Abstract
Glycosylphosphatidylinositols (GPIs), the anchor molecules of some membrane proteins of Plasmodium species, have been implicated in the induction of immunopathology during malaria infections. Hence, neutralization of GPIs by antibodies may reduce the severity of clinical attacks of malaria. To test this hypothesis, we have assessed the levels of anti-GPI antibodies in plasma from children and adults living in areas of seasonal malaria transmission in The Gambia. In a prospective study of susceptibility to clinical or asymptomatic infection, the levels of anti-GPI antibodies were measured before and after the transmission season. Samples were also obtained from children recruited into a hospital-based study of severe malaria. We find that in malaria-exposed individuals both the prevalence and the concentration of anti-GPI antibodies increase with age and that antibody levels are significantly higher at the end of the malaria transmission season than at the start of the season. Antibody levels are also higher in children with asymptomatic infections (i.e., those with a degree of clinical immunity) than in children who developed clinical malaria and high parasitemia, although this difference is not statistically significant. Importantly, antibodies appear to be rapidly boosted by clinical malaria infection, but children under the age of two years are seronegative for anti-GPI antibodies, even during an acute infection. While GPIs may be involved in the pathogenesis of human malaria, the data from this study do not provide any strong evidence to support the notion that anti-GPI antibodies confer resistance to mild or severe malarial disease. Further case-control studies, ideally of a prospective nature, are required to elucidate the role of antiglycolipid antibodies in protection from severe malaria.
It is evident that many of the clinical manifestations of malaria (including acute febrile illness, anemia, cerebral malaria, and hypoglycemia) are mediated in part by overproduction of proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), and gamma interferon (IFN-γ) (for a review, see reference 16). The temporal correlation between schizont rupture and acute febrile episodes, with sharp increases in the concentrations of circulating cytokines, in both Plasmodium vivax and P. falciparum infections (13, 14) is consistent with the view that parasite products released from infected erythrocytes at the time of schizogony may trigger the inflammatory cytokine cascade, leading to the onset of symptoms (6). Soluble parasite products (often described as parasite “toxins”) can activate macrophages to release TNF-α and IL-1 (1), and this process is enhanced in the presence of CD3+ T cells (27). More recently, it has been proposed that parasite-derived glycosylphosphatidylinositol (GPI) is the principle mediator of this response (24). GPIs have been identified in mouse, human, and monkey malarias (10, 24, 28), where they serve as membrane anchors for merozoite, trophozoite, and sporozoite surface proteins (4, 17, 18), but large quantities of GPI are also produced as free glycolipid (9). P. falciparum GPI-anchored proteins mediate macrophage activation and cytokine production via the induction of protein tyrosine kinase and protein kinase C phosphorylation pathways by their carbohydrate and lipid moieties, respectively (29); the terminal (fourth) mannose and the lipid moieties synergize for maximal signal transduction (30). The precise cell surface ligand for malarial GPI is not yet known, but GPIs from another intracellular protozoan, Trypanosoma cruzi, have been shown to activate macrophages to produce IL-12 via binding to Toll-like receptor-2 (5).
Malarial GPI thus has a potentially important role in the induction of innate immune responses to malaria, such as macrophage activation, parasite phagocytosis, and killing, and may thus contribute to the rapid control of blood-stage infections. Alternatively, the induction of high levels of proinflammatory cytokines may predispose patients to clinical disease and development of severe malaria (for a review, see reference 21). Malarial GPI is also believed to contribute to hypoglycemia by mimicking the action of insulin (8). This supports the notion that antibodies to malarial toxins such as GPI may contribute to the development of clinical immunity to malaria and that this could be exploited for the development of antidisease vaccines (20). However, before such a step can be taken, the precise protective or pathogenic role of GPI needs to be investigated further.
Previous serological studies with purified parasite extracts or generic phospholipids have suggested some association between antibodies to soluble schizont antigens or lipid moieties and clinical immunity to malaria (12, 22). However, the recently reported purification and characterization of P. falciparum GPI (19) provides us with more precise tools for dissecting the anti-GPI antibody response in populations living in areas of malaria endemicity. Recently, data from a study in Kenya have shown that while malaria-immune adults maintain high levels of anti-GPI antibodies, children at risk of developing clinical malaria and anemia have much lower antibody concentrations (19). In the present study, we measured the anti-GPI immunoglobulin G (IgG) response in plasma from children and adults from The Gambia (where P. falciparum transmission is highly seasonal) before and after the main malaria transmission season, in samples from prospective studies of susceptibility to clinical or asymptomatic malaria infection, and in samples from children recruited into a hospital-based study of severe malaria. This is the first study to directly test the association between the acquisition of antibodies to native P. falciparum GPI and resistance to mild or severe clinical malaria.
MATERIALS AND METHODS
Study area and study design.
Human plasma samples were collected in The Gambia, West Africa. Samples for the age cross-sectional study and the longitudinal study of mild clinical malaria were collected from hamlets around the town of Farafenni on the north bank of the River Gambia (22). Children (n = 233) aged 3 to 8 years were examined and their blood samples obtained at the beginning of the malaria transmission season in May. The children were followed up with fortnightly health questionnaires and physical examinations until the end of the transmission season (October) and were divided into groups on the basis of their malaria history during the follow-up period: (i) Group I consisted of children in whom no evidence of infection was detected during the follow-up period and who may be completely immune or may not have received any infectious bites; (ii) Group II consisted of children who experienced an attack of clinical malaria (axillary temperature of ≥37.5°C with peripheral parasitemia [≥5,000 parasites per μl of blood]) and who were deemed to be nonimmune; (iii) Group III consisted of children who developed asymptomatic infections (i.e., parasitemia or acquired splenomegaly in the absence of clinical symptoms) and who were deemed to have a degree of clinical immunity; and (iv) Group IV consisted of children who developed fever in the presence of only low levels of peripheral parasitemia (<5,000 parasites per μl of blood). The last group was difficult to interpret as these children’s fevers may have been due to causes other than malaria. At the end of the follow-up period (October), a second blood sample was obtained from 61 of the children. At that time, a single blood sample was also collected from 74 adults.
The second study was conducted among children (aged 6 months to 15 years) admitted to hospitals with severe malaria (severe anemia [n = 22] or cerebral malaria [n = 84], according to World Health Organization definitions; for details, see reference 14) or, as controls, children (n = 65) admitted to the same hospitals with severe nonmalarial illnesses (predominantly respiratory tract infections). Control samples were also obtained from children attending the outpatient clinics of the same hospitals who had mild malaria (n = 90) or mild nonmalarial illness (n = 80) (14).
Plasma samples (malaria nonexposed) were collected from 10 European adults with no history of exposure to malaria. Positive control samples were obtained from a pool of plasma from Gambian adults living in the rural village of Brefet, where there is a high level of malaria exposure.
Anti-GPI IgG enzyme-linked immunosorbent assay.
Anti-GPI IgG was detected as described previously (19). Briefly, P. falciparum GPI purified by high-performance liquid chromatography was dissolved in methanol and coated (at 1 ng/ml) onto flat-bottomed Maxisorp (Nunc, Roskilde, Denmark) microtiter plates. The plates were dried at 37°C and blocked with Tris-buffered saline with 0.5% casein (TBS-casein). Plasma samples were diluted 1:100 in TBS-casein with 0.05% Tween 20, added to antigen-coated plates, and incubated at room temperature for 2 h. The plates were washed extensively with TBS-Tween 20, and specific IgG binding was detected with horseradish peroxidase-conjugated rabbit anti-human IgG with H2O2 as the substrate and o-phenylenediamine as the chromogen. The reaction was stopped after 10 min by the addition of 2 M H2SO4, and the optical densities (ODs) at 492 nm were read. All samples were tested in duplicate or triplicate, and all assays were repeated at least once.
Statistical analysis.
Based on values obtained with control sera from the European adults (mean OD = 0.08 ± 0.01), the cutoff for a positive antibody response was taken to be an OD value of 0.1 (mean plus 2 standard deviations). Comparison of the proportions with a positive response was made by using chi-square analysis. Raw antibody data (OD values) were presented by using the group median and were analyzed by using the Kruskal-Wallis rank test. OD values were log transformed to enable the calculation of geometric means, and the Pearson correlation coefficients were calculated. Analysis of the association between antibody response and clinical outcome was limited to a comparison of Group II results with Group III results and was performed by using analysis of variance on the log-transformed data, both unadjusted and adjusted for the ages of the subjects.
RESULTS
Age-related acquisition of anti-GPI antibodies.
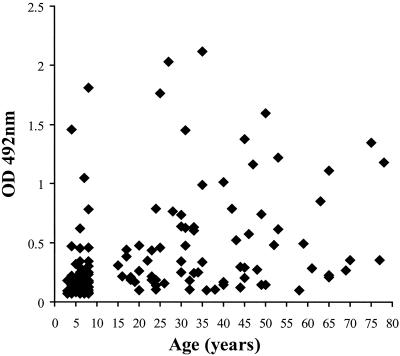
Plasma samples collected at the end of the malaria transmission season (October) from 61 children (aged 3 to 8 years) and 74 adults (aged 15 to more than 75 years) were tested for anti-GPI antibodies. The OD values for the samples from the children (median, 0.147; range, 0.065 to 1.813) were significantly lower than those for the samples from the adults (median, 0.3445; range, 0.098 to 2.117) (Kruskal-Wallis χ2 = 28.5, P < 0.001), with a ratio of the geometric means of 2.16 (t = 5.61; 95% confidence interval [CI], 1.65 to 2.84) (Fig. 1). In addition, the seroprevalence of anti-GPI antibodies (percent of samples giving an OD value greater than the normal range for unexposed European donors) increased steadily with age; approximately 64% of those under 5 years old were seropositive, whereas 85% of those 6 to 8 years old and 97% of adults (>15 years old) were seropositive (χ2 = 19.0, P < 0.001) (Table 1). The proportions of sera with ODs higher than that of the pooled immune serum (OD of 0.4) were 8 of 61 (13%) for those 3 to 8 years old and 33 of 74 (45%) for adults
FIG. 1.
Age-related acquisition of anti-GPI antibodies. Plasma samples from 61 children and 74 adults from the Farafenni district of The Gambia were collected at the end of the malaria transmission season (October) and analyzed for anti-GPI IgG antibodies by enzyme-linked immunosorbent assay. Data are expressed as absolute OD values for samples at a serum dilution of 1:100.
TABLE 1.
Median ODs, range of OD values, and percent positive for anti-GPI antibodies by age for 61 children and 74 adults from the Farafenni district of The Gambiaa
| Age range (yr) | No. of patients | Median OD | Range of OD values | % Respondersb |
|---|---|---|---|---|
| 3-5 | 22 | 0.123 | 0.068-0.474 | 64 |
| 6-8 | 39 | 0.163 | 0.065-1.813 | 85 |
| 15-25 | 21 | 0.214 | 0.101-1.767 | 100 |
| 26-35 | 19 | 0.604 | 0.106-2.117 | 100 |
| 36+ | 34 | 0.355 | 0.098-1.600 | 94 |
Sera were collected in October 1988.
Responders defined as those whose sera gave OD values above the cutoff (OD = 0.1), defined as the mean OD value plus 2 standard deviations for control sera from European donors.
Prospective study of anti-GPI antibodies and malaria morbidity.
Plasma samples collected at the beginning of the malaria transmission season (May) from 233 children aged 3 to 8 years were tested for anti-GPI IgG. There was a highly significant positive association between the OD values for anti-GPI IgG and age (R2 = 0.26, P < 0.001), but the maximal OD values and the proportion of seropositive samples (141 of 233 [61%]) were lower (median, 0.113; range, 0.057 to 0.903) than those in the October (posttransmission) data set.
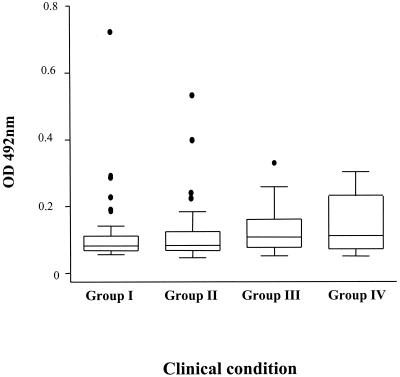
The pretransmission season anti-GPI antibody OD values for samples from 204 children in the four different clinical groups, based on their malaria morbidity between May and October (Fig. 2), were compared. The OD values were as follows: median OD = 0.102 and range = 0.069 to 0.903 for Group I (n = 51), median OD = 0.103 and range = 0.057 to 0.667 for Group II (n = 76), median OD = 0.134 and range = 0.063 to 0.412 for Group III (n = 52), and median OD = 0.139 and range = 0.062 to 0.378 for Group IV (n = 25). Overall, samples from children in Group III (asymptomatic malaria) had higher ODs than those from children in Group II (clinical malaria) (Kruskal-Wallis χ2 = 4.51, P = 0.03), although the mean age of the asymptomatic children was 6 months greater than that of those who developed clinical malaria (5.7 versus 5.2 years). After allowing for the effect of age, we found that the levels of anti-GPI antibodies were higher in the asymptomatic, infected children (Group III) than in the children who developed clinical malaria (Group II), but this difference was not statistically significant (ratio of geometric means = 1.146; 95% CI, 0.977 to 1.344; P < 0.093).
FIG. 2.
Prospective study of the relationship between anti-GPI antibodies and malaria morbidity in Gambian children. Shown are the anti-GPI antibody levels (expressed in terms of OD) in children with no malaria infection (Group I, n = 51), clinical malaria (Group II, n = 76), asymptomatic malaria (Group III, n = 52), or febrile illness with low parasitemia (Group IV, n = 25). Box plots show the medians (25th and 75th percentiles), 95% CI, and outliers.
Boosting of anti-GPI antibodies during the malaria transmission season.
Since we noticed that OD values for anti-GPI antibodies were generally lower in samples collected in May than in those collected in October, we compared the antibody responses of individual children measured on both occasions. Paired samples, collected in May and October from 57 children, were tested in adjacent wells of the same microtiter plate, and their OD values were compared (Table 2). In samples from children with either asymptomatic or low-density malaria infections (Groups III and IV), the OD values remained high during the transmission season and there was no significant difference between the pre- and posttransmission season OD values (ratio of geometric mean ODs = 1.01, paired t = 0.12, df = 15, P > 0.8). However, for samples from children who experienced an attack of clinical malaria (Group II), there was a significant increase in geometric mean OD values during the transmission season (ratio of geometric mean ODs = 1.448, paired t = 2.5384, df = 24, P < 0.018). Since these were the children in whom the highest parasite loads were detected, it may be that boosting is dependent on high levels of antigen exposure. For 10 of the 25 children with clinical malaria, samples collected in October had lower OD values than samples collected in May, suggesting that the boosting effect of reinfection may be offset by the consumption of antibodies in children with high parasite densities.
TABLE 2.
Boosting of anti-GPI antibodies during the malaria transmission season (between May and October 1988) by category of clinical infection
| Group (condition) | No. of patients | GMa anti-GPI IgG OD (95% CI) for sera collected in:
|
Ratio of GM ODsb (95% CI) | |
|---|---|---|---|---|
| May | October | |||
| I (no infection) | 8 | 0.113 (0.081, −0.159) | 0.182 (0.077, −0.433) | 1.610 (0.629, 4.112)c |
| II (clinical malaria) | 25 | 0.129 (0.108, −0.155) | 0.188 (0.140, −0.252) | 1.451 (1.071, 1.962)d |
| III (asymptomatic malaria) | 16 | 0.162 (0.129, −0.204) | 0.164 (0.108, −0.248) | 1.010 (0.748, 1.315)c |
| IV (fever plus low parasitemia) | 8 | 0.195 (0.113, −0.338) | 0.140 (0.082, −0.240) | 0.720 (0.579, 0.895)c |
GM, geometric mean.
October value/May value.
P > 0.05.
P < 0.018.
Association between anti-GPI antibodies and resistance to severe malaria.
Given the clear association between high levels of proinflammatory cytokines and the outcome of malaria infection, especially cerebral malaria (14), it was of interest to determine whether high levels of anti-GPI antibodies might protect against the development of severe disease (Table 3). Anti-GPI antibody levels in children admitted to hospitals with severe malaria (mainly cerebral malaria but also including some severe anemia cases) (Fig. 3a) or severe nonmalarial illnesses (Fig. 3b) were determined and compared with antibody levels in children with mild malaria (Fig. 3c) or mild nonmalarial illnesses (Fig. 3d) (14). This study included several children below the age of 2 years (i.e., significantly younger than the youngest children in the prospective study), and we noticed that very few children under the age of 2 years were seropositive for anti-GPI antibodies; this is in accordance with the known inability of children under the age of 2 years to make substantial IgG responses to carbohydrate or glycolipid antigens (3).
TABLE 3.
Case-control analysis of the association between anti-GPI antibody levels on admission and severity of clinical malaria
| Condition | No. of children | Median age in yrs (range) | Anti-GPI IgG antibodiesa
|
No. (%) of children for whom the ODa was:
|
Comparison with mild nonmalariac
|
Comparison with mild malariac
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median OD | Range | GMb OD | >0.1 | >0.15 | Unadjusted | Adjusted for age | Unadjusted | Adjusted for age | |||
| Nonmalaria | |||||||||||
| Mild | 80 | 3.50 (0.6-12.0) | 0.083 | 0.060-0.964 | 0.099 | 18 (23) | 12 (15) | ||||
| Severe | 65 | 3.00 (0.7-9.9) | 0.091 | 0.058-2.153 | 0.109 | 24 (37) | 8 (12) | 0.4 | 0.25 | ||
| Malaria | |||||||||||
| Mild | 90 | 2.75 (0.4-12.0) | 0.107 | 0.060-1.227 | 0.142 | 50 (56) | 28 (31) | <0.001 | <0.001 | ||
| Severe anemia | 22 | 2.90 (0.4-9.0) | 0.124 | 0.060-0.756 | 0.140 | 15 (68) | 6 (27) | 0.03 | 0.003 | 0.9 | 0.9 |
| Cerebral malaria | 84 | 4.70 (0.5-11.0) | 0.143 | 0.065-0.875 | 0.169 | 67 (80) | 39 (46) | <0.001 | <0.001 | 0.1 | 0.9 |
Plasma was tested at a dilution of 1:100; OD values are for IgG.
GM, geometric mean.
P values for analysis of variance using log-transformed OD values.
FIG. 3.
Anti-GPI antibody levels in children with mild or severe malaria and in nonmalaria controls. Shown are the anti-GPI levels (OD at a 1:100 plasma dilution) in plasma samples from Gambian children (aged 2 to 15 years) with severe (a) or mild (c) malaria or with severe (b) or mild (d) nonmalarial disease. The numbers of children in each group are given in Table 2. The dotted lines indicate the cutoffs for seropositive samples (OD = 0.1).
Taking together the samples from children of all ages, we found the prevalence of samples with ODs above 0.1 to be 42 of 145 (29%) for the groups without malaria (Fig. 3b and d) and 132 of 196 (67%) for the groups with malaria (Fig. 3a and c). After adjusting for age, we found that the geometric mean OD values were significantly higher for samples from children with either mild or severe malaria (n = 196, geometric mean OD = 0.153) than for samples from the nonmalaria control groups (n = 145, geometric mean OD = 0.104) (ratio of geometric mean ODs = 1.44, t = 5.44, P < 0.001); this presumably reflects the rapid boosting effect of malaria infection on anti-GPI antibody levels. As has been noted previously (14), children with cerebral malaria tend to be slightly older on average than those with severe anemia. Although anti-GPI antibody levels increase with age, the geometric mean OD values for anti-GPI antibodies were only slightly higher in children with cerebral malaria (n = 84, geometric mean OD = 0.169) than in those with severe anemia (n = 22, geometric mean OD = 0.140), and this difference was not significant (t = 1.24, df = 104, P = 0.2). Indeed, the geometric mean OD for samples from children with severe anemia was similar to that of samples from children with mild malaria (n = 90, geometric mean OD = 0.142), who were closely matched in terms of age. After adjusting for age, we found no significant differences in the anti-GPI antibody levels or prevalence between those with mild and those with severe malaria (mild cases versus all severe cases, ratio of geometric mean ODs = 1.036 and P > 0.7; mild cases versus cerebral cases, ratio of geometric mean ODs = 1.062 and P > 0.5).
DISCUSSION
In this study of the anti-GPI antibody levels in children and adults in an area of highly seasonal malaria transmission, we have demonstrated that anti-GPI antibodies are acquired in an age-dependent fashion, with maximum antibody prevalence being reached by the age of 15 to 20 years. This is in keeping with the findings from a study of an area in Kenya where malaria is highly endemic (19). Our data also indicate that antibody levels are boosted by recent malaria infection; comparison of antibody prevalence and concentration in children with acute, clinical malaria with that in children without malaria indicates that this boosting happens very rapidly during a malaria infection.
Importantly, anti-GPI IgG antibodies are rarely found in children under the age of 2 years, even in those with acute malaria infection. It is well known that children under 2 years of age have a limited capacity to produce antibodies to nonprotein antigens but are able to produce antibodies to the same antigens when they are conjugated to a protein carrier (3); this indicates that the defect is in the recognition of nonprotein antigens by helper T cells rather than by B cells. In this case, the lack of IgG antibodies to GPI in children under 2 years of age raises the possibility that the predominant form of malarial GPI responsible for inducing anti-GPI antibodies is free glycolipid rather than protein-GPI complexes. If physically attached to their GPI anchors, parasite surface proteins might be expected to provide T-cell help for anti-GPI antibody production. On the other hand, the rapid boosting of anti-GPI IgG antibodies by malaria infection suggests that there is a good memory response to GPI, which somewhat contradicts previous evidence that antibodies to parasite toxins are predominantly IgM, T-cell independent, and very short lived (2). The nature of the T-cell help is unknown; cognate interactions with regular αβ T helper cells seem unlikely given the lack of response in very young children. One recent study with P. berghei suggests that T-cell help for production of antibodies to GPI-anchored proteins is provided by a population of NK T cells that directly recognize glycolipid antigens presented in the context of CD1d (25), but others have failed to confirm this finding and have shown that cognate B-cell help is provided by classical major histocompatibility complex class II-restricted CD4+ T cells (17).
Data from the prospective study indicate that children with partial immunity to malaria (as measured by their ability to tolerate malaria parasitemia without obvious febrile symptoms) have somewhat higher levels of anti-GPI antibodies than do children with little or no immunity (as measured by their failure to control peripheral parasitemia and the development of an acute febrile illness). Although this observation failed to reach the level of statistical significance, it is in line with the results of a previous study with the same group of children which showed that antibodies to carbohydrate-containing soluble exoantigens released from malaria schizont-infected erythrocytes correlate with resistance to clinical malaria (22). However, it is still not clear whether resistance to febrile disease is associated with anti-GPI antibodies per se or is secondary to the ability to keep parasite densities below the levels required to trigger a febrile response. One hypothesis is that antiparasite responses serve to limit parasite growth while antitoxic responses raise the parasite density threshold for induction of clinical malaria. If so, then the inability of infants to make anti-GPI antibodies may explain their very low parasite density threshold for onset of clinical malaria (15).
Although a direct link between GPI and immunopathology has not yet been established in human malaria, experimental studies have provided evidence for a causal relationship between the two. For example, purified GPI injected into mice induces three of the major clinical manifestations of severe malaria, hypoglycemia, severe anemia, and cachexia (8, 23, 26). The study in Kenya (19) reported an association between anti-GPI antibody levels and protection from severe anemia in children, suggesting that GPI may mediate similar toxic effects in mice and humans. By contrast, the link to cerebral disease in mice is not yet confirmed; vaccination of mice with semipurified extracts of P. yoelii schizonts can protect against early mortality with minimal effect on parasitemia (7), but the effect of anti-GPI antibody responses still needs to be evaluated in a reliable model of murine cerebral malaria (11). Although our study provides no evidence to support the hypothesis that anti-GPI antibodies protect humans against severe P. falciparum malaria, we cannot exclude a role for such antibodies. First, anti-GPI antibody levels may have been too low to confer any significant protective effect. Antibody levels were very low even in positive sera (OD values tended to be well below 0.5 when plasma samples were tested at a dilution of 1:100); this may explain the differences between our observations and the data from the Kenyan study (19), which was conducted in an area of much higher malaria transmission. Second, it is quite possible that preinfection antibody levels differed between children with mild malaria and those with severe malaria but that rapid boosting of antibodies by concurrent malaria infections masked these differences by the time the children arrived in the hospitals. Samples from prospective studies of severe malaria would need to be analyzed for anti-GPI antibodies in order to test the hypothesis that these antibodies protect humans against severe P. falciparum malaria. Alternatively, it may be that naturally acquired anti-GPI antibodies are not able to neutralize the toxic activities of GPI, possibly because they target functionally irrelevant epitopes. In that case, vaccination with modified synthetic immunogens may be able to direct the immune response to functionally important components of the GPI molecule.
In summary, this study has shown that anti-GPI antibodies are acquired in an age- and exposure-dependent manner. The antibodies are rapidly boosted by acute malaria infection but are infrequently found in children under the age of two years. Although the results were not statistically significant, this study lends support to the notion of a link between anti-GPI antibodies and resistance to mild clinical malaria, but the link to protection against cerebral malaria or severe anemia remains to be established. More-detailed case-control studies are required to determine the precise role of antibodies against GPIs and other parasite-derived molecules in the prevention of cerebral malaria. The relatively low concentration of anti-GPI antibodies in this population, and their rather short-lived nature, suggests that they may provide only transient immunity to clinical symptoms of malaria in endemic settings. If the importance of these antibodies can be confirmed in other studies, then the challenge for vaccine development will be to induce high levels of long-lasting, neutralizing anti-GPI antibodies.
Acknowledgments
The epidemiological and hospital studies referred to in the text were carried out by a large team of researchers from the Medical Research Council Laboratories in The Gambia and by staff of the Royal Victoria Hospital, Banjul, The Gambia. In particular, we acknowledge the roles of Steve Allen, Adrian Hill, Steve Bennett, and Brian Greenwood in the initiation, implementation, and analysis of these studies. We thank Elizabeth King for technical assistance, Kirk Rockett for helpful advice and sample management, and Patrick Corran for useful discussions.
We thank the Medical Research Council (United Kingdom), Wellcome Trust, and World Health Organization Special Programme for Research on Tropical Disease (WHO/TDR) for funding the original studies and University College London, the Wellcome Trust, and the NIH (NIAID) (grant no. AI41139) for funding the present study.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Bate, C. A., J. Taverne, and J. H. L. Playfair. 1988. Malarial parasites induce TNF production by macrophages. Immunology 64:227-231. [PMC free article] [PubMed] [Google Scholar]
- 2.Bate, C. A., J. Taverne, A. Davé, and J. H. L. Playfair. 1990. Malaria exoantigens induce T-independent antibody that blocks their ability to induce TNF. Immunology 70:315-320. [PMC free article] [PubMed] [Google Scholar]
- 3.Borrow, R., D. Goldblatt, N. Andrews, P. Richmond, J. Southern, and E. Miller. 2001. Influence of prior meningococcal C polysaccharide vaccination on the response and generation of memory after meningococcal C conjugate vaccination in young children. J. Infect. Dis. 184:377-380. [DOI] [PubMed] [Google Scholar]
- 4.Burns, J., C. Belk, and P. Dunn. 2000. A protective glycosylphosphatidylinositol-anchored membrane protein of Plasmodium yoelii trophozoites and merozoites contains two epidermal growth factor-like domains. Infect. Immun. 68:6189-6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campos, M., I. Almeida, O. Takeuchi, S. Akira, E. Valente, D. Procopio, L. Travassos, J. Smith, D. Golenbock, and R. Gazzinelli. 2001. Activation of Toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J. Immunol. 167:416-423. [DOI] [PubMed] [Google Scholar]
- 6.Clark, I. A., J. L. Virelizier, E. A. Carswell, and P. R. Wood. 1981. Possible importance of macrophage-derived mediators in acute malaria. Infect. Immun. 32:1058-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Souza, J. B., and J. H. L. Playfair. 1988. Immunization of mice against blood-stage Plasmodium yoelii malaria with isoelectrically focused antigens and correlation of immunity with T cell priming in vivo. Infect. Immun. 56:88-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elased, K., K. Gumaa, J. B. de Souza, H. Rahmoune, J. H. L. Playfair, and T. Rademacher. 2001. Reversal of type 2 diabetes in mice by products of malaria parasites. II. Role of inositol phosphoglycans (IPGs). Mol. Genet. Metab. 73:248-258. [DOI] [PubMed] [Google Scholar]
- 9.Gerold, P., A. Dieckmann-Schuppert, and R. Schwartz. 1994. Glycosylphosphatidylinositols synthesized by asexual erythrocytic stages of the malarial parasite, Plasmodium falciparum. Candidates for plasmodial glycosylphosphatidylinositol membrane anchor precursors and pathogenicity factors. J. Biol. Chem. 269:2597-2606. [PubMed] [Google Scholar]
- 10.Gerold, P., L. Vivas, S. Ogun, N. Azzouz, K. Brown, A. Holder, and R. Schwarz. 1997. Glycosylphosphatidylinositols of Plasmodium chabaudi chabaudi: a basis for the study of malarial glycolipid toxins in a rodent model. Biochem. J. 328:905-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hearn, J., N. Rayment, D. Landon, D. Katz, and J. B. de Souza. 2000. Immunopathology of cerebral malaria: morphological evidence of parasite sequestration in murine brain microvasculature. Infect. Immun. 68:5364-5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jakobsen, P. H., S. D. Morris-Jones, L. Hviid, T. G. Theander, M. Hoier-Madsen, R. A. Bayoumi, and B. M. Greenwood. 1993. Anti-phospholipid antibodies in patients with Plasmodium falciparum malaria. Immunology 79:653-657. [PMC free article] [PubMed] [Google Scholar]
- 13.Karunaweera, N. D., G. E. Grau, P. Gamage, R. Carter, and K. N. Mendis. 1992. Dynamics of fever and serum levels of tumor necrosis factor are closely associated during clinical paroxysms in Plasmodium vivax malaria. Proc. Natl. Acad. Sci. USA 89:3200-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwiatkowski, D., A. V. S. Hill, I. Sambou, P. Twumasi, J. Castracane, K. R. Manogue, A. Cerami, D. Brewster, and B. M. Greenwood. 1990. TNF concentration in fatal, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet 336:1201-1204. [DOI] [PubMed] [Google Scholar]
- 15.McGuinness, D., K. Koram, S. Bennett, G. Wagner, F. K. Nkrumah, and E. M. Riley. 1998. Clinical case definitions for malaria: clinical malaria associated with very low parasite densities in African infants. Trans. R. Soc. Trop. Med. Hyg. 92:527-531. [DOI] [PubMed] [Google Scholar]
- 16.Miller, L. H., M. F. Good, and G. Milon. 1994. Malaria pathogenesis. Science 264:1878-1883. [DOI] [PubMed] [Google Scholar]
- 17.Molano, A., S. Park, Y. Chiu, S. Nosseir, A. Bendelac, and M. Tsuji. 2000. The IgG response to the circumsporozoite protein is MHC class II-dependent and CD1d-independent: exploring the role of GPIs in NK T cell activation and antimalarial responses. J. Immunol. 164:5005-5009. [DOI] [PubMed] [Google Scholar]
- 18.Moran, P., and I. Caras. 1994. Requirements for glycosylphosphatidylinositol attachment are similar but not identical in mammalian cells and parasitic protozoa. J. Cell Biol. 125:333-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naik, R., O. Branch, A. Woods, M. Vijaykumar, D. Perkins, B. Nahlen, A. Lal, R. Cotter, C. Costello, C. Ockenhouse, E. Davidson, and D. Gowda. 2000. Glycosylphosphatidylinositol anchors of Plasmodium falciparum: molecular characterization and naturally elicited antibody response that may provide immunity to malaria pathogenesis. J. Exp. Med. 192:1563-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Playfair, J. H. L., J. Taverne, C. A. W. Bate, and J. B. de Souza. 1990. The malaria vaccine: antiparasite or antidisease? Immunol. Today 11:25-27. [DOI] [PubMed] [Google Scholar]
- 21.Riley, E. M. 1999. Is T cell priming required for initiation of pathology in malaria infections? Immunol. Today 20:228-233. [DOI] [PubMed] [Google Scholar]
- 22.Riley, E. M., P. H. Jakobsen, S. J. Allen, J. G. Wheeler, S. Bennett, and B. M. Greenwood. 1991. Immune responses to soluble exoantigens of Plasmodium falciparum may contribute to both pathogenesis and protection in clinical malaria: evidence from a longitudinal, prospective study of semi-immune African children. Eur. J. Immunol. 21:1019-1025. [DOI] [PubMed] [Google Scholar]
- 23.Rudin, W., V. Quesniaux, N. Favre, and G. Bordmann. 1997. Malaria toxins from P. chabaudi chabaudi AS and P. berghei ANKA causes dyserythropoiesis in C57/BL6 mice. Parasitology 115:467-474. [DOI] [PubMed] [Google Scholar]
- 24.Schofield, L., and F. Hackett. 1993. Signal transduction in host cells by a glycosylphosphatidylinositol toxin of malaria parasites. J. Exp. Med. 177:145-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schofield, L., M. McConville, D. Hansen, A. Campbell, B. Fraser-Reid, M. Grusby, and S. D. Tachado. 1999. CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NKT cells. Science 283:225-229. [DOI] [PubMed] [Google Scholar]
- 26.Schofield, L., L. Vivas, F. Hackett, P. Gerold, R. Schwartz, and S. D. Tachado. 1993. Neutralizing monoclonal antibodies to glycosylphosphatidylinositol, the dominant TNF-alpha-inducing toxin of Plasmodium falciparum: prospects for the immunotherapy of severe malaria. Ann. Trop. Med. Parasitol. 87:617-626. [DOI] [PubMed] [Google Scholar]
- 27.Scragg, I., M. Hensmann, C. Bate, and D. Kwiatkowski. 1999. Early cytokine induction by Plasmodium falciparum is not a classical endotoxin-like process. Eur. J. Immunol. 29:2636-2644. [DOI] [PubMed] [Google Scholar]
- 28.Sherwood, J., S. Spitalnik, S. Aley, I. A. Quakyi, and R. J. Howard. 1986. Plasmodium falciparum and P. knowlesi: initial identification and characterization of malaria synthesized glycolipids. Exp. Parasitol. 62:127-141. [DOI] [PubMed] [Google Scholar]
- 29.Tachado, S. D., P. Gerold, M. J. McConville, T. Baldwin, D. Quilici, R. H. Schwartz, and L. Schofield. 1996. Glycosylphosphatidylinositol toxin of Plasmodium induces nitric oxide synthase expression in macrophages and vascular endothelial cells by a protein tyrosine kinase-dependent and protein kinase C-dependent signaling pathway. J. Immunol. 156:1897-1907. [PubMed] [Google Scholar]
- 30.Vijaykumar, M., R. Naik, and D. Gowda. 2001. Plasmodium falciparum glycosylphosphatidylinositol-induced TNF-alpha secretion by macrophages is mediated without membrane insertion or endocytosis. J. Biol. Chem. 276:6909-6912. [DOI] [PubMed] [Google Scholar]