Abstract
The Th1/Th2 cytokines involved in human periodontitis remain unclear; therefore, we established a humanized mouse model to investigate this issue in Actinobacillus actinomycetemcomitans-mediated periodontal infection. Quantitative-PCR analysis clearly demonstrates a predominantly mixed Th1 and Th2 expression profile associated with pathogen-specific cell-mediated immunity via osteoprotegerin ligand (or RANK-L)-mediated alveolar bone destruction in vivo.
Human periodontal disease (i.e., periodontitis) results from interplay between specific subgingival microorganisms and inflammatory and immune responses (2). Actinobacillus actinomycetemcomitans, a gram-negative facultative capnophilic rod, is the major etiological pathogen of localized juvenile periodontitis (LJP; until recently known as localized aggressive periodontitis) and rapidly progressing periodontitis (34). A. actinomycetemcomitans releases several virulence factors, such as endotoxin and leukotoxin (6), and the infection is accompanied by local and systemic humoral immune responses (5). Earlier studies demonstrated an altered CD4/CD8 T-cell ratio and autologous mixed-lymphocyte reaction in LJP (14), suggesting a potential regulatory role of T cells in periodontitis. Some A. actinomycetemcomitans-specific CD4+ T cells could migrate to rat periodontal tissues and mediate immune protection (4, 33), suggesting that CD4+ T cells play a role in host periodontal defense. However, previous studies of Th1 versus Th2 cytokine expression in periodontitis have shown extremely mixed results (7, 9, 12, 18, 21, 23, 24, 30), which were confounded by uncertainties regarding the presence of ongoing disease activity, the specific microorganisms involved, and other undefined immune parameters.
We have established a humanized mouse model to study periodontal immune cell-parasite interactions (8, 25, 26) where (i) nonobese diabetic (NOD)-SCID mice can be reconstituted by human peripheral blood leukocytes (HuPBL; 30 to 60% chimerism); (ii) activated human CD4+ T cells are essential mediators of alveolar bone destruction; (iii) oral inoculation of HuPBL-NOD-SCID mice (which received HuPBL engraftments from LJP subjects) with A. actinomycetemcomitans leads to increased expression of osteoprotegerin ligand (OPGL, or RANK-L), a key mediator of osteoclastogenesis and osteoclast activation, by periodontal CD4+ T cells; and (iv) inhibition of OPGL via antagonistic osteoprotegerin (OPG) significantly reduces alveolar bone destruction after bacterial infection. These results suggest the critical role of microorganism-reactive human CD4+ T cells in periodontal pathogenesis. Further, a majority of the T-cell receptor (TCR) genes used by periodontal CD4+ T cells in these mice overlap (83% of TCR Vα; 91% of TCR Vβ) with those used by periodontal T cells in LJP patients (8). This suggests that a pathogen-associated human immune repertoire can be established in these mice.
To characterize the potential association of Th1 and Th2 cytokines in periodontitis, we studied their expression profiles by quantitative-PCR analysis of periodontal tissues of six different groups of A. actinomyctemcomitans-inoculated HuPBL-NOD-SCID mice (10 to 16 mice per donor group) whose autologous HuPBL were obtained from four LJP patients and two periodontitis-free healthy subjects, individually. Briefly, four LJP patients [LJP1 to LJP4; mean age, 21 ± 4.4 years] and two disease-free healthy subjects [N1 and N2; ages, 19 and 22 years] had been described previously (8, 26). Informed consent was obtained from all of the patients, and all protocols were approved by the human ethics and animal experimentation committees of the University of Western Ontario. Eighty-nine female NOD-SCID mice, 8 to 9 weeks old, were obtained from the breeding suites of the animal colony and housed in a specific-pathogen-free unit. The experimental protocols were described in detail previously (8, 25), and the levels of individual HuPBL engraftment in the mice were comparable (≥30%) to those reported in our previous studies.
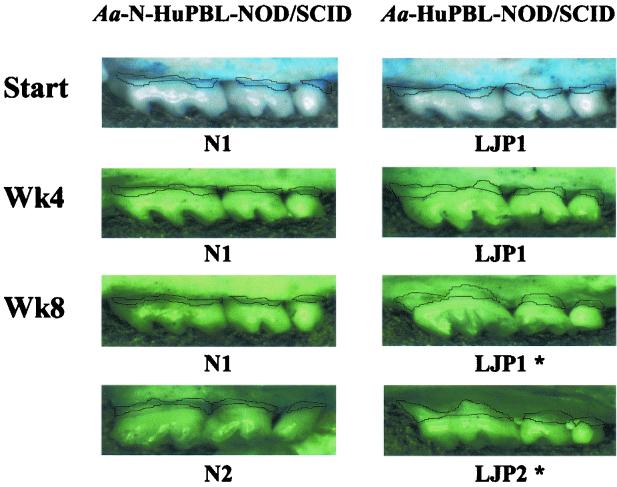
Among all of the A. actinomycetemcomitans-inoculated HuPBL-NOD-SCID mice studied, significant periodontal inflammation and bone destruction were detected by the end of 8 weeks (25, 26). However, when HuPBL samples from N1 and N2 were used, there was very little periodontal inflammatory infiltrate and alveolar bone loss detected by 8 weeks (26). Due to this factor, periodontal and cervical lymph node-derived CD4+ T cells were collected from a pool of 10 to 14 A. actinomycetemcomitans-inoculated N-HuPBL-NOD-SCID mice (engrafted with autologous HuPBL from N1 or N2 [26]) as the cellular sources for PCR analyses (8). This was further supported in our previous study (26), where periodontal CD4+ T cells isolated from A. actinomycetemcomitans-inoculated N-HuPBL-NOD-SCID mice showed very little OPGL expression by fluorescence-activated cell sorter analysis, in contrast to those isolated from A. actinomycetemcomitans-inoculated HuPBL-NOD-SCID mice. Subsequently, the amount of alveolar bone loss on the upper molars, the surface areas (in square micrometers) between the cement-enamel junction (CEJ) and the alveolar bone crest (ABC) measured at ×16 magnification, was quantitated using a Leica MZ95 stereomicroscope with a Hamamatsu digital camera and Openlab version 3.0.8 software. As a result, the net alveolar bone loss detected in A. actinomycetemcomitans-inoculated HuPBL-NOD-SCID mice by week 8 was significantly greater than that in A. actinomycetemcomitans-inoculated N-HuPBL-NOD-SCID mice (26). For the controls, where both groups were sham infected, only background levels of alveolar bone loss were detected by 8 weeks (26). Collectively, these findings are summarized in Fig. 1, where the amount of alveolar bone loss is indicated. These results are consistent with those of other mouse studies, in which progression to active alveolar bone destruction occurred in 6 to 8 weeks (1, 32).
FIG. 1.
Net alveolar bone loss detected at various time points (start, week 4 [Wk4], and week 8 [Wk8]) in A. actinomycetemcomitans-inoculated N-HuPBL-NOD-SCID (HuPBL samples from N1 and N2 subjects) versus A. actinomycetemcomitans-inoculated HuPBL-NOD-SCID mice (HuPBL samples from subjects LJP1 and LJP2) by using a digital imaging system measuring the exposed root surfaces between CEJ and ABC (see the text for details). The blue stains represent the exposed root surface areas (CEJ-ABC) on the upper molars. The solid lines indicate the surface areas measured (in square micrometers) for alveolar bone loss. The bottom two images from each group represent the typical result of alveolar bone loss at week 8 (from subjects N1, N2, LJP1, and LJP2). *, statistical significance compared to other group of samples (P < 0.01) (26).
To study pathogen-specific T-cell immunity, we have previously employed quantitative PCR to assess the expression levels of individual human TCR gene transcripts in LJP patients and humanized mice (8). In the present study, to investigate which (Th1 or Th2) cytokine expression profile was associated with alveolar bone destruction in vivo, periodontal CD4+ T cells were purified (>90 to 95%) as described previously and then subjected to in vitro stimulation with autologous irradiated monocytes/macrophages and A. actinomycetemcomitans sonicate antigens in 24-well plates (8, 25). After 48 h, 2 × 106 to 3 × 106 A. actinomycetemcomitans-reactive periodontal CD4+ T cells were collected by Ficoll gradient centrifugation, followed by fluorescence-activated cell sorter scanning and sorting (FACSVantage; BD Biosciences) for OPGL-expressing CD4+ T cells by using OPG-Fc-fluorescein isothiocyanate conjugate (purity, >95 to 97%) (26). Subsequently, total RNA was prepared from individual pools of 106 A. actinomycetemcomitans-reactive OPGL-producing periodontal CD4+ T cells representing individual groups of A. actinomycetemcomitans-inoculated N-HuPBL-NOD-SCID mice and A. actinomycetemcomitans-inoculated HuPBL-NOD-SCID mice for quantitative-PCR analysis (8, 27). Later, first-strand cDNA was prepared from 2 μg of total RNA and then precipitated and diluted in 40 μl of Tris-EDTA (8). cDNA from each sample (2 μl) was aliquoted into PCR tubes containing specific forward and reverse primers of human Th1 and Th2 cytokine genes, as described elsewhere (3). The amplified human β-actin (hβ-actin) gene products were used as the internal controls for all PCRs and subsequent quantitation. Another endogenous control, hTCR-Cα primers (8), was included to compare the total amounts of transcripts derived from each of the 106 A. actinomycetemcomitans-specific OPGL-expressing CD4+ T cells described above for quantitative-PCR analyses. Based on our previous studies (8), all PCRs were carried out for 30 cycles under the following conditions: 94°C denaturation for 1 min, 60°C annealing for 1 min, and 72°C extension for 1.5 min (RoboCycler 96 gradient; Stratagene, La Jolla, Calif.) with an additional 7 min at 72°C after the last cycle. The resulting PCR products were analyzed by electrophoresis in 2% agarose gels for their respective sizes (3, 8). All PCRs and subsequent quantifications were performed at least two or three times from the same cDNA sources to ensure consistent and reproducible measurements. The identities of the cytokine transcripts amplified were confirmed by Southern blot analysis using specific probes. The fluorescence intensities of the amplified PCR products were captured by an Ultra-Violet Products digital camera and quantitated via image acquisition and analysis software, LabWorks (Upland, Calif.) version 3.0.2. The resulting signal intensities were then normalized to the mean values of the internal control, hβ-actin, or hTCR-Vα gene, which was set as 1. Repeated (two or three times) PCR analyses of individually amplified cytokine transcripts did not change the results obtained, suggesting high reproducibility of the study.
The results of the quantitative-PCR analyses showed that a predominantly mixed Th1-Th2 expression profile was manifest, not a Th0 profile (as interleukin-2 [IL-2] expression was not high throughout) (Fig. 2 and 3); moreover, this was associated with A. actinomycetemcomitans-specific OPGL-mediated alveolar bone destruction (Fig. 1) (26). As these findings were obtained under the same microbial and immune specificities defined throughout (8, 25, 26), the present study provides unambiguous significance not evident in previous studies (7, 9, 12, 18, 21, 23, 24, 30) for their association with a cytokine expression pattern in “active” periodontitis. Although the A. actinomycetemcomitans-reactive periodontal CD4+-T-cell repertoire is relatively widespread, with a few dominant genes shared by LJP patients (8), the results obtained here suggest that each bacterial antigen and epitope stimulates a different Th1 and/or Th2 response (10). As a result, different bacterial antigen-specific Th1 or Th2 cells may elicit destructive and/or protective immunity during disease pathogenesis (13). A Th2 clone that mediated protection in a rat periodontitis model (4, 33) has been reported; however, it has also been shown that both Th1 and Th2 cells are capable of triggering destruction in different diseases (10, 16). Experiments are under way to clarify whether (i) A. actinomycetemcomitans-specific antigens associated with periodontal destruction (Y.-T. A. Teng, W. Hu, and G. Xing, submitted for publication) induces either a Th1 or Th2 cytokine profile, or both, at the clonal level and (ii) antigen-specific human Th1 or Th2 cells are capable of mediating alveolar bone destruction.
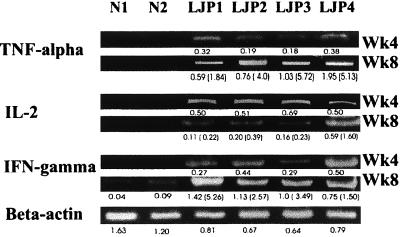
FIG. 2.
Predominant Th1 cytokine tumor necrosis factor (TNF) alpha and IFN-γ, not IL-2, expression from week 4 (Wk4) to week 8 (Wk8) in A. actinomycetemcomitans-inoculated HuPBL-NOD-SCID mice (individually engrafted with autologous HuPBL from LJP1 to LJP4) in contrast to that detected in A. actinomycetemcomitans-inoculated N-HuPBL-NOD-SCID mice (individually engrafted with autologous HuPBL from N1 and N2). The numbers below each band show the fluorescence intensities of the quantified PCR signals by computation, after normalizing to the mean value of all of the control hβ-actin signals. The numbers within parentheses indicate the ratios of the fluorescence intensities of individual cytokine expression between week 8 and week 4 to demonstrate changes over time. The results obtained by using hTCR-Vα gene products for control and quantitation were the same (data not shown). The results shown here illustrate representative data from one of three independent experiments.
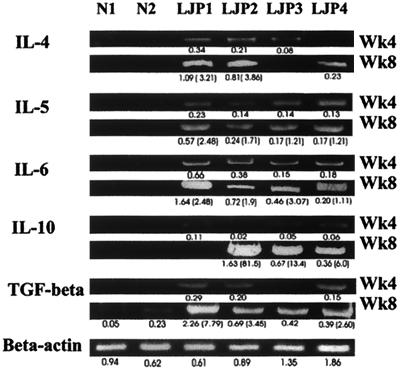
FIG. 3.
Predominant Th2 cytokine expression from week 4 (Wk4) to week 8 (Wk8) in A. actinomycetemcomitans-inoculated HuPBL-NOD-SCID mice in contrast to that detected in A. actinomycetemcomitans-inoculated N-HuPBL-NOD-SCID mice. The numbers below each band show the fluorescence intensities of the quantified PCR signals by computation, after normalizing to the mean value of all of the control hβ-actin. The numbers within parentheses indicate the ratios of the fluorescence intensities of individual cytokine expression between week 8 and week 4 to demonstrate changes over time. The results shown here illustrate representative data from one of three independent experiments. The results obtained by using hTCR Vα gene products for control and quantitation were the same (data not shown). TGF, transforming growth factor.
Only recently has T-cell-mediated OPGL-induced osteoclastogenesis been considered a prime regulator in microorganism-mediated osteolytic diseases, such as periodontitis, adjuvant-induced arthritis, and human arthritic diseases (15, 26, 29). Conceivably, Th1 and/or Th2 cytokines may participate in a counterregulatory feedback loop, along with OPGL-OPG, for homeostatic control of bone destruction (15, 29). Some Th1-Th2 cytokines have been shown to be associated with periodontitis (1, 7); however, both IL-4 and gamma interferon (IFN-γ) can inhibit osteoclastogenesis (22, 31). Thus, the precise mechanisms by which these cytokines modulate alveolar bone destruction during the onset or early stage of periodontitis are not clear. In the present study, tumor necrosis factor alpha and IFN-γ expression levels became higher when significant alveolar bone destruction was detected by 8 weeks (Fig. 1 and 2) (26). One possibility may be that increasing Th1 expression is required before progression to periodontal destruction, while certain Th2 antiinflammatory cytokines (i.e., IL-10 and transforming growth factor β [Fig. 3]) are involved in protection from and repair of tissue loss (7, 12, 23). Our recent studies using sandwich enzyme-linked immunosorbent assays for protein expression in cultures showed the same Th1-Th2 profiles (data not shown). Further studies are needed to explore the signaling interrelationships between OPGL-OPG and specific Th1-Th2 cytokine expressions in order to understand this issue.
The present study is the first describing a clear microorganism-specific Th1 and Th2 expression profile associated with cell-mediated immunity for tissue destruction in periodontitis. The majority of the lymphocytes in A. actinomycetemcomitans-inoculated HuPBL-NOD-SCID mice manifest an activated-memory phenotype (CD45RO+) (25); therefore, this model may have limited value for studying the primary immune response. Nevertheless, the present approach does not deal with the initiation of periodontal inflammation and infection. It remains to be seen whether Th1 or Th2 cytokines are involved at the onset stage preceding significant alveolar bone destruction. Alternatively, other cell types (i.e., dendritic cells, macrophages, or resident cells 11, 17, 19, 20), or the local microenvironment (28) may be involved in directing Th1 or Th2 differentiation for periodontal inflammation or destruction.
In summary, the present study reveals that both Th1 and Th2 cytokines are associated with A. actinomycetemcomitans-specific human T-cell-mediated immunity for periodontal destruction in vivo. It will be of interest to determine whether directing the microorganism-specific immunity to a Th1 or Th2 profile would have any influence on the initiation or progression of periodontal disease.
Acknowledgments
I thank J. Penninger at the University of Toronto for a generous gift of OPG-fluorescein isothiocyanate, Stephen Sims at the University of Western Ontario for critical review of the manuscript, and Giujuan Gao for her technical assistance.
This work was supported by grants to Y.-T.A.T. from the Ministry of Health of Ontario, Canada; the London Health Sciences Center (IRF-029-00); the Canadian Institute of Health Research (CIHR) (MOP-37960); the National Institutes of Health (NIH), Bethesda, Md. (DE12969-01 and DE14473-01); and the University of Western Ontario, London, Ontario, Canada.
Editor: T. R. Kozel
REFERENCES
- 1.Baker, P. J., M. Dixon, R. T. Evans, L. Dufour, and D. C. Roopenian. 1999. CD4+ T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect. Immun. 67:2804-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark, W. B., and H. Loe. 1993. Mechanisms of initiation and progression of periodontal disease. Periodontol. 2000 2:72-82. [DOI] [PubMed] [Google Scholar]
- 3.Coligan, J. E., A. M. Kruisbeek, D. H. Margulies, E. M. Shevach, and W. Strober. 1996. Current protocols in immunology, vol. 2, suppl. 10, p. 10.23.2. John Wiley & Sons, Inc., New York, N.Y.
- 4.Eastcott, J. W., K. Yamashita, M. A. Taubman, J. Harada, and D. J. Smith. 1994. Adoptive transfer of cloned T helper cells ameliorates periodontal disease in nude rats. Oral Microbiol. Immunol. 9:284-289. [DOI] [PubMed] [Google Scholar]
- 5.Ebersole, J. L., and M. A. Taubman. 1994. The protective nature of host responses in periodontal diseases. Periodontol. 2000 5:112-141. [DOI] [PubMed] [Google Scholar]
- 6.Fives-Tayler, P., D. Meyer, and K. Mintz. 1996. Virulence factors of the periodontopathogen Actinobacillus actinomycetemcomitans. J. Periodontol. 67:291-297. [DOI] [PubMed] [Google Scholar]
- 7.Fujihashi, K., M. Yamamoto, T. Hiroi, T. V. Bamberg, J. R. McGhee, and H. Kiyono. 1996. Selected Th1 and Th2 cytokine mRNA expression by CD4(+) T cells isolated from inflamed human gingival tissues. Clin. Exp. Immunol. 103:422-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao, X., and Y.-T., A. Teng. T-cell-receptor gene usage of Actinobacillus actinomycetemcomitans-reactive periodontal CD4+ T cells from localized juvenile periodontitis patients and human peripheral blood leukocyte-reconstituted NOD/SCID mice. J. Periodontol. Res., in press. [DOI] [PubMed]
- 9.Germmell, E., and G. J. Seymour. 1998. Cytokine profiles of cells extracted from humans with periodontal diseases. J. Dent. Res. 77:16-26. [DOI] [PubMed] [Google Scholar]
- 10.Iqbal, N., J. R. Oliver, F. H. Wagner, A. S. Lazenby, C. O. Elson, and C. T. Weaver. 2002. T helper 1 and T helper 2 cells are pathogenic in an antigen-specific model of colitis. J. Exp. Med. 195:71-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalinski, P., C. M. Hilkens, E. A. Wierenga, and M. Kapsenberg. 1999. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol. Today 20:561-567. [DOI] [PubMed] [Google Scholar]
- 12.Kawai, T., R. Eisen-Lev, M. Seki, J. W. Eastcott, M. E. Wilson, and M. A. Taubman. 2000. Requirement of B7 costimulation for Th1-mediated inflammatory bone resorption in experimental periodontitis. J. Immunol. 164:2102-2109. [DOI] [PubMed] [Google Scholar]
- 13.Kelso, A. 1995. Th1 and Th2 subsets—paradigms lost. Immunol. Today 16:374-379. [DOI] [PubMed] [Google Scholar]
- 14.Kinane, D. F., F. A. Jonston, and C. W. Evans. 1989. Depressed helper-to-suppressor T-cell ratios in early-onset forms of periodontal disease. J. Periodontol. Res. 24:161-164. [DOI] [PubMed] [Google Scholar]
- 15.Kong, Y. Y., U. Feige, I. Sarosi, B. Bolon, A. Tafuri, S. Morony, C. Capparelli, J. Li, R. Elliott, S. McCabe, et al. 1999. Activated T cells regulate bone loss and joint destruction in arthritis via OGPL. Nature 402:304-309. [DOI] [PubMed] [Google Scholar]
- 16.Lafaille, J. J., F. V. Keere, A. L. Hsu, J. L. Baron, W. Haas, C. S. Raine, and S. Tonegawa. 1997. Myelin basic protein-specific T helper 2 (Th2) cells cause experimental autoimmune encephalomyelitis in immunodeficient hosts rather than protect them from the disease. J. Exp. Med. 186:307-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacDonald, A. S., and E. Pearce. 2002. Cutting edge: polarized Th cell response induction by transferred antigen-pulsed dendritic cells is dependent on IL-4 or IL-12 production by recipient cells. J. Immunol. 168:3127-3130. [DOI] [PubMed] [Google Scholar]
- 18.Manhart, S. S., R. A. Reinhardt, J. B. Payne, G. J. Seymour, E. Gemmell, J. K. Dyer, et al. 1994. Gingival cell IL-2 and IL-4 in early-onset periodontitis. J. Periodontol. 65:807-813. [DOI] [PubMed] [Google Scholar]
- 19.Rani, C. S., and M. MacDougall. 2000. Dental cells express factors that regulate bone resorption. Mol. Cell. Biol. Res. Commun. 3:145-152. [DOI] [PubMed] [Google Scholar]
- 20.Reis e Sousa, C., A. Sher, and P. Kaye. 1999. The role of dendritic cells in the induction and regulation of immunity to microbial infection. Curr. Opin. Immunol. 11:392-399. [DOI] [PubMed] [Google Scholar]
- 21.Sigusch, B., G. Klinger, E. Glockmann, and S. Hu. 1998. Early-onset and adult periodontitis associated with abnormal cytokine production by activated T lymphocytes. J. Periodontal. 69:1098-1104. [DOI] [PubMed] [Google Scholar]
- 22.Takayanagi, H., K. Ogasawara, S. Hida, T. Chiba, S. Murata, K. Sato, et al. 2000. T cell mediated regulation of osteoclastogenesis by signaling cross-talk between RANKL and IFN-γ. Nature 408:600-605. [DOI] [PubMed] [Google Scholar]
- 23.Takeichi, O., J. Haber, T. Kawai, D. J. Smith, I. Moro, and M. A. Taubman. 2000. Cytokine profile of T lymphocytes from gingival tissues with pathological pocketing. J. Dent. Res. 79:1548-1555. [DOI] [PubMed] [Google Scholar]
- 24.Takeichi, O., M. A. Taubman, J. Haber, D. J. Smith, and I. Moro. 1994. Cytokine profiles of CD4 and CD8 T cells isolated from adult periodontitis gingivae. J. Dent. Res. 73:205-211. [Google Scholar]
- 25.Teng, Y.-T. A., H. Nguyen, A. Hassanloo, R. P. Ellen, H. Hozumi, and R. M. Gorczynski. 1999. Periodontal immune responses of human lymphocytes in Actinobacillus actinomycetemcomitans-inoculated NOD-SCID mice engrafted with peripheral blood leukocytes of periodontitis patients. J. Periodontol. Res. 34:54-61. [DOI] [PubMed] [Google Scholar]
- 26.Teng, Y.-T. A., H. Nguyen, X. Gao, Y.-Y. Kong, R. M. Gorczynski, B. Singh, R. P. Ellen, and J. M. Penninger. 2000. Functional human T-cell immunity and osteoprotegerin-ligand control alveolar bone destruction in periodontal infection. J. Clin. Investig. 106:R59-R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teng, Y.-T., D. Williams, N. Hozumi, and R. M. Gorczynski. 1996. Multiple levels of regulation for self-tolerance in beef insulin transgenic mice. Cell Immunol. 173:183-191. [DOI] [PubMed] [Google Scholar]
- 28.Teng, Y.-T. A., S. Iwasaki, D. Williams, R. Gorczynski, and N. Hozumi. 1995. Evidence for Th2 cell-mediated suppression of antibody responses in transgenic, beef-insulin tolerant mice. Eur. J. Immunol 25:2522-2527. [DOI] [PubMed] [Google Scholar]
- 29.Theill, L. E., W. J. Boyle, and J. M. Penninger. 2002. RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu. Rev. Immunol. 20:795-823. [DOI] [PubMed] [Google Scholar]
- 30.Tokoro, Y., Y. Matsuki, T. Yamamoto, T. Suzuki, and K. Hara. 1997. Relevance of local Th2-type cytokine mRNA expression in immunocompetent infiltrates in inflamed gingival tissue to periodontal diseases. Clin. Exp. Immunol. 107:166-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei, S., M. W. H. Wang, S. L. Teitelbaum, and F. P. Ross. 2002. Interleukin-4 reversibly inhibits osteoclastogenesis via inhibition of NF-κB and mitogen-activated protein kinase signaling. J. Biol. Chem. 277:6622-6630. [DOI] [PubMed] [Google Scholar]
- 32.Wray, D., and L. Graeme. 1992. Periodontal bone loss in mice induced by different periodontopathic organisms. Arch. Oral Biol. 37:435-438. [DOI] [PubMed] [Google Scholar]
- 33.Yamashita, K., J. W. Eastcott, M. A. Taubman, J. D. Smith, and D. S. Cox. 1991. Effect of adoptive transfer of cloned Actinobacillus actinomycetemcomitans-specific T helper cells on periodontal disease. Infect. Immun. 59:1529-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zambon, J. J., T. Umemoto, E. De Nardin, F. Nakazawa, J. A. Christersson, and R. J. Genco. 1988. Actinobacillus actinomycetemcomitans in the pathogenesis of human periodontal disease. Adv. Dent. Res. 2:269-274. [DOI] [PubMed] [Google Scholar]