Abstract
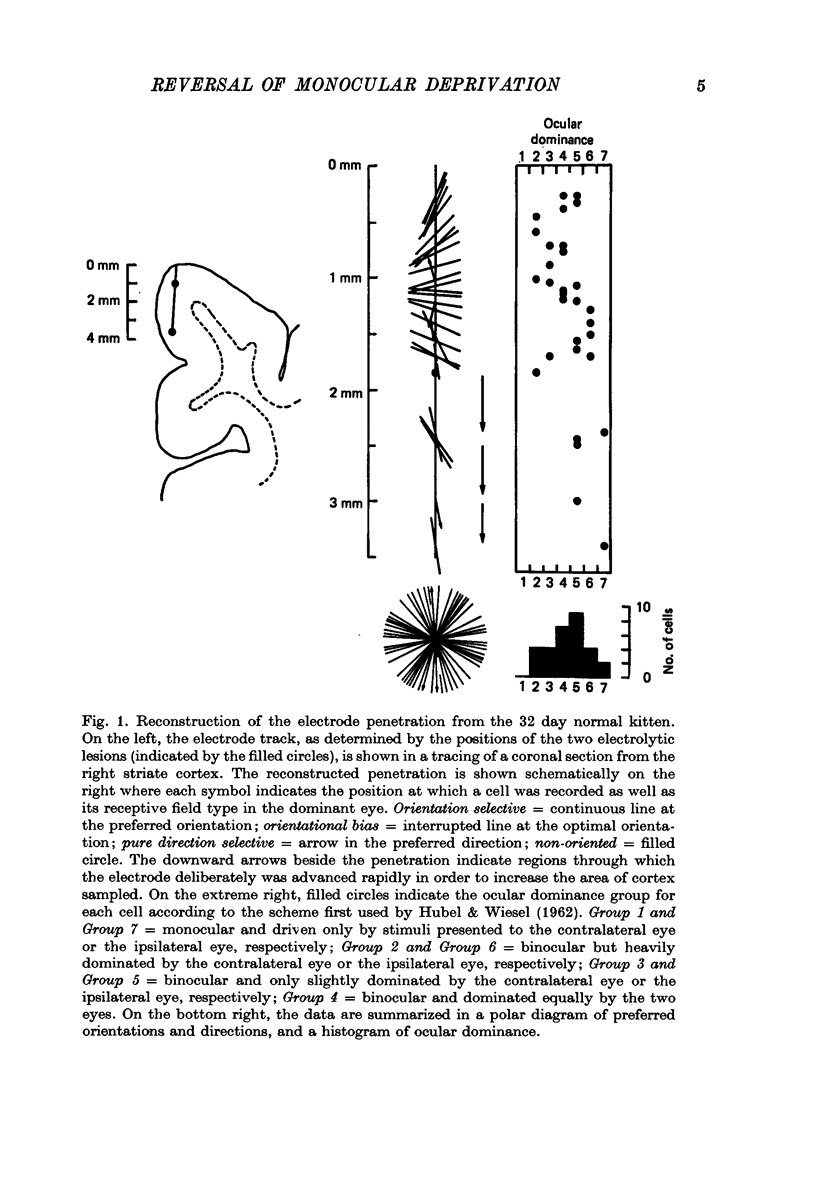
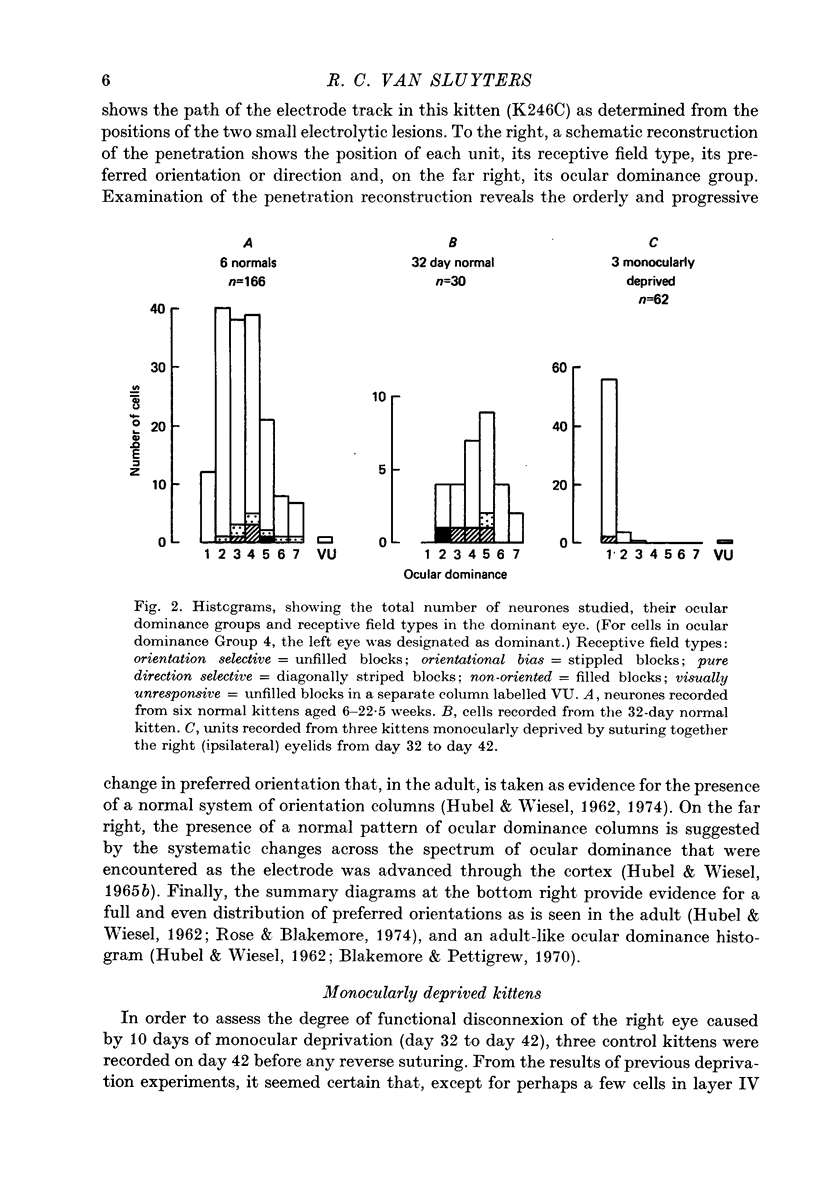
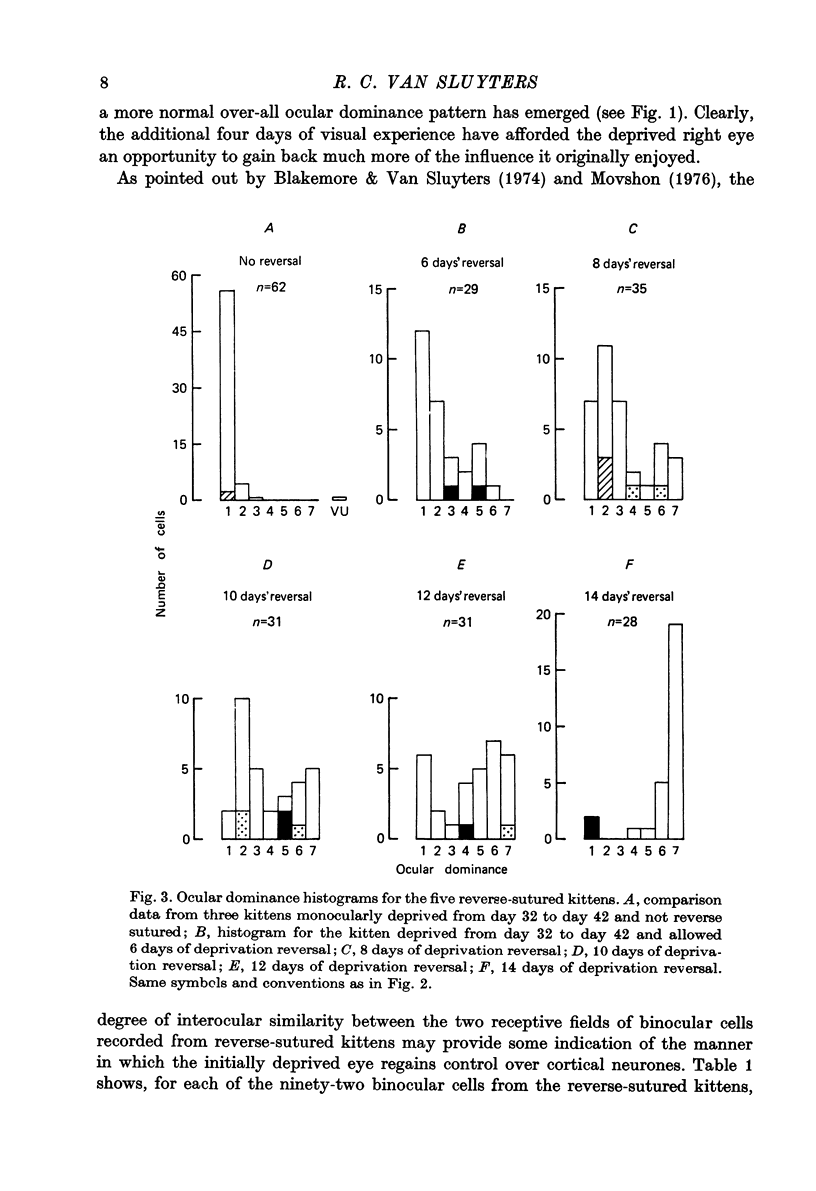
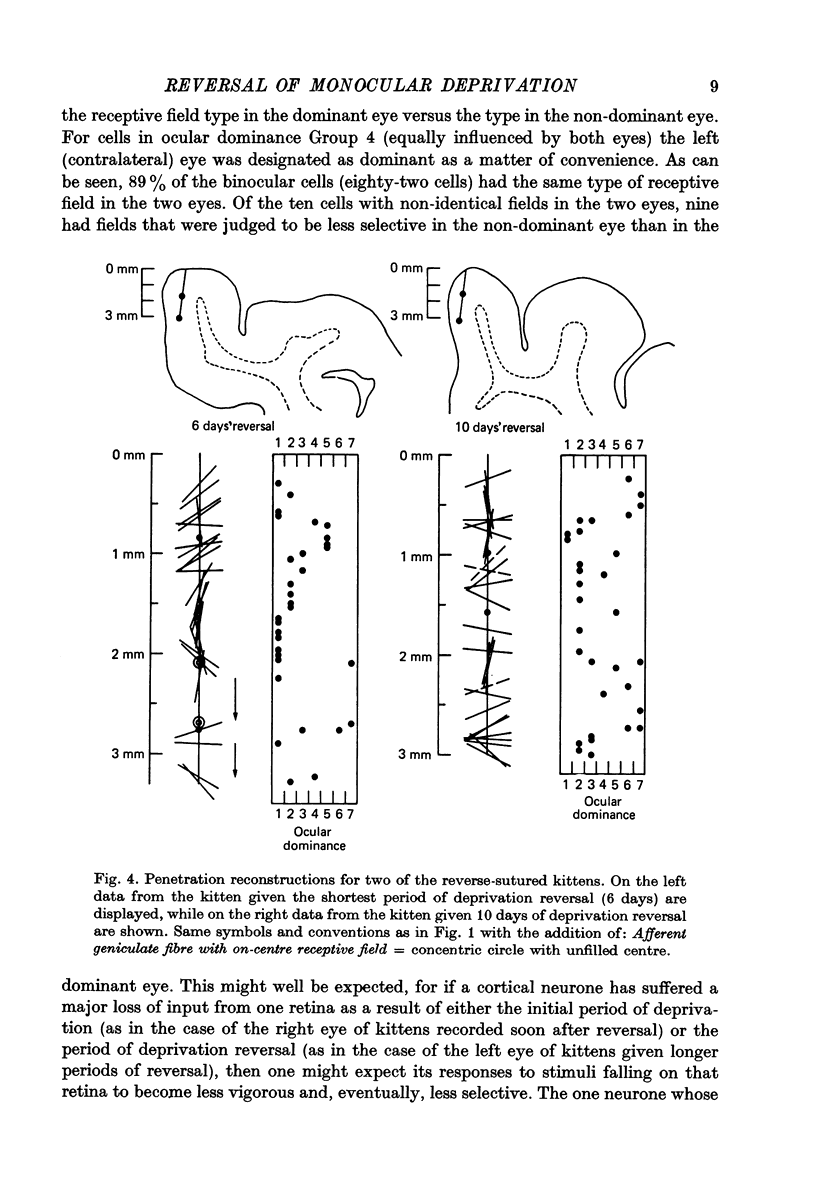
1. Five normally reared kittens were monocularly deprived from day 32 to day 42 and then subjected to varying periods (from 6 to 14 days) of deprivation reversal using the technique of reverse suturing. Data obtained from single unit recordings in striate cortex of these kittens were compared to control data from seven normal kittens (aged 32 days to 22.5 weeks) and three normally reared kittens given monocular deprivation from day 32 to day 42. 2. Analysis of cortical ocular dominance patterns in these reverse-sutured kittens revealed a progressively greater shift of influence away from the originally experienced eye and toward the originally deprived eye as the duration of the period of deprivation reversal increased. Most cortical cells were monocularly driven in these animals, but at each stage of the reversal process some binocular neurones were found. 3. The distribution of interocular differences in preferred orientation for binocular cells from these kittens was not significantly different from that for normal kittens, indicating that the pattern of connexions established by the originally deprived eye during reinnervation of striate cortex was very similar to that present before deprivation. 4. Previous studies on reversal of the effects of longer periods of monocular deprivation have indicated that during reinnervation of cortex by fibres representing the originally deprived eye an entirely new and different pattern of connexions is formed. On the basis of these results, it is hypothesized that short-term monocular deprivation causes a reversible silencing of otherwise intact synapses, while terminals are physically disrupted when deprivation is prolonged. 5. This hypothesis is supported by the results of an additional set of experiments in which receptive fields, many of which were highly specific, could be demonstrated for about one-half of the cells studied after removal of the non-deprived eye in two normally reared kittens given 10 days of monocular deprivation beginning on day 32. Similar experiments in two kittens deprived from eye opening to day 42 revealed functional input to less than 20% of the cells studied and no evidence of response specificity.
Full text
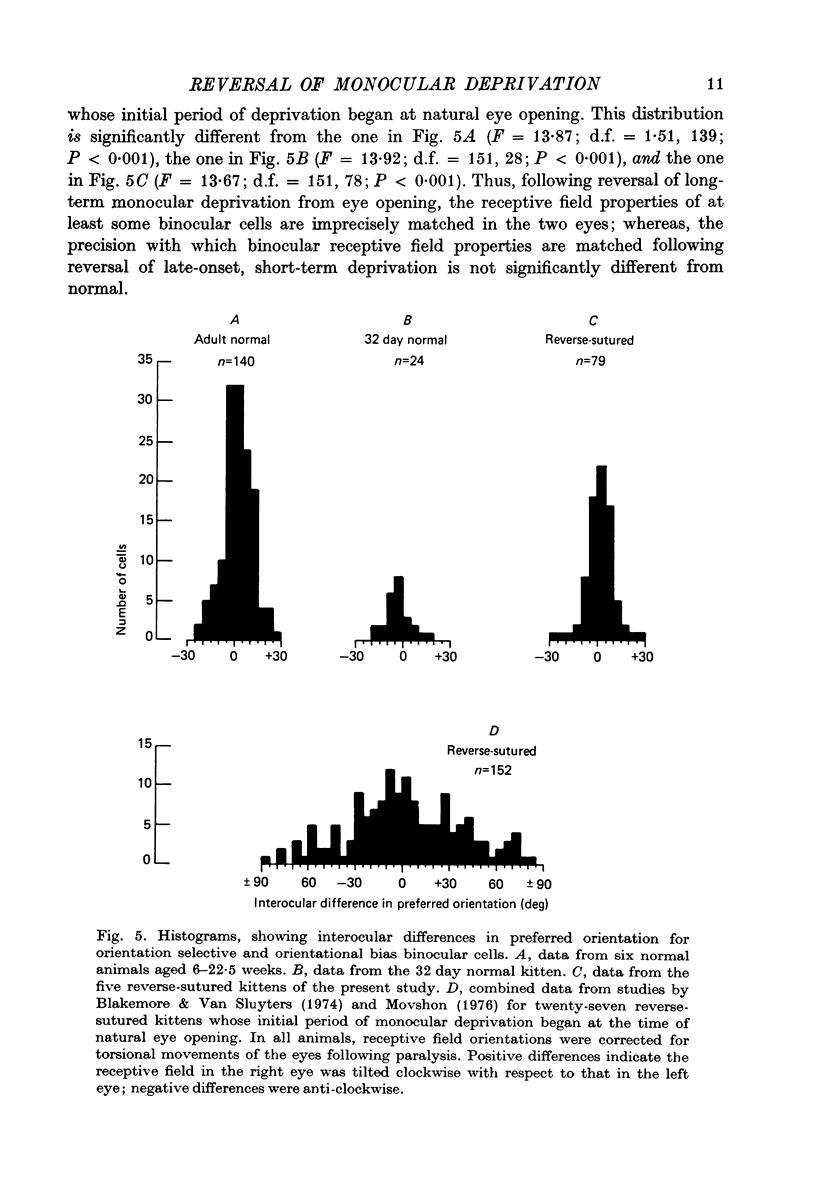
PDF










Selected References
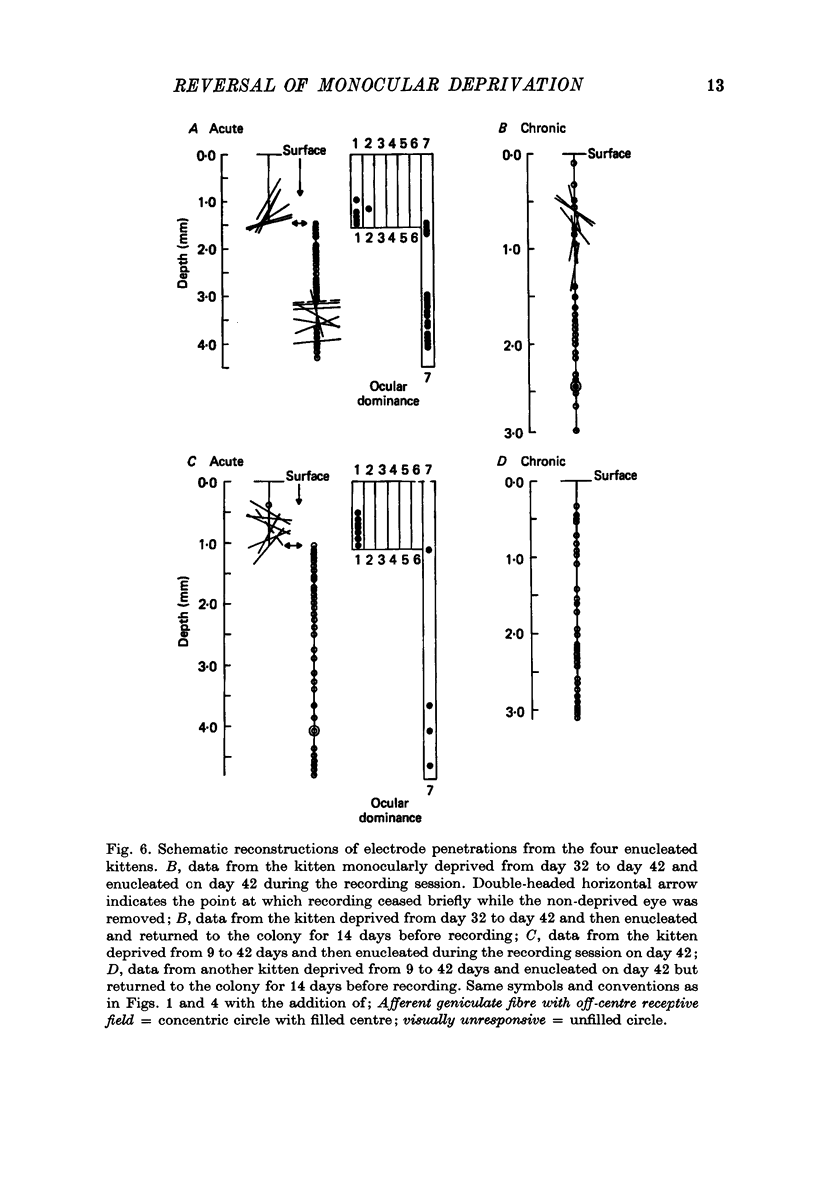
These references are in PubMed. This may not be the complete list of references from this article.
- Anker R. L., Cragg B. G. Development of the extrinsic connections of the visual cortex in the cat. J Comp Neurol. 1974 Mar 1;154(1):29–41. doi: 10.1002/cne.901540103. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Fiorentini A., Maffei L. A second neural mechanism of binocular depth discrimination. J Physiol. 1972 Nov;226(3):725–749. doi: 10.1113/jphysiol.1972.sp010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C. Genetic instructions and developmental plasticity in the kitten's visual cortex. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):425–434. doi: 10.1098/rstb.1977.0052. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Pettigrew J. D. Eye dominance in the visual cortex. Nature. 1970 Jan 31;225(5231):426–429. doi: 10.1038/225426a0. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Van Sluyters C. V., Movshon J. A. Synaptic competition in the kitten's visual cortex. Cold Spring Harb Symp Quant Biol. 1976;40:601–609. doi: 10.1101/sqb.1976.040.01.056. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Van Sluyters R. C. Innate and environmental factors in the development of the kitten's visual cortex. J Physiol. 1975 Jul;248(3):663–716. doi: 10.1113/jphysiol.1975.sp010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Van Sluyters R. C. Reversal of the physiological effects of monocular deprivation in kittens: further evidence for a sensitive period. J Physiol. 1974 Feb;237(1):195–216. doi: 10.1113/jphysiol.1974.sp010478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisseret P., Imbert M. Visual cortical cells: their developmental properties in normal and dark reared kittens. J Physiol. 1976 Feb;255(2):511–525. doi: 10.1113/jphysiol.1976.sp011293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow K. L., Stewart D. L. Reversal of structural and functional effects of long-term visual deprivation in cats. Exp Neurol. 1972 Mar;34(3):409–433. doi: 10.1016/0014-4886(72)90038-6. [DOI] [PubMed] [Google Scholar]
- Cragg B. G. The development of synapses in cat visual cortex. Invest Ophthalmol. 1972 May;11(5):377–385. [PubMed] [Google Scholar]
- Cragg B. G. The development of synapses in the visual system of the cat. J Comp Neurol. 1975 Mar 15;160(2):147–166. doi: 10.1002/cne.901600202. [DOI] [PubMed] [Google Scholar]
- Crewther D. P., Crewther S. G., Pettigrew J. D. A role for extraocular afferents in post-critical period reversal of monocular deprivation. J Physiol. 1978 Sep;282:181–195. doi: 10.1113/jphysiol.1978.sp012456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dews P. B., Wiesel T. N. Consequences of monocular deprivation on visual behaviour in kittens. J Physiol. 1970 Feb;206(2):437–455. doi: 10.1113/jphysiol.1970.sp009023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS AND FUNCTIONAL ARCHITECTURE IN TWO NONSTRIATE VISUAL AREAS (18 AND 19) OF THE CAT. J Neurophysiol. 1965 Mar;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann K. P., Cynader M. Functional aspects of plasticity in the visual system of adult cats after early monocular deprivation. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):411–424. doi: 10.1098/rstb.1977.0051. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Binocular interaction in striate cortex of kittens reared with artificial squint. J Neurophysiol. 1965 Nov;28(6):1041–1059. doi: 10.1152/jn.1965.28.6.1041. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Sequence regularity and geometry of orientation columns in the monkey striate cortex. J Comp Neurol. 1974 Dec 1;158(3):267–293. doi: 10.1002/cne.901580304. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970 Feb;206(2):419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz K. E., Spear P. D. Postcritical-period reversal of effects of monocular deprivation on striate cortex cells in the cat. J Neurophysiol. 1976 May;39(3):501–511. doi: 10.1152/jn.1976.39.3.501. [DOI] [PubMed] [Google Scholar]
- Nelson J. I., Kato H., Bishop P. O. Discrimination of orientation and position disparities by binocularly activated neurons in cat straite cortex. J Neurophysiol. 1977 Mar;40(2):260–283. doi: 10.1152/jn.1977.40.2.260. [DOI] [PubMed] [Google Scholar]
- Olson C. R., Freeman R. D. Monocular deprivation and recovery during sensitive period in kittens. J Neurophysiol. 1978 Jan;41(1):65–74. doi: 10.1152/jn.1978.41.1.65. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. D. The effect of visual experience on the development of stimulus specificity by kitten cortical neurones. J Physiol. 1974 Feb;237(1):49–74. doi: 10.1113/jphysiol.1974.sp010469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose D., Blakemore C. An analysis of orientation selectivity in the cat's visual cortex. Exp Brain Res. 1974 Apr 30;20(1):1–17. doi: 10.1007/BF00239014. [DOI] [PubMed] [Google Scholar]
- Smith D. C., Spear P. D., Kratz K. E. Role of visual experience in postcritical-period reversal of effects of monocular deprivation in cat striate cortex. J Comp Neurol. 1978 Mar 15;178(2):313–328. doi: 10.1002/cne.901780207. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N., HUBEL D. H. EFFECTS OF VISUAL DEPRIVATION ON MORPHOLOGY AND PHYSIOLOGY OF CELLS IN THE CATS LATERAL GENICULATE BODY. J Neurophysiol. 1963 Nov;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]


