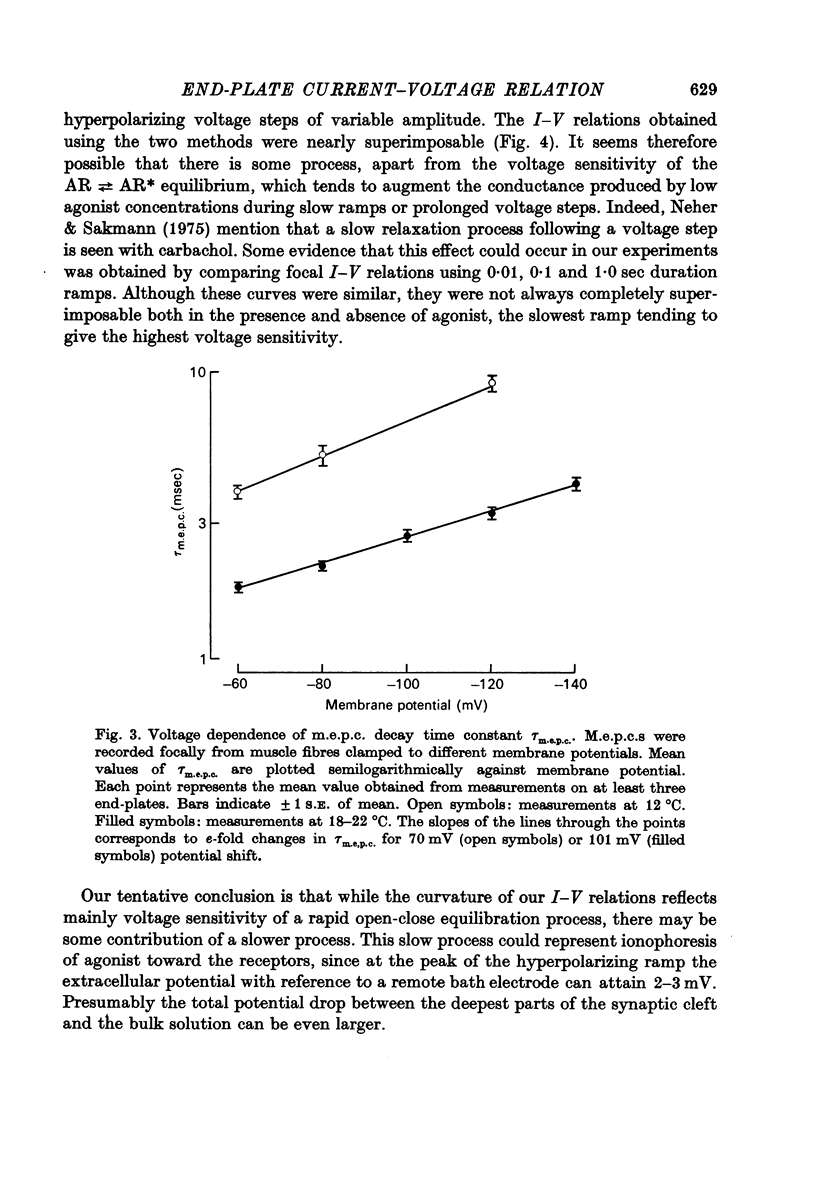
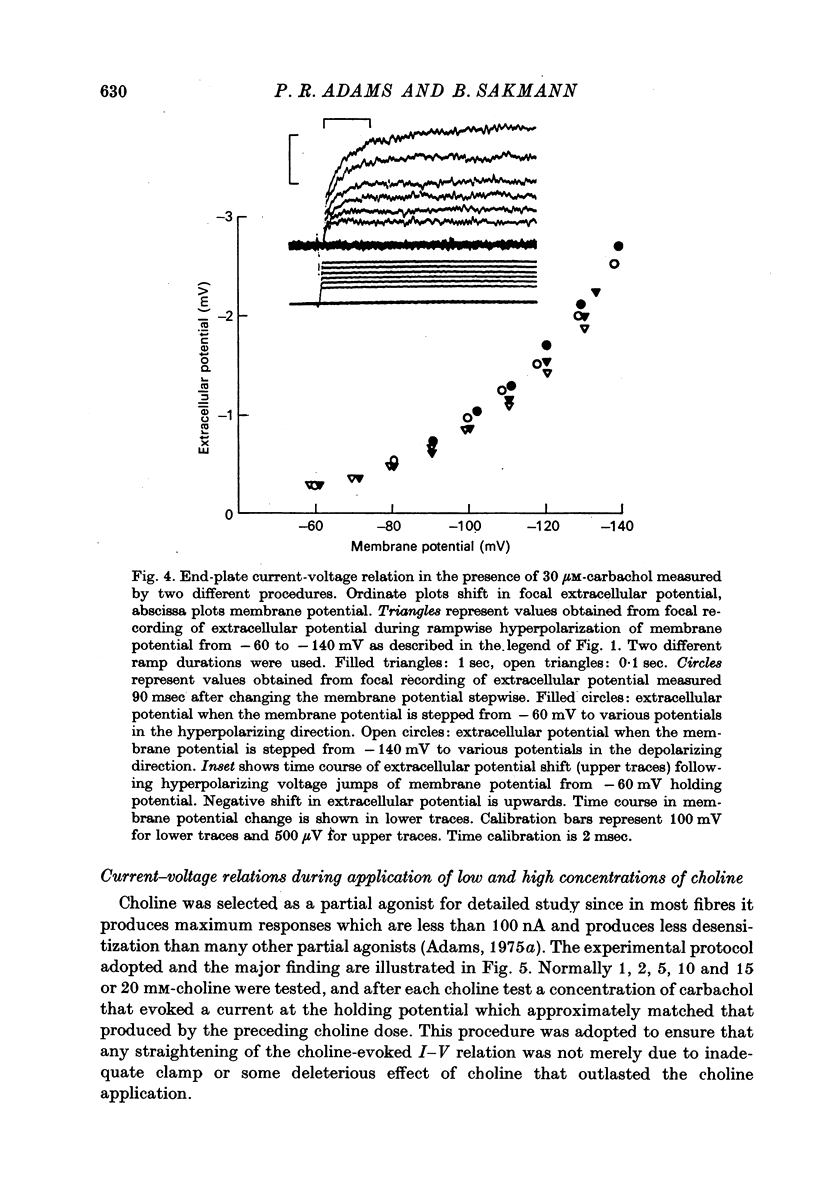






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Kinetics of agonist conductance changes during hyperolarization at frog endplates. Br J Pharmacol. 1975 Feb;53(2):308–310. doi: 10.1111/j.1476-5381.1975.tb07364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R. Relaxation experiments using bath-applied suberyldicholine. J Physiol. 1977 Jun;268(2):271–289. doi: 10.1113/jphysiol.1977.sp011857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Sakmann B. Decamethonium both opens and blocks endplate channels. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2994–2998. doi: 10.1073/pnas.75.6.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R. Voltage dependence of agonist responses at voltage-clamped frog endplates. Pflugers Arch. 1976 Jan 30;361(2):145–151. doi: 10.1007/BF00583458. [DOI] [PubMed] [Google Scholar]
- Adams P. R. Voltage jump analysis of procaine action at frog end-plate. J Physiol. 1977 Jun;268(2):291–318. doi: 10.1113/jphysiol.1977.sp011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen O. S., Fuchs M. Potential energy barriers to ion transport within lipid bilayers. Studies with tetraphenylborate. Biophys J. 1975 Aug;15(8):795–830. doi: 10.1016/S0006-3495(75)85856-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam K. G. A voltage-clamp study of the effect of two lidocaine derivatives on the time course of end-plate currents. J Physiol. 1976 Jun;258(2):279–300. doi: 10.1113/jphysiol.1976.sp011420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. W. Changes in the structure of neuromuscular junctions caused by variations in osmotic pressure. J Cell Biol. 1976 Jun;69(3):521–538. doi: 10.1083/jcb.69.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Dionne V. E., Steinbach J. H., Stevens C. F. Conductance of channels opened by acetylcholine-like drugs in muscle end-plate. Nature. 1975 Jan 17;253(5488):204–206. doi: 10.1038/253204a0. [DOI] [PubMed] [Google Scholar]
- Crawford A. C., McBurney R. N. On the elementary conductance event produced by L-glutamate and quanta of the natural transmitter at the neuromuscular junctions of Maia squinado. J Physiol. 1976 Jun;258(1):205–225. doi: 10.1113/jphysiol.1976.sp011415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Interaction at end-plate receptors between different choline derivatives. Proc R Soc Lond B Biol Sci. 1957 May 7;146(924):369–381. doi: 10.1098/rspb.1957.0018. [DOI] [PubMed] [Google Scholar]
- Del Castillo J., Sobrino J. A. Carbonyl binding sites in the cholinergic receptors of motor-end-plate. Int J Neurosci. 1973 Aug;6(2):67–75. doi: 10.3109/00207457309147650. [DOI] [PubMed] [Google Scholar]
- Dionne V. E., Stevens C. F. Voltage dependence of agonist effectiveness at the frog neuromuscular junction: resolution of a paradox. J Physiol. 1975 Oct;251(2):245–270. doi: 10.1113/jphysiol.1975.sp011090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F., Walther C., Peper K. Junctional and extrajunctional acetylcholine receptors in normal and denervated frog muscle fibres. Noise analysis experiments with different agonists. Pflugers Arch. 1976 Oct 15;366(1):1–9. doi: 10.1007/BF02486555. [DOI] [PubMed] [Google Scholar]
- Hutter O. F., Warner A. E. The voltage dependence of the chloride conductance of frog muscle. J Physiol. 1972 Dec;227(1):275–290. doi: 10.1113/jphysiol.1972.sp010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINSON D. H. The antagonism between tubocurarine and substances which depolarize the motor end-plate. J Physiol. 1960 Jul;152:309–324. doi: 10.1113/jphysiol.1960.sp006489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The characteristics of 'end-plate noise' produced by different depolarizing drugs. J Physiol. 1973 May;230(3):707–717. doi: 10.1113/jphysiol.1973.sp010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of procaine on the action of acetylcholine at the neuromuscular junction. J Physiol. 1975 Jul;249(2):269–284. doi: 10.1113/jphysiol.1975.sp011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll W., Stark G. An extended kinetic analysis of valinomycin-induced Rb-transport through monoglyceride membranes. J Membr Biol. 1975;25(3-4):249–270. doi: 10.1007/BF01868578. [DOI] [PubMed] [Google Scholar]
- Kordás M., Brzin M., Majcen Z. A comparison of the effect of cholinesterase inhibitors on end-plate current and on cholinesterase activity in frog muscle. Neuropharmacology. 1975 Nov;14(11):791–800. doi: 10.1016/0028-3908(75)90106-9. [DOI] [PubMed] [Google Scholar]
- Krasne S., Eisenman G. Influence of molecular variations of ionophore and lipid on the selective ion permeability of membranes: I. Tetranactin and the methylation of nonactin-type carriers. J Membr Biol. 1976 Dec 25;30(1):1–44. doi: 10.1007/BF01869658. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Dreyer F., Peper K. Current-voltage relation and reversal potential at junctional and extrajunctional ACh-receptors of the frog neuromuscular junction. Pflugers Arch. 1976 Mar 11;362(1):43–47. doi: 10.1007/BF00588679. [DOI] [PubMed] [Google Scholar]
- Manalis R. S. Voltage-dependent effect of curare at the frog neuromuscular junction. Nature. 1977 May 26;267(5609):366–368. doi: 10.1038/267366a0. [DOI] [PubMed] [Google Scholar]
- Nastuk W. L., Parsons R. L. Factors in the inactivation of postjunctional membrane receptors of frog skeletal muscle. J Gen Physiol. 1970 Aug;56(2):218–249. doi: 10.1085/jgp.56.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Noise analysis of drug induced voltage clamp currents in denervated frog muscle fibres. J Physiol. 1976 Jul;258(3):705–729. doi: 10.1113/jphysiol.1976.sp011442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976 Apr 29;260(5554):799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Voltage-dependence of drug-induced conductance in frog neuromuscular junction. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2140–2144. doi: 10.1073/pnas.72.6.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Active phase of frog's end-plate potential. J Neurophysiol. 1959 Jul;22(4):395–411. doi: 10.1152/jn.1959.22.4.395. [DOI] [PubMed] [Google Scholar]
- Trautmann A., Zilber-Gachelin N. F. Further investigations on the effect of denervation and pH on the conductance change at the neuromuscular junction of the frog. Pflugers Arch. 1976 Jun 29;364(1):53–58. doi: 10.1007/BF01062911. [DOI] [PubMed] [Google Scholar]


