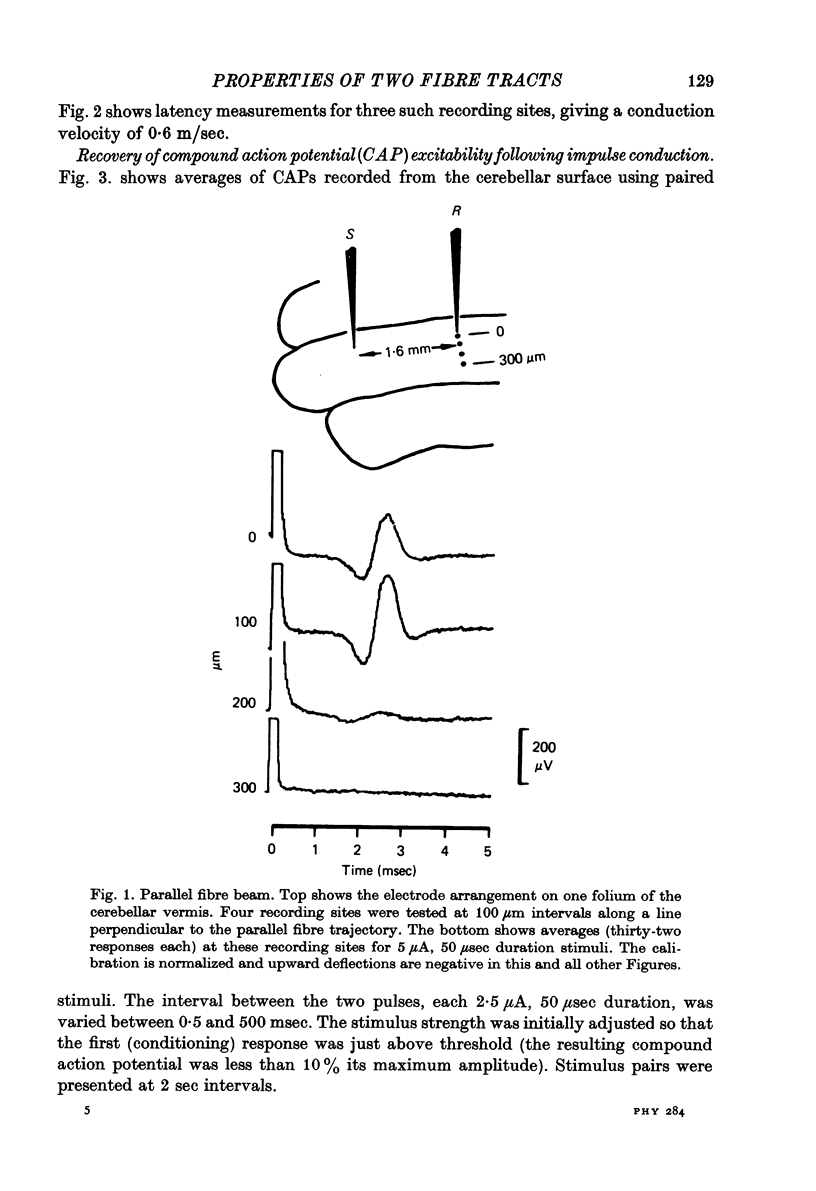
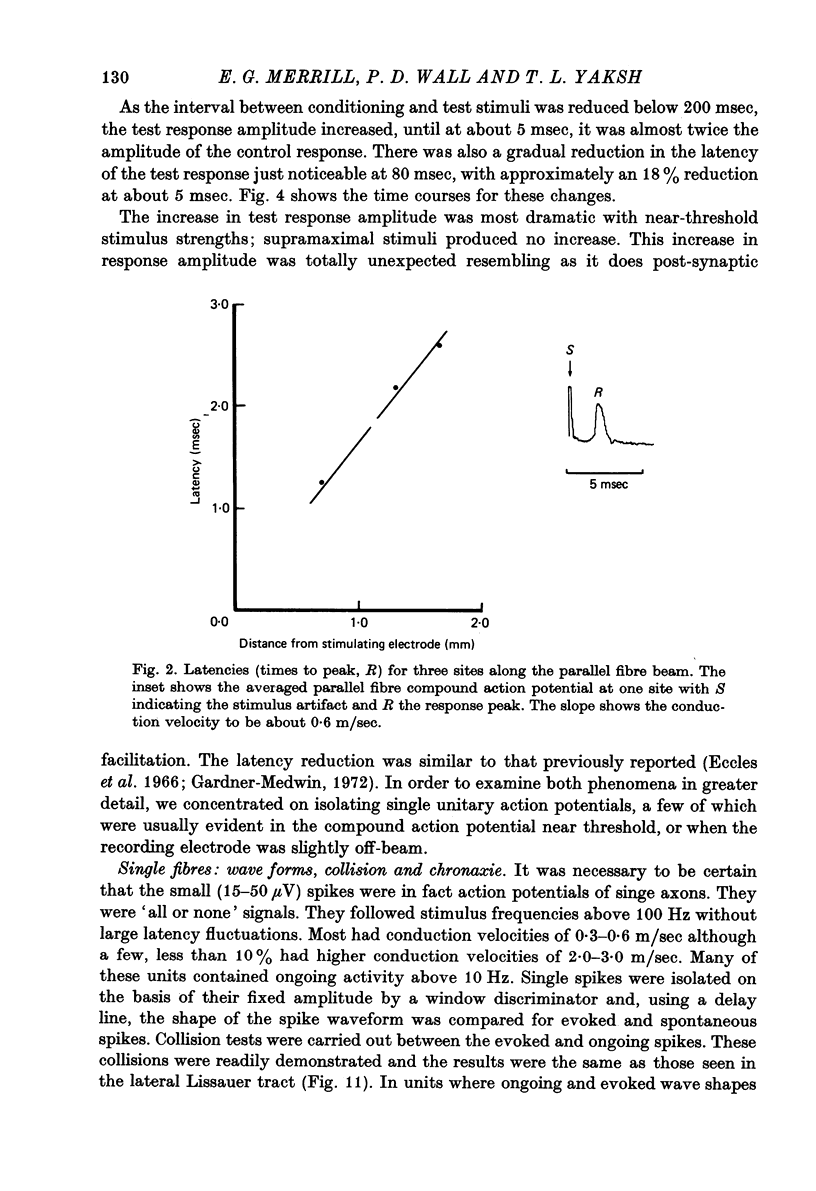
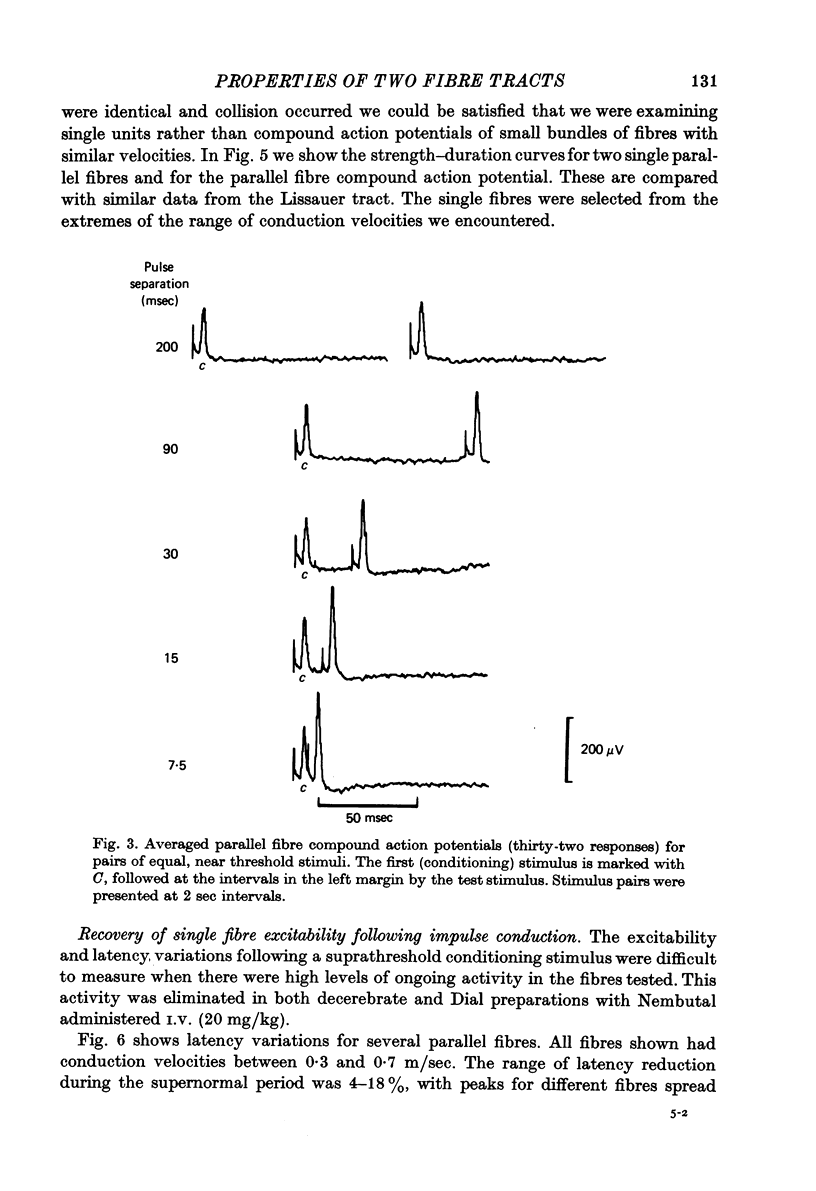
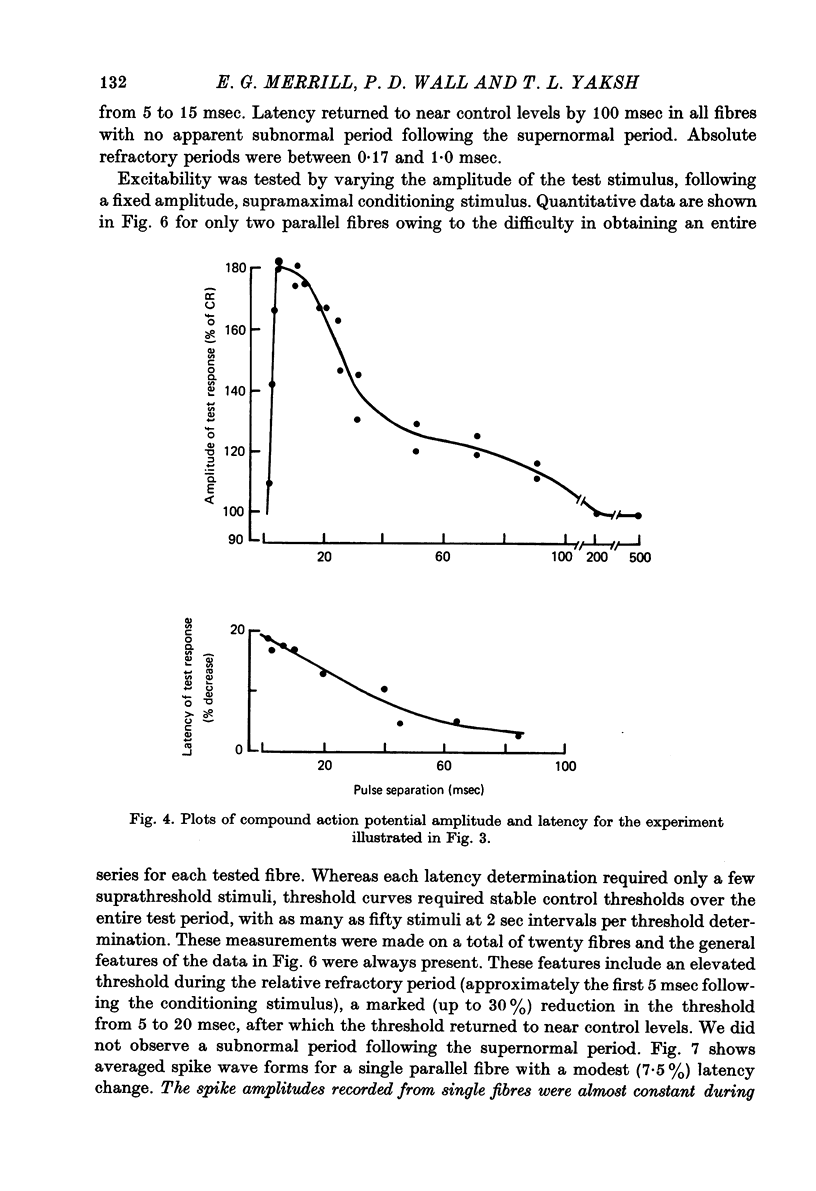
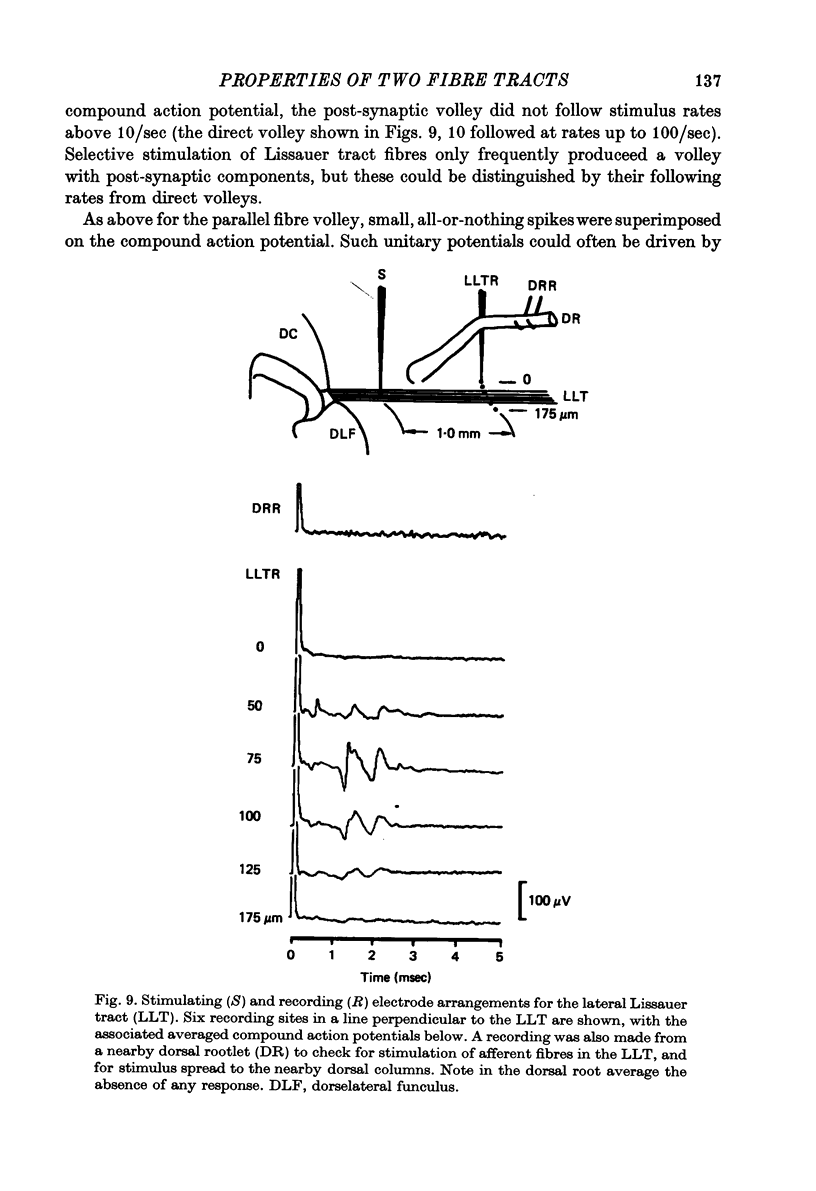
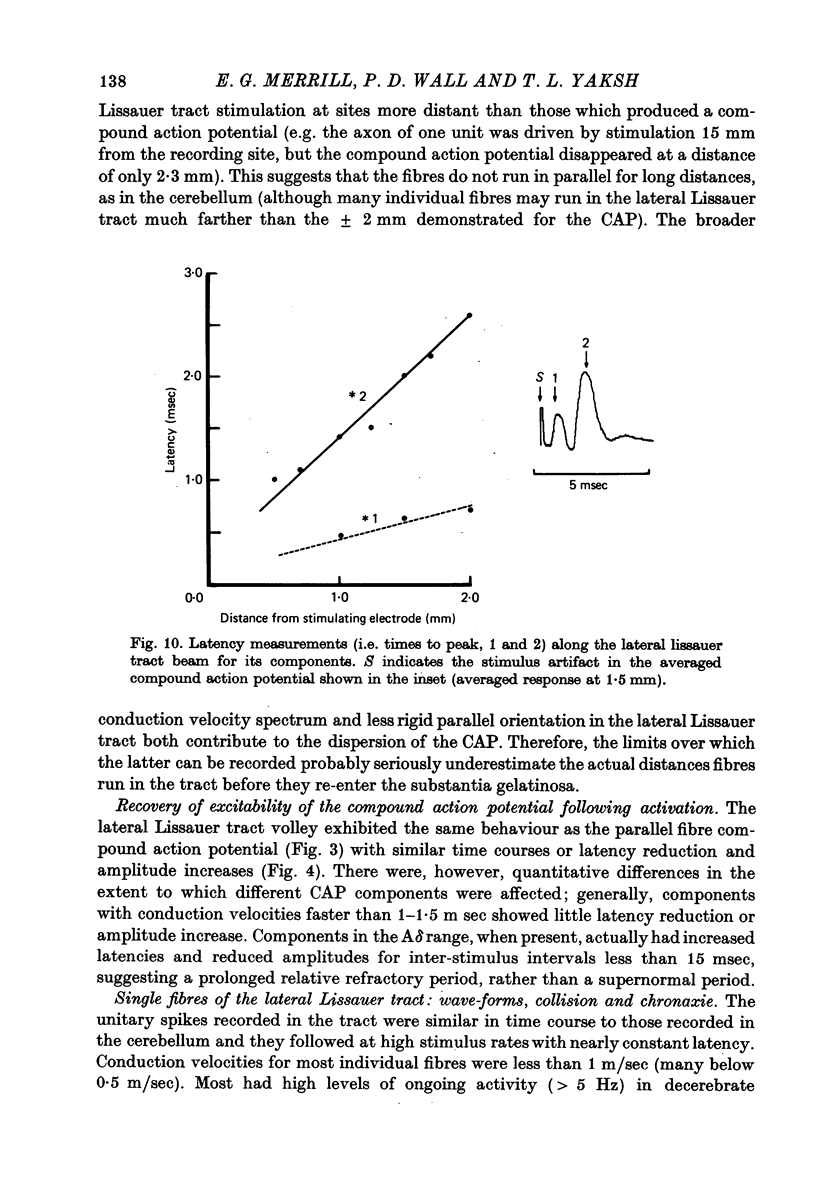
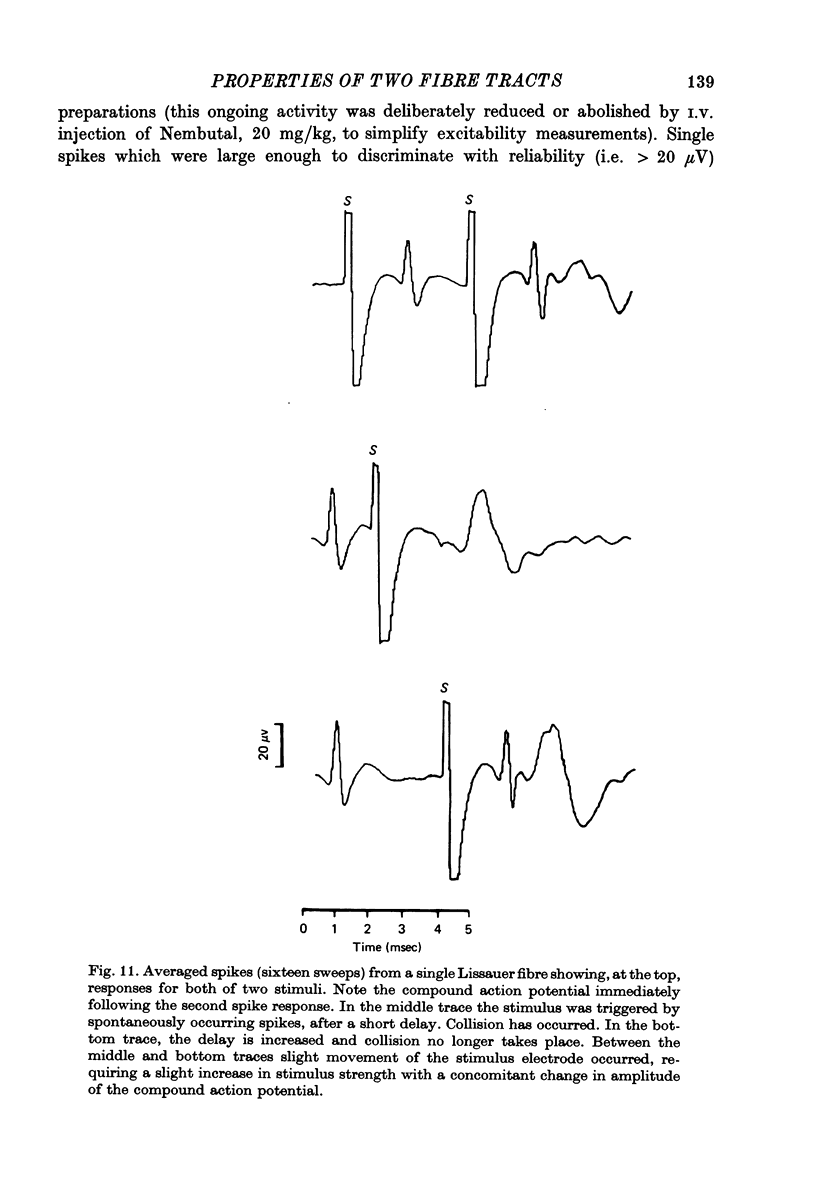
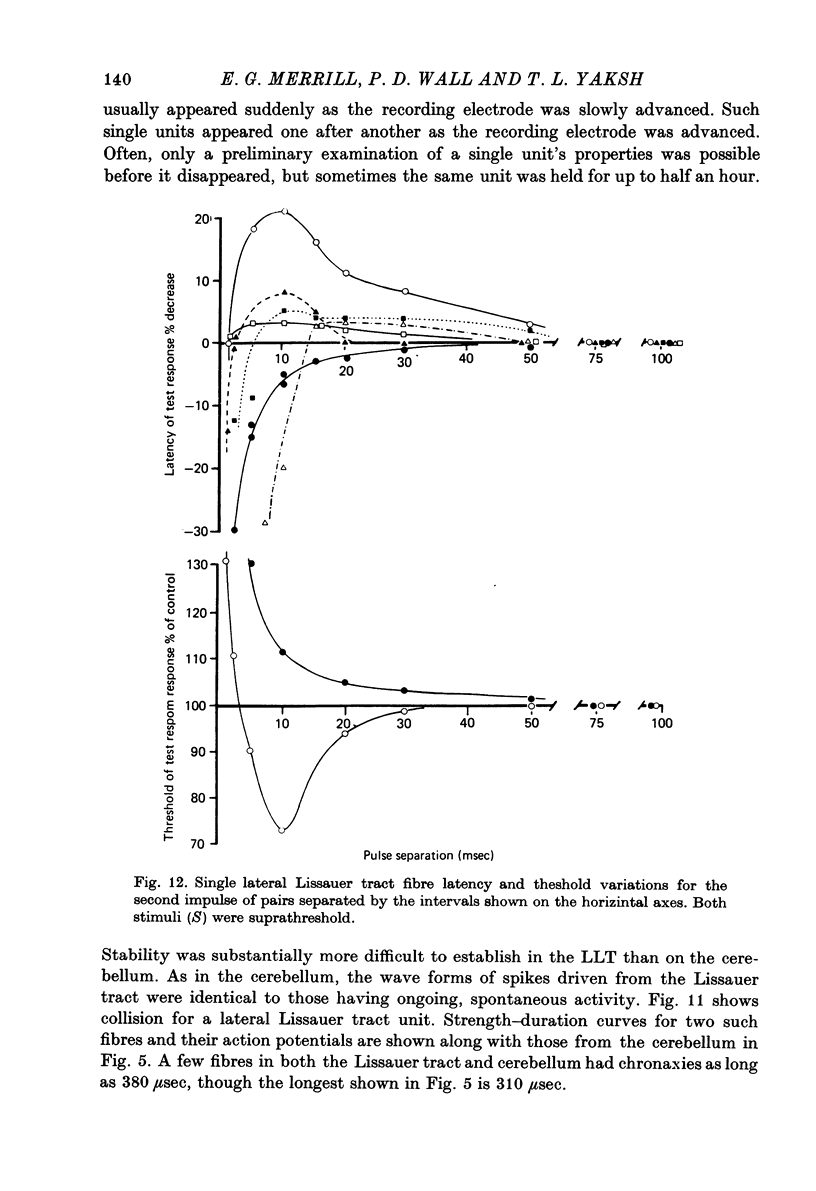
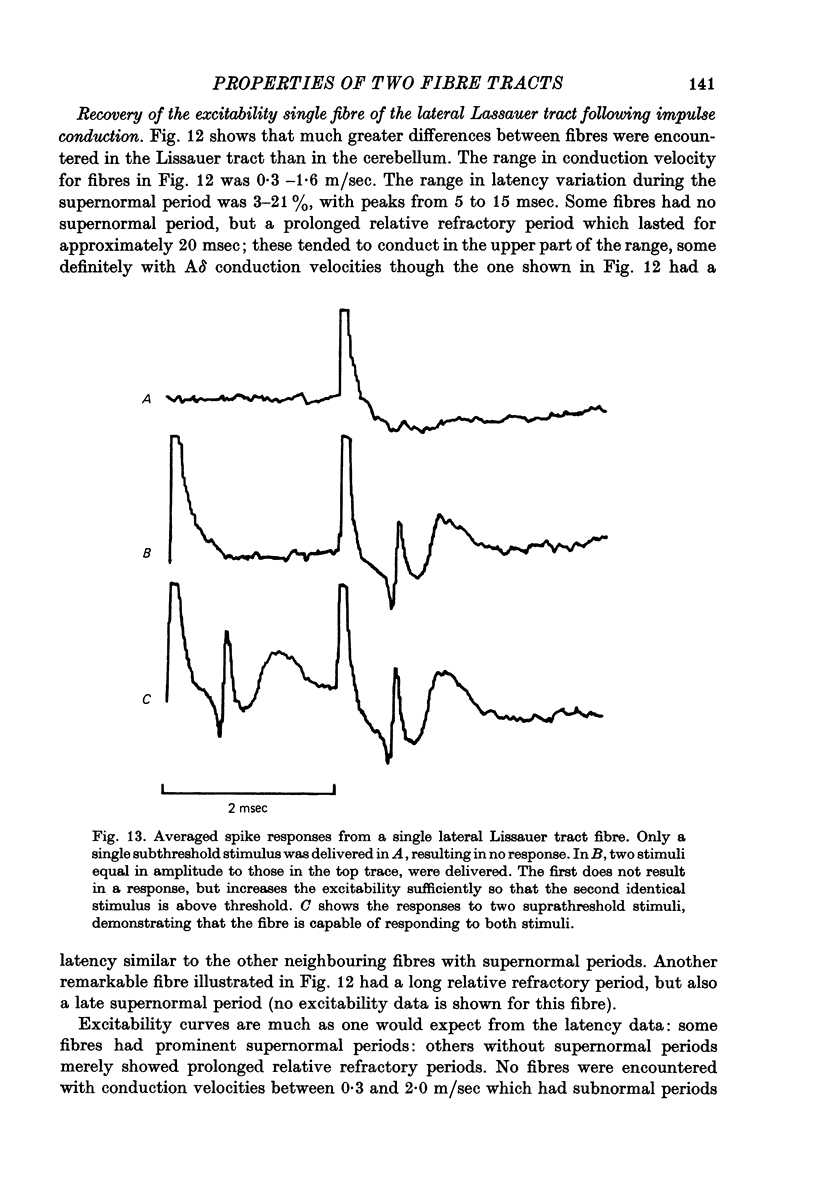
Abstract
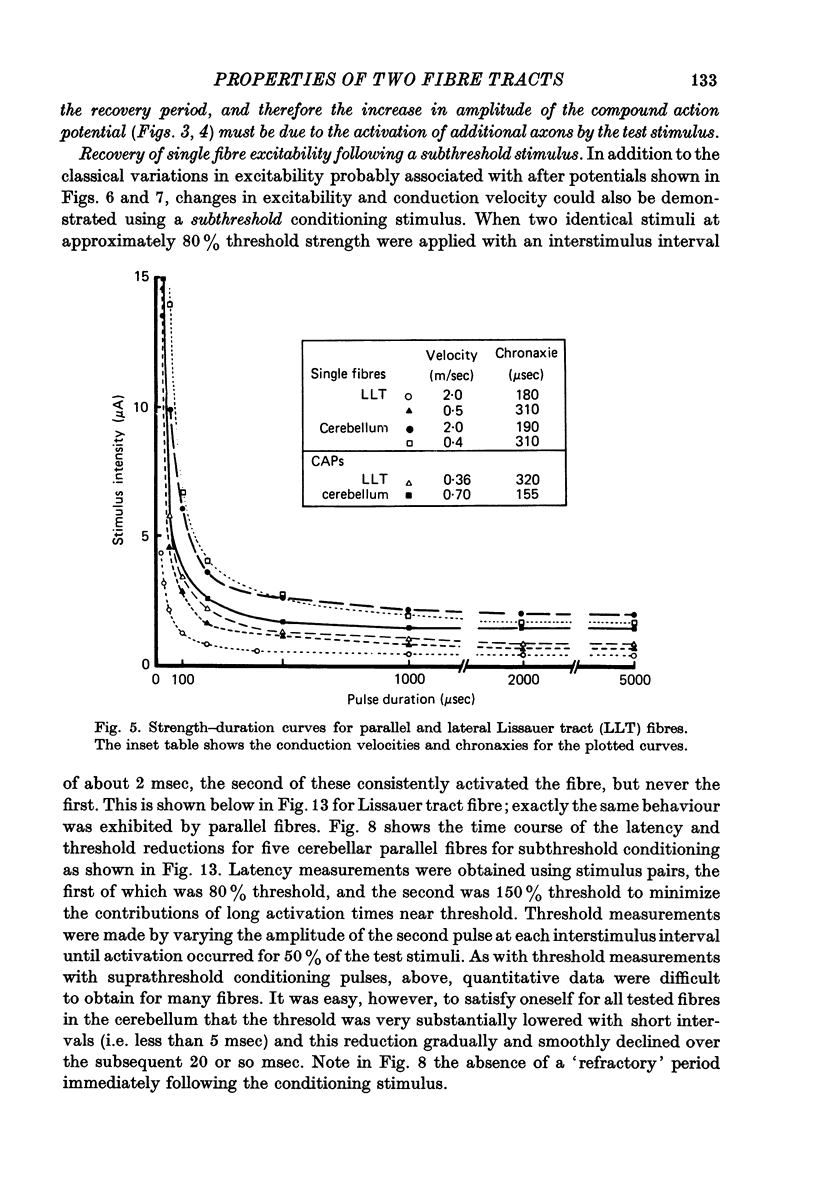
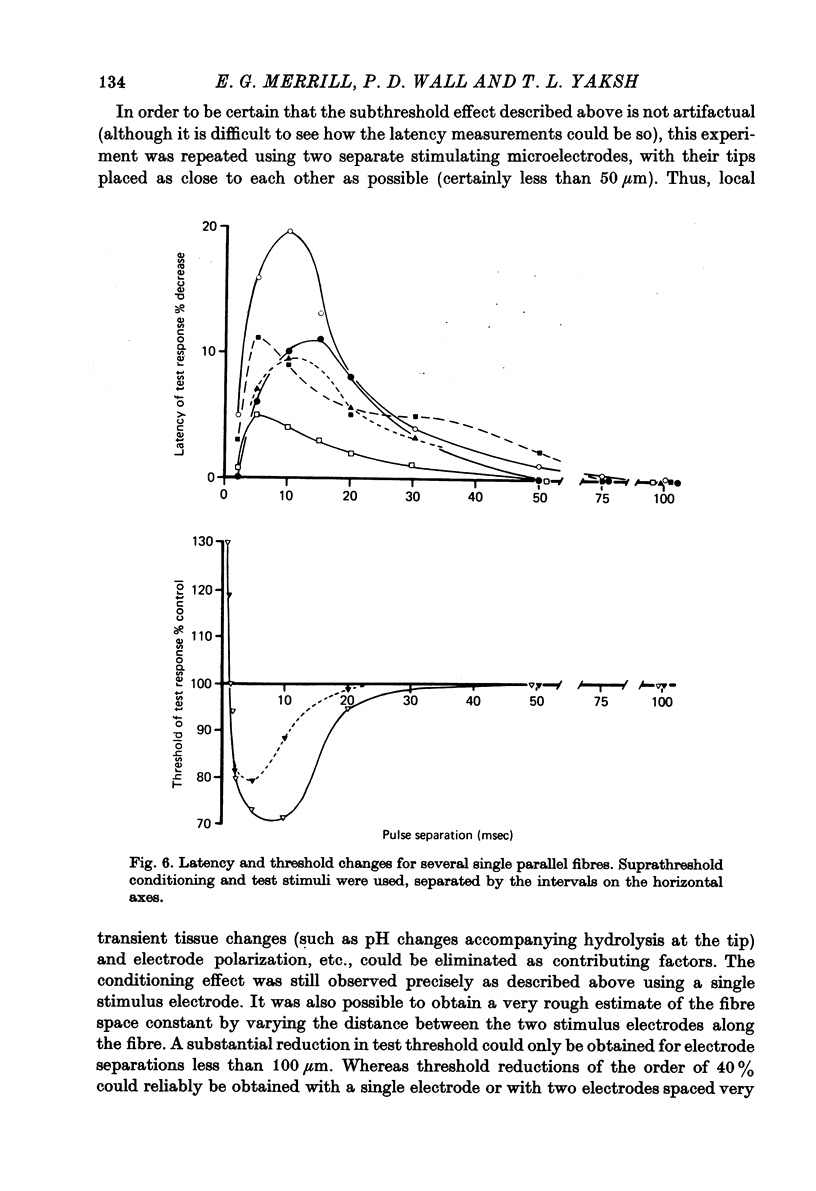
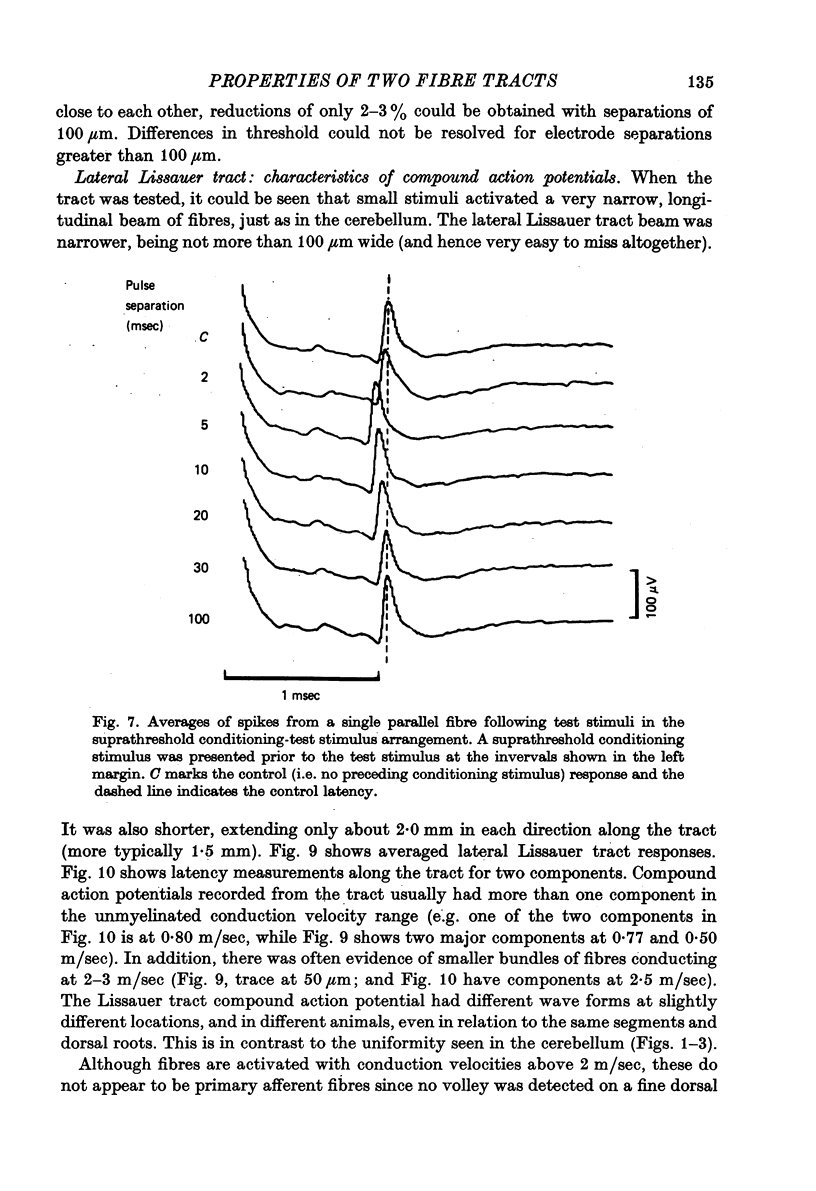
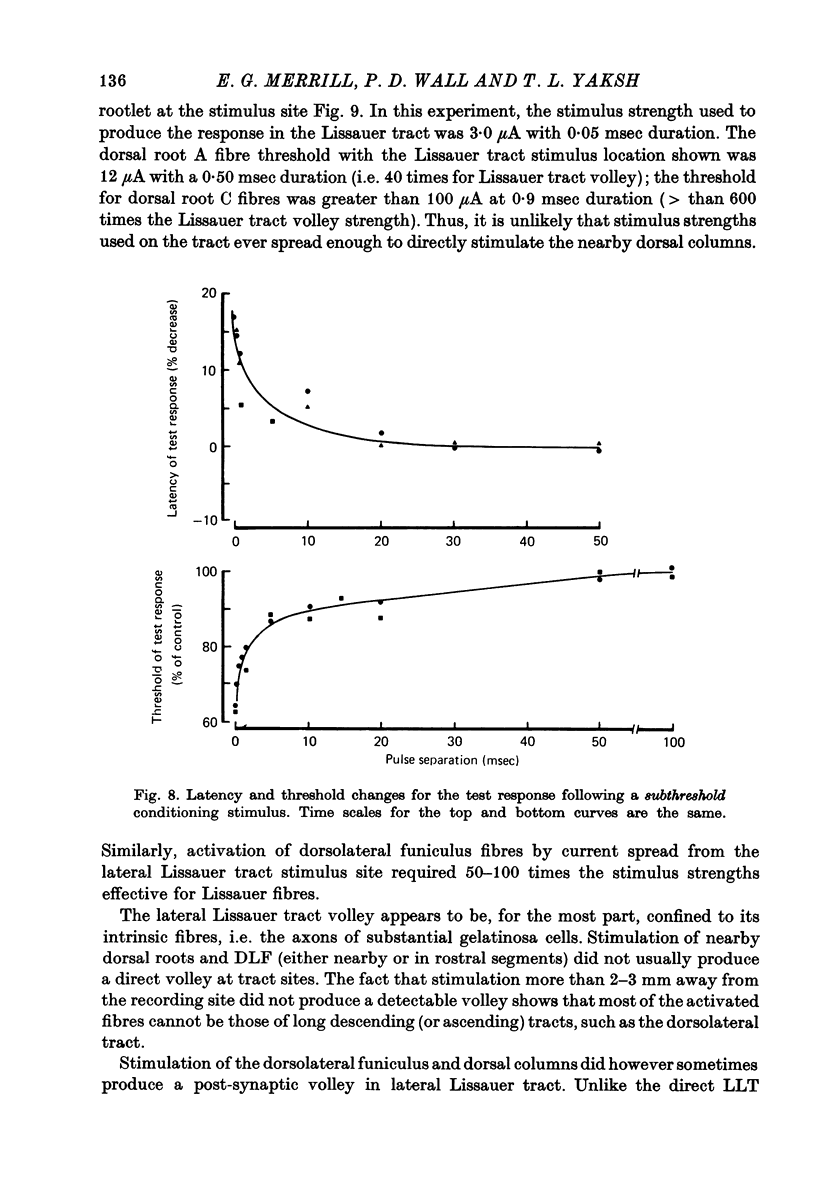
1. Monoplar tungsten micro-electrodes were used to stimulate and platinun plated tungsten micro-electrodes to record from single, unmyelinated cerebellar parallel fibres and lateral Lissauer tract axons in cats. 2. Stimulation of the lateral Lissauer tract resulted in the activation of a narrow, longitudinal 'beam', much as on the cerebellar surface. 3. Following impulse conduction, parallel and Lissauer tract fibres showed a supernormal conduction velocity (up to 25% increase) and increased excitability (up to 40% increase). No subnormality was encountered following supernormality. Some Lissauer tract fibres had prolonged relative refractory periods and no supernormal periods. 4. Chronaxies ranged from 155 to 380 microseconds. 5. Single fibres exhibited a remarkable increase in conduction velocity (up to 18% and excitability (up to 40%) following a single subthreshold stimulus. The duration of this effect (up to 20 msec) was much longer than expected from membrane time constant estimates.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bliss T. V., Rosenberg M. E. Proceedings: Supernormal conduction velocity in the olfactory nerve of the tortoise. J Physiol. 1974 May;239(1):60P–61P. [PubMed] [Google Scholar]
- Chung S. H., Bliss T. V., Keating M. J. The synaptic organization of optic afferents in the amphibian tectum. Proc R Soc Lond B Biol Sci. 1974 Nov 19;187(1089):421–447. doi: 10.1098/rspb.1974.0086. [DOI] [PubMed] [Google Scholar]
- Chung S. H., Keating M. J., Bliss T. V. Functional synaptic relations during the development of the retino-tectal projection in amphibians. Proc R Soc Lond B Biol Sci. 1974 Nov 19;187(1089):449–459. doi: 10.1098/rspb.1974.0087. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. Parallel fibre stimulation and the responses induced thereby in the Purkinje cells of the cerebellum. Exp Brain Res. 1966;1(1):17–39. doi: 10.1007/BF00235207. [DOI] [PubMed] [Google Scholar]
- Franz D. N., Iggo A. Conduction failure in myelinated and non-myelinated axons at low temperatures. J Physiol. 1968 Dec;199(2):319–345. doi: 10.1113/jphysiol.1968.sp008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY E. G. The granule cells, mossy synapses and Purkinje spine synapses of the cerebellum: light and electron microscope observations. J Anat. 1961 Jul;95:345–356. [PMC free article] [PubMed] [Google Scholar]
- Gardner-Medwin A. R. An extreme supernormal period in cerebellar parallel fibres. J Physiol. 1972 Apr;222(2):357–371. doi: 10.1113/jphysiol.1972.sp009802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koslow M., Bak A., Li C. L. C-fiber excitability in the cat. Exp Neurol. 1973 Dec;41(3):745–753. doi: 10.1016/0014-4886(73)90065-4. [DOI] [PubMed] [Google Scholar]
- MATURANA H. R., LETTVIN J. Y., MCCULLOCH W. S., PITTS W. H. Anatomy and physiology of vision in the frog (Rana pipiens). J Gen Physiol. 1960 Jul;43(6):129–175. doi: 10.1085/jgp.43.6.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill E. G., Ainsworth A. Glass-coated platinum-plated tungsten microelectrodes. Med Biol Eng. 1972 Sep;10(5):662–672. doi: 10.1007/BF02476084. [DOI] [PubMed] [Google Scholar]
- Merrill E. G., Wall P. D. Impulses recorded in cat substantia gelatinosa. J Physiol. 1975 Feb;245(2):82P–83P. [PubMed] [Google Scholar]
- Merrill E. G., Yaksh T. L. Properties of cerebellum parallel fibres and the Lissauer tract of the cat [proceedings]. J Physiol. 1978 Feb;275:71P–72P. [PubMed] [Google Scholar]
- RUDIN D. O., EISENMAN G. After-potential of spinal axons in vivo. J Gen Physiol. 1953 May;36(5):643–657. doi: 10.1085/jgp.36.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranck J. B., Jr Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res. 1975 Nov 21;98(3):417–440. doi: 10.1016/0006-8993(75)90364-9. [DOI] [PubMed] [Google Scholar]
- Richards C. D., Sercombe R. Calcium, magnesium and the electrical activity of guinea-pig olfactory coex in vitro. J Physiol. 1970 Dec;211(3):571–584. doi: 10.1113/jphysiol.1970.sp009294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow H. A. Systematic variations in the conduction velocity of slowly conducting axons in the rabbit corpus callosum. Exp Neurol. 1974 May;43(2):445–451. doi: 10.1016/0014-4886(74)90183-6. [DOI] [PubMed] [Google Scholar]
- Wall P. D. Impulses originating in the region of dendrites. J Physiol. 1965 Sep;180(1):116–133. [PMC free article] [PubMed] [Google Scholar]


