Abstract
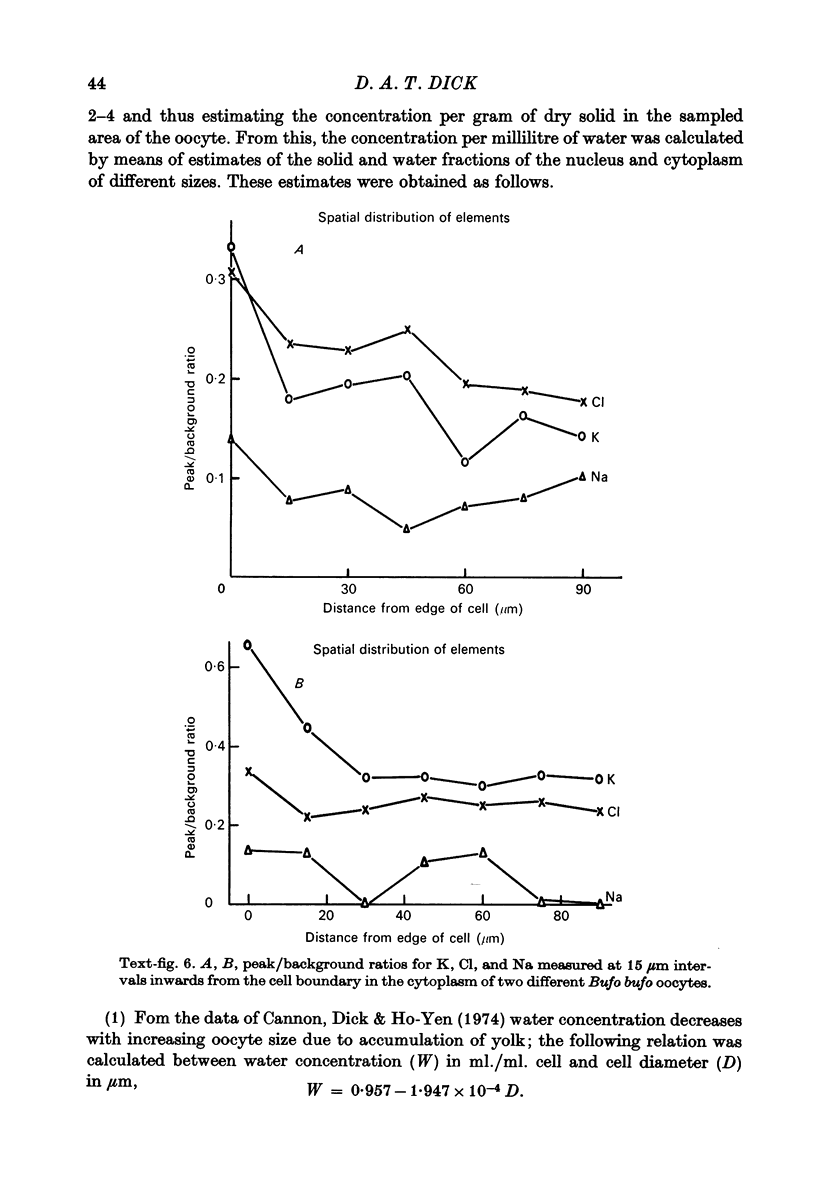
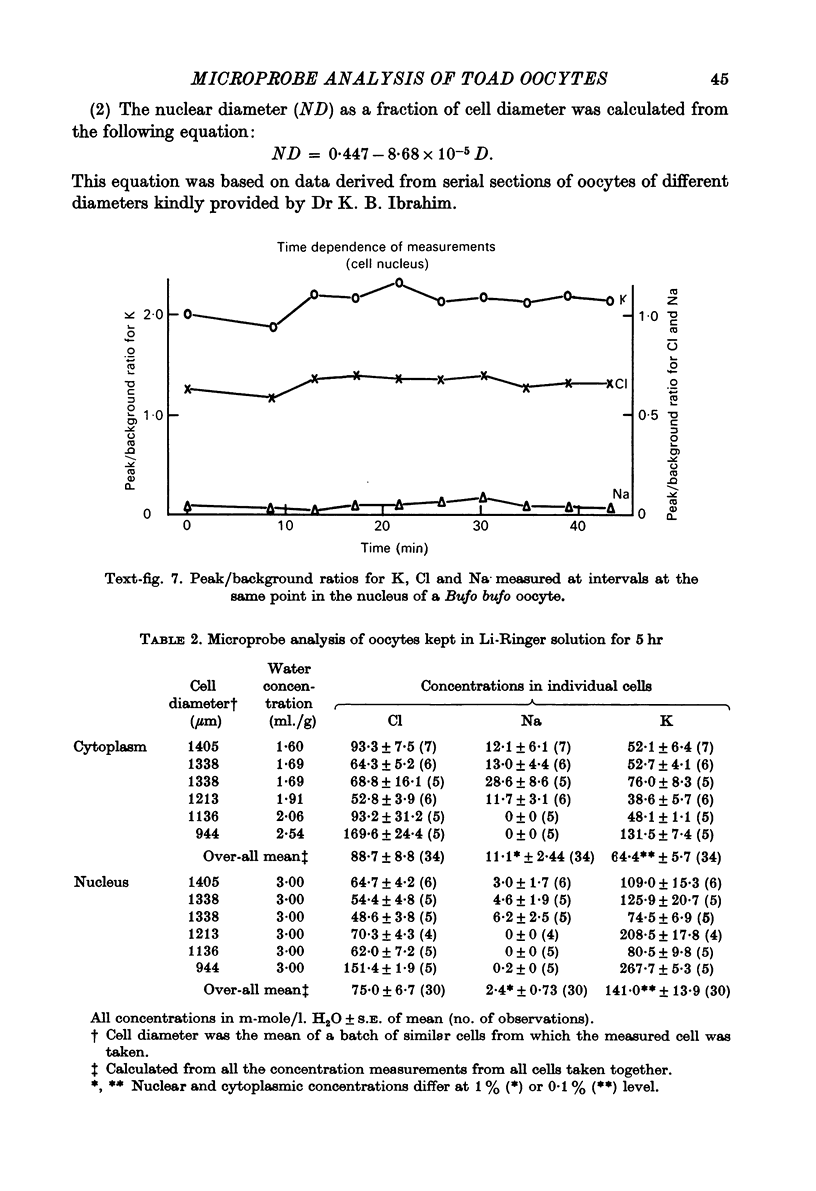
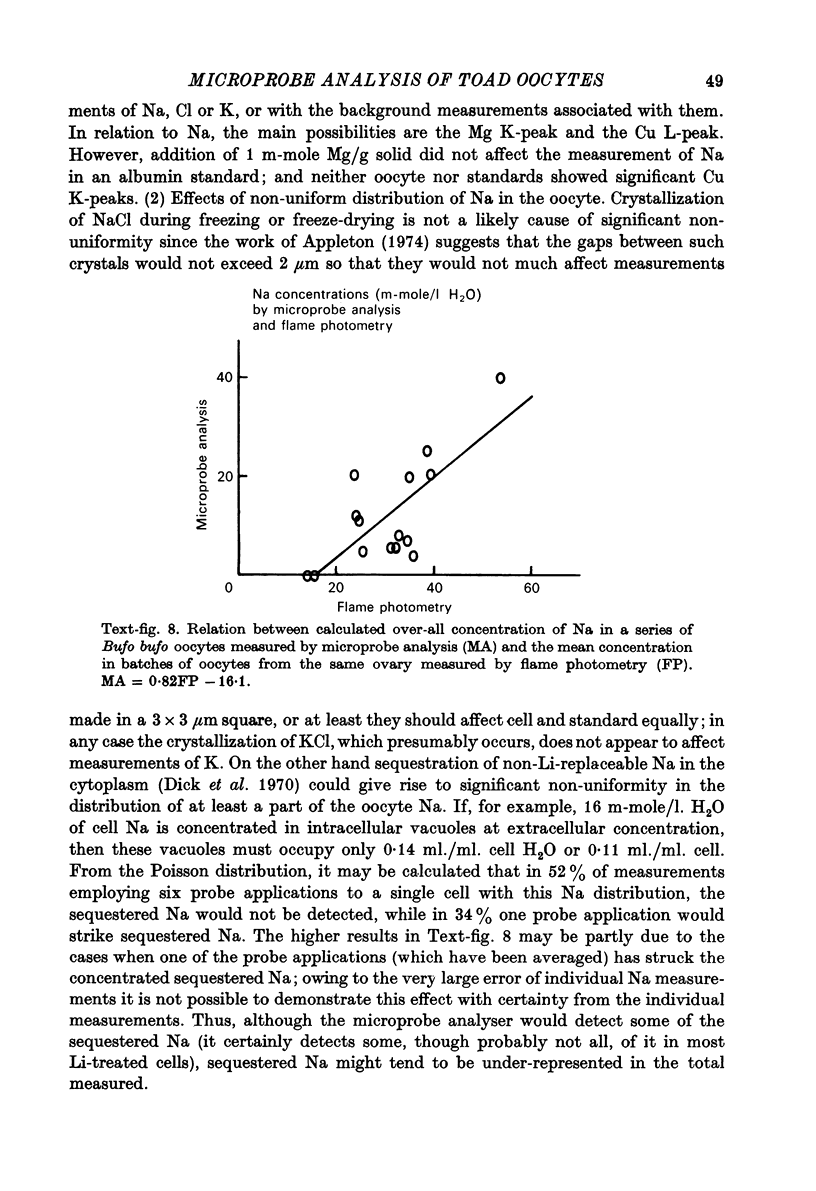
1. Measurements of cytoplasmic and nuclear Na, K and Cl have been made by electron microprobe analysis on freeze-dried sections of oocytes of Bufo bufo, using standards of bovine plasma albumin and gamma-globulin. Concentrations were obtained per kilogram of dry mass, were converted to concentrations per litre of water content using known figures for water and solid concentration of nucleus and cytoplasm, and were then compared with measurements on cells from the same animal obtained by flame photometry. 2. In fresh oocytes concentrations were (mean +/- S.E. of mean in m-mole/l. H2O) in cytoplasm Na 10.9 +/- 1.95, K 70.2 +/- 3.22, Cl 98.8 +/- 11.0, and in nucleus Na 10.4 +/- 1.79, K 266.4 +/- 22.8, Cl 91.3 +/- 9.0. 3. After treatment with Na-free Ringer (Li substituted for Na) for 5 hr, concentrations were in cytoplasm Na 11.1 +/- 2.44, K 64.4 +/- 5.7, Cl 88.7 +/- 8.8, and in nucleus Na 2.4 +/- 0.73, K 141 +/- 13.9, Cl 75.0 +/- 6.7. Na inexchangeable with Li therefore lay in the cytoplasm but not in the nucleus as previously shown by autoradiography. 4. For K electron microscopic analysis measurements agreed well with those obtained by flame photometry but the former measured only 35% of Na measured by flame photometry. This discrepancy may be due either to technical difficulties with the electron microprobe analysis or to localization of Na in the cytoplasm.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleton T. C. A cryostat approach to ultrathin "dry" frozen sections for electron microscopy: a morphological and x-ray analytical study. J Microsc. 1974 Jan;100(1):49–74. doi: 10.1111/j.1365-2818.1974.tb03913.x. [DOI] [PubMed] [Google Scholar]
- Cannon J. D., Dick D. A., Ho-Yen D. O. Intracellular sodium and potassium concentrations in toad and frog oocytes during development. J Physiol. 1974 Sep;241(2):497–508. doi: 10.1113/jphysiol.1974.sp010668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Century T. J., Fenichel I. R., Horowitz S. B. The concentrations of water, sodium and potassium in the nucleus and cytoplasm of amphibian oocytes. J Cell Sci. 1970 Jul;7(1):5–13. doi: 10.1242/jcs.7.1.5. [DOI] [PubMed] [Google Scholar]
- Century T. J., Horowitz S. B. Sodium exchange in the cytoplasm and nucleus of amphibian oocytes. J Cell Sci. 1974 Nov;16(2):465–471. doi: 10.1242/jcs.16.2.465. [DOI] [PubMed] [Google Scholar]
- DICK D. A., LEA E. J. NA FLUXES IN SINGLE TOAD OOCYTES WITH SPECIAL REFERENCE TO THE EFFECT OF EXTERNAL AND INTERNAL NA CONCENTRATION ON NA EFFLUX. J Physiol. 1964 Oct;174:55–90. doi: 10.1113/jphysiol.1964.sp007474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick D. A., Fry D. J. Location of inexchangeable sodium in the nucleus and cytoplasm of oocytes of Bufo bufo exposed to sodium-free solutions. J Physiol. 1973 May;231(1):19–29. doi: 10.1113/jphysiol.1973.sp010217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick D. A., Fry D. J. Sodium fluxes in single amphibian oocytes: further studies and a new model. J Physiol. 1975 May;247(1):91–116. doi: 10.1113/jphysiol.1975.sp010922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick D. A., John P. N., Fry D. J., Rogers A. W. Autoradiographic demonstration of inhomogeneous distribution of sodium in single oocytes of Bufo bufo. J Physiol. 1970 Sep;210(2):305–319. doi: 10.1113/jphysiol.1970.sp009212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick D. A., McLaughlin S. G. The activities and concentrations of sodium and potassium in toad oocytes. J Physiol. 1969 Nov;205(1):61–78. doi: 10.1113/jphysiol.1969.sp008951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick D. A. Proceedings: The distribution of Na, K and Cl in Bufo bufo oocytes measured by electron microprobe analysis. J Physiol. 1976 Jun;258(2):102P–103P. [PubMed] [Google Scholar]
- Gupta B. L., Hall T. A., Maddrell S. H., Moreton R. B. Distribution of ions in a fluid-transporting epithelium determined by electron-probe X-ray microanalysis. Nature. 1976 Nov 18;264(5583):284–287. doi: 10.1038/264284a0. [DOI] [PubMed] [Google Scholar]
- Horowitz S. B., Fenichel I. R. Analysis of sodium transport in the amphibian oocyte by extractive and radioautographic techniques. J Cell Biol. 1970 Oct;47(1):120–131. doi: 10.1083/jcb.47.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOEWENSTEIN W. R., KANNO Y. The electrical conductance and potential across the membrane of some cell nuclei. J Cell Biol. 1963 Feb;16:421–425. doi: 10.1083/jcb.16.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. O., Armstrong W. M. State and distribution of potassium and sodium ions in frog skeletal muscle. J Membr Biol. 1974;15(4):331–362. doi: 10.1007/BF01870094. [DOI] [PubMed] [Google Scholar]
- Morrill G. A., Ziegler D., Zabrenetzky V. S. An analysis of transport, exchange, and binding of sodium and potassium in isolated amphibian follicles and denuded oocytes. J Cell Sci. 1977 Aug;26:311–322. doi: 10.1242/jcs.26.1.311. [DOI] [PubMed] [Google Scholar]
- NAORA H., NAORA H., IZAWA M., ALLFREY V. G., MIRSKY A. E. Some observations on differences in composition between the nucleus and cytoplasm of the frog oocyte. Proc Natl Acad Sci U S A. 1962 May 15;48:853–859. doi: 10.1073/pnas.48.5.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann W., Muir C., Macgregor H. C. Sodium and potassium in oocytes of Triturus cristatus. J Cell Sci. 1969 Mar;4(2):299–304. doi: 10.1242/jcs.4.2.299. [DOI] [PubMed] [Google Scholar]
- Saubermann A. J., Echlin P. The preparation, examination and analysis of frozen hydrated tissue sections by scanning transmission electron microscopy and x-ray microanalysis. J Microsc. 1975 Nov;105(2):155–191. doi: 10.1111/j.1365-2818.1975.tb04048.x. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Shuman H., Somlyo A. P. Elemental distribution in striated muscle and the effects of hypertonicity. Electron probe analysis of cryo sections. J Cell Biol. 1977 Sep;74(3):828–857. doi: 10.1083/jcb.74.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischnitzer S. The ultrastructure of the cytoplasm of the developing amphibian egg. Adv Morphog. 1966;5:131–179. doi: 10.1016/b978-1-4831-9952-8.50008-0. [DOI] [PubMed] [Google Scholar]
- de Laat S. W., Buwalda R. J., Habets A. M. Intracellular ionic distribution, cell membrane permeability and membrane potential of the Xenopus egg during first cleavage. Exp Cell Res. 1974 Nov;89(1):1–14. doi: 10.1016/0014-4827(74)90180-3. [DOI] [PubMed] [Google Scholar]



