Abstract
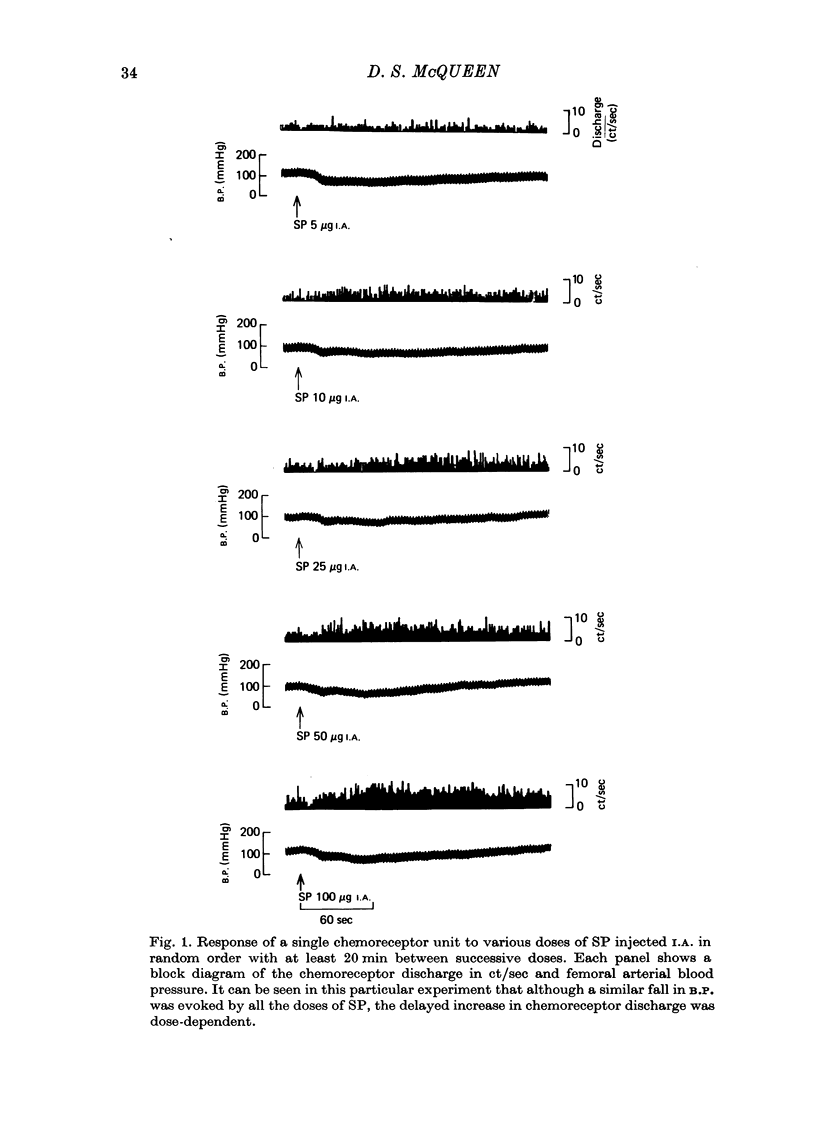
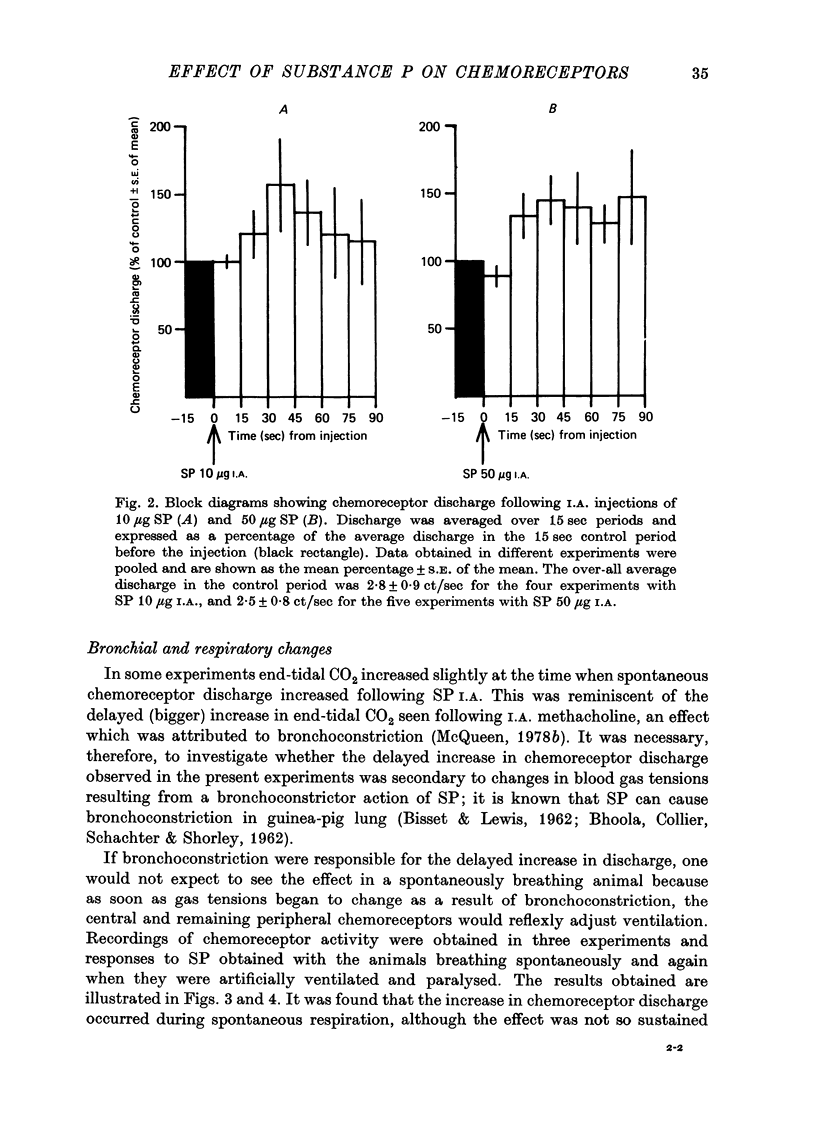
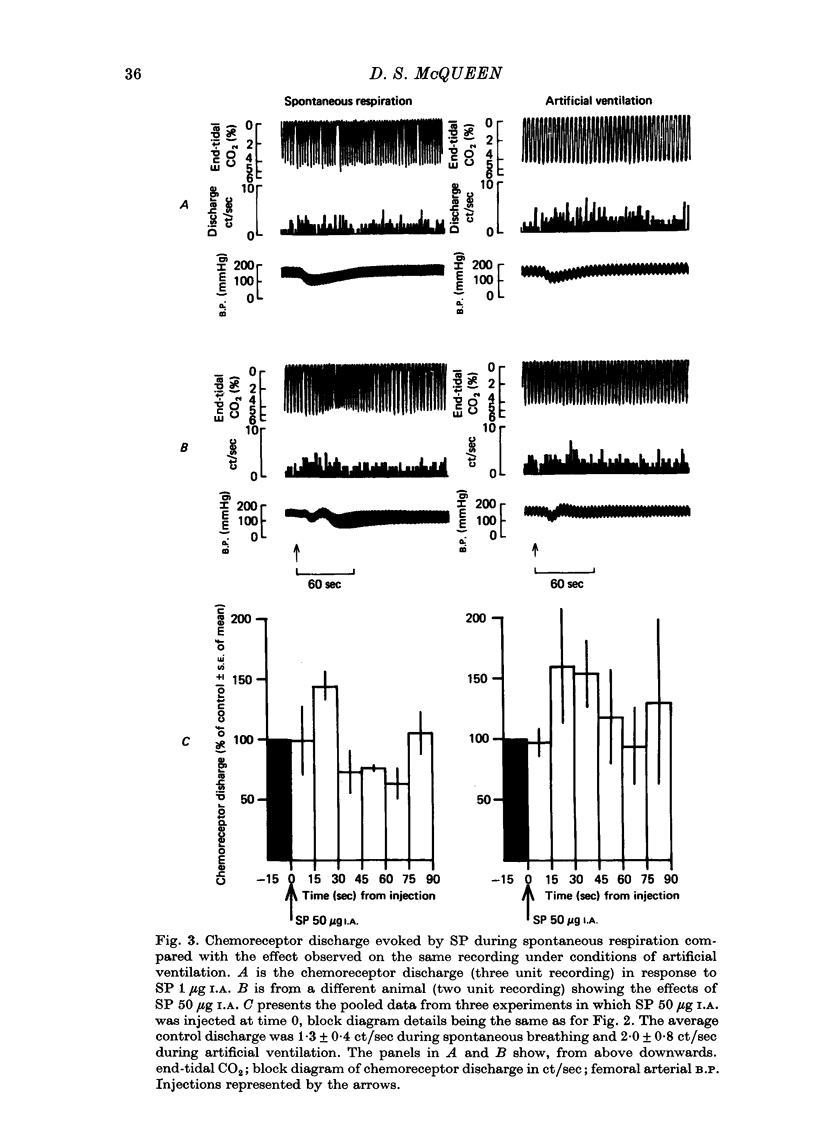
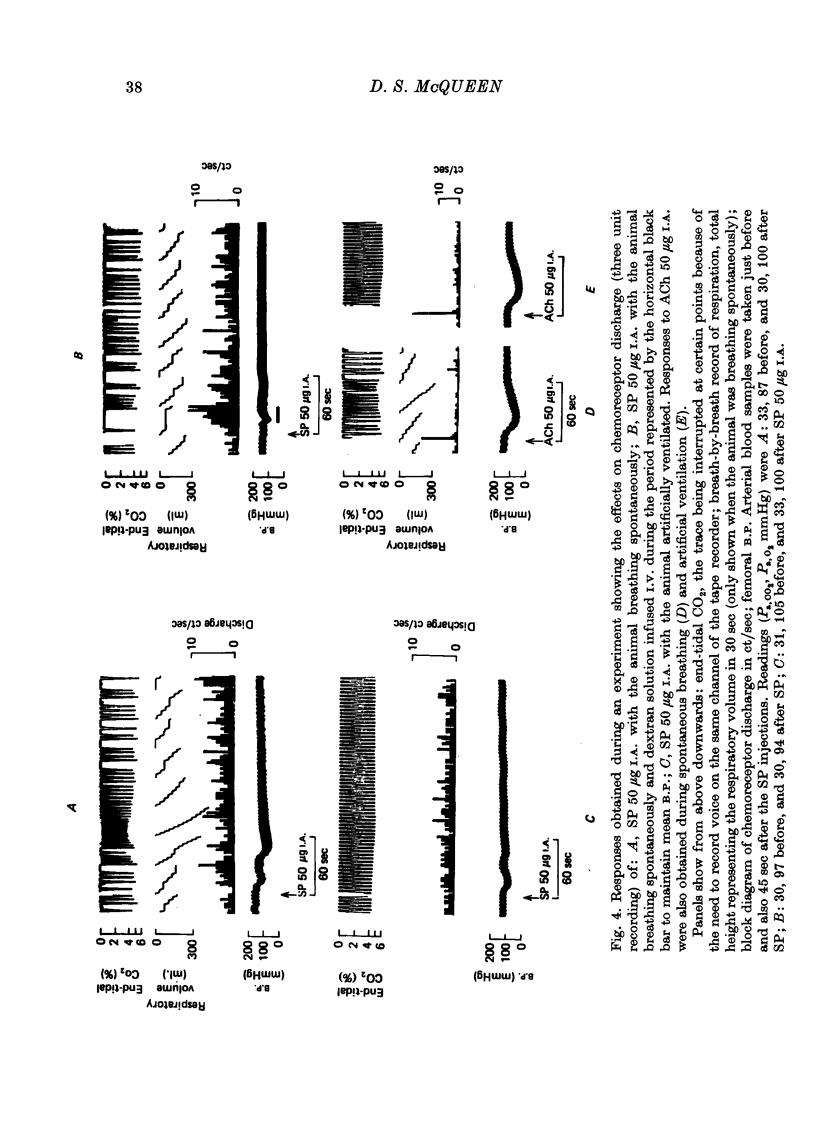
1. The influence of substance P (SP) on spontaneous chemosensory discharge and on responses of the carotid chemoreceptors to various drugs has been investigated in pentobarbitone anaesthetized casts in which chemoreceptor activity was recorded from the peripheral end of a sectioned sinus nerve. 2. After an initial slight inhibition during the first 5--15 sec following the injection, SP (0.1--100 microgram I.A.) caused a dose-related increase in discharge which lasted for 45--300 sec in artificially ventilated cats, discharge being increased by about 50% on average. The increase was of shorter duration when the animals were allowed to breathe spontaneously. 3. The delayed increase in discharge was not secondary to the hypotension caused by SP, nor was it entirely due to changes in bronchomotor tone resulting from direct or indirect actions of SP, although such changes contributed to the response. It was not possible to determine whether the excitation was due to a direct effect of SP on the chemoreceptors. 4. Chemosensory excitation evoked by NaCN (5 microgram I.A.) was potentiated during I.A. infusions of SP and also 10--20 min after SP (10 microgram I.A.) had been injected. In contrast, responses to ACh (50 microgram I.A.) were inhibited. These effects may be due to a nicotinic-blocking action of SP on the carotid chemoreceptors. It was also found that the inhibitory action of dopamine (5 microgram I.A.) was reduced during SP infusion whereas that of 5-HT (10 microgram I.A.) was potentiated. 5. A sample of crude SP had effects on spontaneous chemoreceptor discharge and responses to NaCN and ACh which were qualitatively similar to those obtained using synthetic SP. 6. The physiological significance of the results is discussed and it is concluded that the interpretation depends upon whether or not SP is present in the cat's carotid body.
Full text
PDF




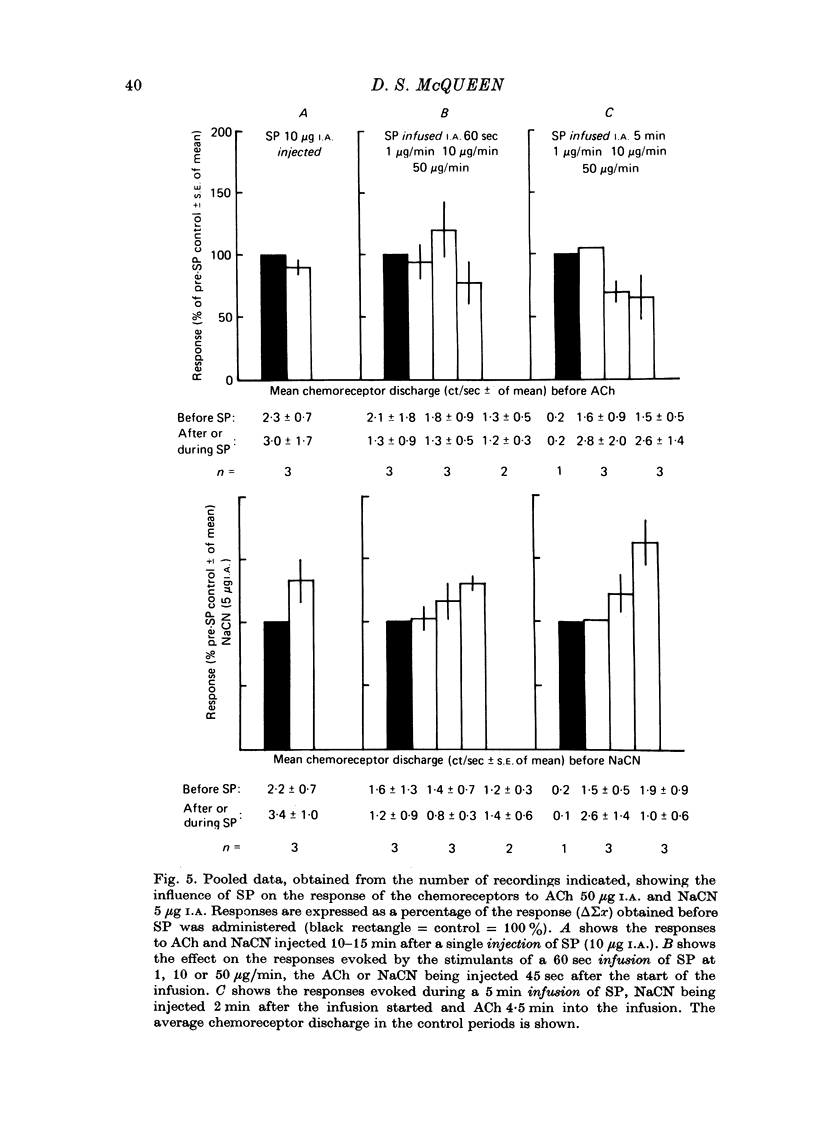
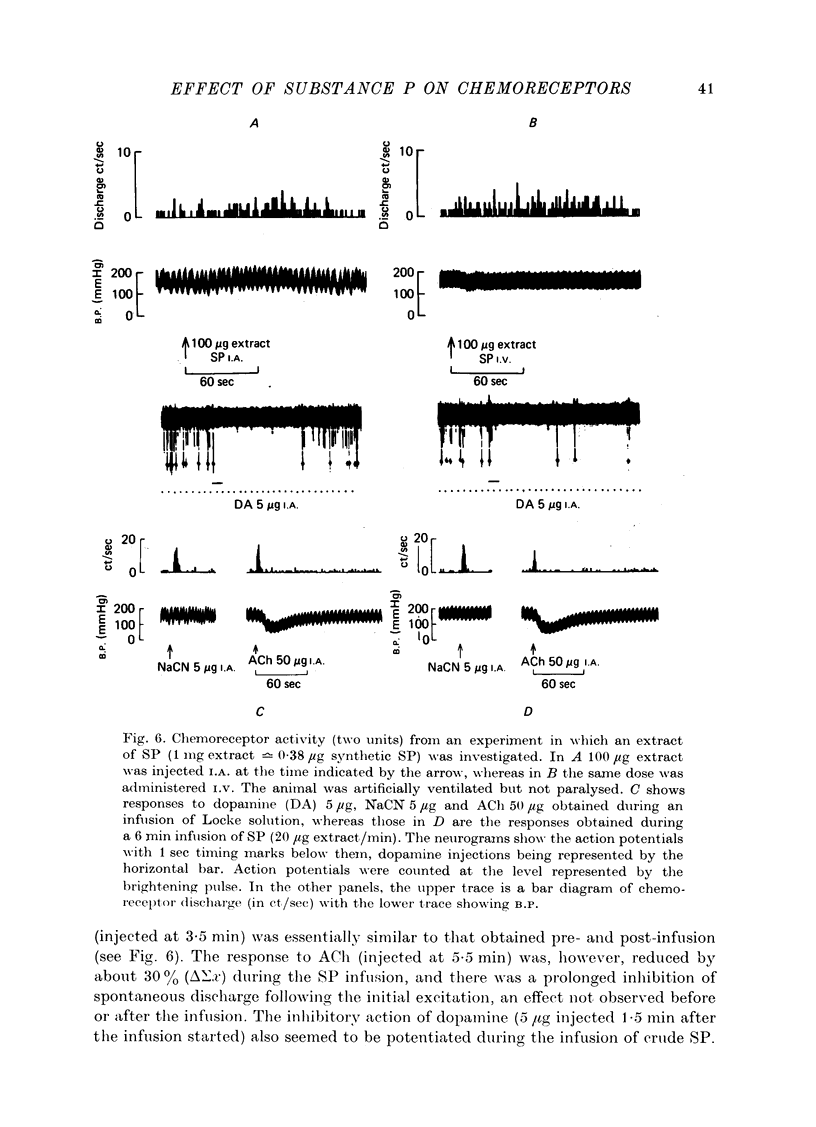






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acker H., Keller H. P., Lübbers D. W., Bingmann D., Schulze H., Caspers H. The relationship between neuronal activity of chemoreceptor fibers and tissue PO2 of the carotid body of the cat during changes in arterial PO2 and blood pressure. Pflugers Arch. 1973 Nov 8;343(4):287–296. doi: 10.1007/BF00595816. [DOI] [PubMed] [Google Scholar]
- BHOOLA K. D., COLLIER H. O., SCHACHTER M., SHORLEY P. G. Actions of some peptides on bronchial muscle. Br J Pharmacol Chemother. 1962 Aug;19:190–197. doi: 10.1111/j.1476-5381.1962.tb01439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISSET G. W., LEWIS G. P. A spectrum of pharmacological activity in some biologically active peptides. Br J Pharmacol Chemother. 1962 Aug;19:168–182. [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Purves M. J. Observations on carotid body chemoreceptor activity and cervical sympathetic discharge in the cat. J Physiol. 1967 Jun;190(3):413–424. doi: 10.1113/jphysiol.1967.sp008218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Purves M. J., Sampson S. R. The frequency of nerve impulses in single carotid body chemoreceptor afferent fibres recorded in vivo with intact circulation. J Physiol. 1970 May;208(1):121–131. doi: 10.1113/jphysiol.1970.sp009109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capella C., Solcia E. Optical and electron microscopical study of cytoplasmic granules in human carotid body, carotid body tumours and glomus jugulare tumours. Virchows Arch B Cell Pathol. 1971;7(1):37–53. doi: 10.1007/BF02892077. [DOI] [PubMed] [Google Scholar]
- Chiocchio S. R., Biscardi A. M., Tramezzani J. H. 5-hydroxytryptamine in the carotid body of the cat. Science. 1967 Nov 10;158(3802):790–791. doi: 10.1126/science.158.3802.790. [DOI] [PubMed] [Google Scholar]
- Chiocchio S. R., King M. P., Carballo L., Angelakos E. T. Monoamines in the carotid body cells of the cat. J Histochem Cytochem. 1971 Oct;19(10):621–626. doi: 10.1177/19.10.621. [DOI] [PubMed] [Google Scholar]
- Cuello A. C. Enkephalin and substance P containing neurones in the trigeminal and extrapyramidal systems. Adv Biochem Psychopharmacol. 1978;18:111–123. [PubMed] [Google Scholar]
- DALY M. de B., SCHWEITZER A. Reflex bronchomotor responses to stimulation of receptors in the regions of the carotid sinus and arch of the aorta in the dog and cat. J Physiol. 1951 May;113(4):442–462. doi: 10.1113/jphysiol.1951.sp004587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty R. J., McQueen D. S. Inhibitory action of dopamine on cat carotid chemoreceptors. J Physiol. 1978 Jun;279:425–436. doi: 10.1113/jphysiol.1978.sp012354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EYZAGUIRRE C., LEWIN J. Chemoreceptor activity of the carotid body of the cat. J Physiol. 1961 Dec;159:222–237. doi: 10.1113/jphysiol.1961.sp006804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EYZAGUIRRE C., LEWIN J. Effect of different oxygen tensions on the carotid body in vitro. J Physiol. 1961 Dec;159:238–250. doi: 10.1113/jphysiol.1961.sp006805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EYZAGUIRRE C., LEWIN J. The effect of sympathetic stimulation on carotid nerve activity. J Physiol. 1961 Dec;159:251–267. doi: 10.1113/jphysiol.1961.sp006806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELDBERG W., LEWIS G. P. THE ACTION OF PEPTIDES ON THE ADRENAL MEDULLA. RELEASE OF ADRENALINE BY BRADYKININ AND ANGIOTENSIN. J Physiol. 1964 May;171:98–108. doi: 10.1113/jphysiol.1964.sp007364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLOYD W. F., NEIL E. The influence of the sympathetic innervation of the carotid bifurcation on chemoceptor and baroceptor activity in the cat. Arch Int Pharmacodyn Ther. 1952 Sep 1;91(1-2):230–239. [PubMed] [Google Scholar]
- HORNBEIN T. F., GRIFFO Z. J., ROOS A. Quantitation of chemoreceptor activity: interrelation of hypoxia and hypercapnia. J Neurophysiol. 1961 Nov;24:561–568. doi: 10.1152/jn.1961.24.6.561. [DOI] [PubMed] [Google Scholar]
- Hanbauer I. Regulation of tyrosine hydroxylase in carotid body. Adv Biochem Psychopharmacol. 1977;16:275–280. [PubMed] [Google Scholar]
- Hökfelt T., Ljungdahl A., Steinbusch H., Verhofstad A., Nilsson G., Brodin E., Pernow B., Goldstein M. Immunohistochemical evidence of substance P-like immunoreactivity in some 5-hydroxytryptamine-containing neurons in the rat central nervous system. Neuroscience. 1978;3(6):517–538. doi: 10.1016/0306-4522(78)90017-9. [DOI] [PubMed] [Google Scholar]
- Juan H., Lembeck F. Action of peptides and other algesic agents on paravascular pain receptors of the isolated perfused rabbit ear. Naunyn Schmiedebergs Arch Pharmacol. 1974;283(2):151–164. doi: 10.1007/BF00501142. [DOI] [PubMed] [Google Scholar]
- LEMBECK F. Zur Frage der zentralen Ubertragung afferenter Impulse. III. Das Vorkommen und die Bedeutung der Substanz P in den dorsalen Wurzeln des Rückenmarks. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1953;219(3):197–213. [PubMed] [Google Scholar]
- Lewis G. P., Reit E. Further studies on the actions of peptides on the superior cervical ganglion and suprarenal medulla. Br J Pharmacol Chemother. 1966 Feb;26(2):444–460. doi: 10.1111/j.1476-5381.1966.tb01925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livett B. G., Kozousek V., Mizobe F., Dean D. M. Substance P inhibits nicotinic activation of chromaffin cells. Nature. 1979 Mar 15;278(5701):256–257. doi: 10.1038/278256a0. [DOI] [PubMed] [Google Scholar]
- McQueen D. S. A quantitative study of the effects of cholinergic drugs on carotid chemoreceptors in the cat. J Physiol. 1977 Dec;273(2):515–532. doi: 10.1113/jphysiol.1977.sp012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen D. S. Effects of methacholine on the carotid chemoreceptors. Q J Exp Physiol Cogn Med Sci. 1978 Apr;63(2):171–178. doi: 10.1113/expphysiol.1978.sp002429. [DOI] [PubMed] [Google Scholar]
- McQueen D. S. Effects of substance P on carotid chemoreceptor and baroreceptor activity in the cat [proceedings]. J Physiol. 1978 Nov;284:164P–165P. [PubMed] [Google Scholar]
- Otsuka M., Konishi S., Takahashi T. Hypothalamic substance P as a candidate for transmitter of primary afferent neurons. Fed Proc. 1975 Sep;34(10):1922–1928. [PubMed] [Google Scholar]
- Pearse A. G., Polak J. M., Rost F. W., Fontaine J., Le Lièvre C., Le Douarin N. Demonstration of the neural crest origin of type I (APUD) cells in the avian carotid body, using a cytochemical marker system. Histochemie. 1973;34(3):191–203. doi: 10.1007/BF00303435. [DOI] [PubMed] [Google Scholar]
- Pearse A. G. The cytochemistry and ultrastructure of polypeptide hormone-producing cells of the APUD series and the embryologic, physiologic and pathologic implications of the concept. J Histochem Cytochem. 1969 May;17(5):303–313. doi: 10.1177/17.5.303. [DOI] [PubMed] [Google Scholar]
- Reubi J. C., Emson P. C., Jessell T. M., Iversen L. L. Effects of GABA, dopamine, and substance P on the release of newly synthesized 3H-5-hydroxytryptamine from rat substantia nigra in vitro. Naunyn Schmiedebergs Arch Pharmacol. 1978 Oct;304(3):271–275. doi: 10.1007/BF00507968. [DOI] [PubMed] [Google Scholar]
- Ryall R. W., Belcher G. Substance P selectively blocks nicotinic receptors on Renshaw cells: a possible synaptic inhibitory mechanism. Brain Res. 1977 Dec 2;137(2):376–380. doi: 10.1016/0006-8993(77)90350-x. [DOI] [PubMed] [Google Scholar]
- Saito K., Konishi S., Otsuka M. Antagonism between Lioresal and substance P in rat spinal cord. Brain Res. 1975 Oct 24;97(1):177–180. doi: 10.1016/0006-8993(75)90928-2. [DOI] [PubMed] [Google Scholar]
- VOGLER K., HAEFELY W., HURLIMANN A., STUDER R. O., LERGIER W., STRASSLE R., BERNEIS K. H. A new purification procedure and biological properties of substance P. Ann N Y Acad Sci. 1963 Feb 4;104:378–390. doi: 10.1111/j.1749-6632.1963.tb17682.x. [DOI] [PubMed] [Google Scholar]
- WIDDICOMBE J. G. Regulation of tracheobronchial smooth muscle. Physiol Rev. 1963 Jan;43:1–37. doi: 10.1152/physrev.1963.43.1.1. [DOI] [PubMed] [Google Scholar]


