Abstract
The Brucella abortus virB locus is required for establishing chronic infection in the mouse. Using in vitro and in vivo models, we investigated whether virB is involved in evasion of the bactericidal activity of NADPH oxidase and the inducible nitric oxide synthase (iNOS) in macrophages. Elimination of NADPH oxidase or iNOS activity in macrophages in vitro increased recovery of wild-type B. abortus but not recovery of a virB mutant. In mice lacking either NADPH oxidase or iNOS, however, B. abortus infected and persisted to the same extent as it did in congenic C57BL/6 mice up until 60 days postinfection, suggesting that these host defense mechanisms are not critical for limiting bacterial growth in the mouse. A virB mutant did not exhibit increased survival in either of the knockout mouse strains, indicating that this locus does not contribute to evasion of nitrosative or oxidative killing mechanisms in vivo.
Brucella species are facultatively intracellular pathogens whose host range includes livestock, wild animals, and humans. Following infection of susceptible hosts, bacteria localize to tissues of the reticuloendothelial system and survive intracellularly within professional macrophages. The mechanisms by which brucellae are able to avoid killing by macrophages are unknown. The ability of Brucella abortus to persist in the macrophage suggests that it is able to avoid or withstand phagocyte killing mechanisms, such as lysosomal enzymes and products of the oxidative burst (3).
The B. abortus virB locus, a region on chromosome II with homology to type IV secretion systems, is required for establishing and maintaining persistent infection in the mouse model (11, 22). This locus has also been found to be required for intracellular survival in macrophages and HeLa cells (17, 30, 39). Recent evidence suggests that the virB locus mediates secretion of an unknown effector(s) required for establishment of an intracellular compartment favorable for bacterial replication in HeLa cells (5, 10). This hypothesis is supported by the finding that expression of the Brucella suis virB operon is induced intracellularly (4). To determine whether B. abortus is able to avoid intracellular degradative pathways, the trafficking of B. abortus was studied in nonphagocytic cells (12-14, 32-35). Results of these studies suggest that B. abortus avoids fusion with lysosomes and localizes to a compartment containing proteins characteristic of rough endoplasmic reticulum and autophagosomes.
Studies of trafficking in macrophages suggest that the intracellular fate of B. abortus in these cells appears to be distinct from the well-described localization in nonphagocytic cells (2). Recent work suggests that B. abortus and B. suis survive in a compartment that acidifies but has delayed fusion with lysosomes (2, 37). Thus, as in nonphagocytic cells, Brucella appears to delay or modify the maturation of its vacuolar compartment. In addition, B. abortus has been found to inhibit bactericidal functions of phagocytes, including phagolysosomal fusion, neutrophil degranulation, and the oxidative burst (2, 3, 28).
Brucella spp. share the ability to modulate the composition of intracellular compartments with several other intracellular bacterial pathogens, including Legionella, Mycobacterium, and Salmonella. Salmonella enterica serovar Typhimurium prevents assembly of the NADPH oxidase complex on phagosomes, which allows it to avoid the toxic products of the oxidative burst, and this activity is dependent on the Salmonella pathogenicity island 2 type III secretion system (40). Interestingly, an ultrastructural study examining the localization of NADPH oxidase in B. abortus-infected macrophages found that vacuoles containing B. abortus tended not to be associated with NADPH oxidase (18), suggesting that B. abortus might employ similar mechanisms for survival within phagocytes. In the present study, we sought to determine whether the virB locus contributes to evasion of NADPH oxidase- and inducible nitric oxide synthase (iNOS)-mediated control of bacterial growth during B. abortus infection of cultured macrophages and of mice.
MATERIALS AND METHODS
Bacterial strains, media, and culture conditions.
The bacterial strains which we used are B. abortus 2308 and its isogenic mutant BA41, which has an insertion of mTn5Km-2 at nucleotide 1232 of the B. abortus virB locus (GenBank accession number AF226278). This insertion is located 59 bp downstream of the virB1 gene and is polar on the expression of downstream genes in the virB operon (data not shown). Strains were cultured on tryptic soy agar (TSA) (Difco/Becton-Dickinson, Sparks, Md.) or in tryptic soy broth at 37°C on a rotary shaker. Bacterial inocula used for infection of mice were cultured on potato infusion agar, as growth on this medium prevents appearance of spontaneous rough mutants (1). Antibiotics, when required, were added at the following concentrations: carbenicillin, 100 mg/liter; kanamycin, 100 mg/liter; and chloramphenicol, 5 to 30 mg/liter. All work with live B. abortus was performed at biosafety level 3.
Cell lines.
Two clones of the mouse macrophage-like cell line J774, J774.16 and J774.D9, were cultured in Dulbecco's modified Eagle's medium (Gibco, Rockville, Md.) supplemented with 10% heat-inactivated equine serum, 1% nonessential amino acids, and 1 mM glutamine (DMEMsup). The ability or inability of these cell lines to generate oxygen radicals via the hexose monophosphate shunt (oxidative burst) after induction with phorbol myristyate acetate (Sigma) was confirmed as described elsewhere (9, 19).
For macrophage killing assays, 24-well microtiter plates were seeded with macrophages at a concentration of 5 × 105 cells/well in 0.5 ml of DMEMsup and incubated overnight at 37°C in the presence of 5% CO2. For experiments with activated macrophages, 10 U of recombinant murine gamma interferon (IFN-γ) (Endogen, Woburn, Mass.) per ml was added to the medium 24 h before infection, and this concentration of IFN-γ was maintained throughout the assay by replacing the medium every 24 h. Bacteria were grown for 48 h and then diluted in DMEMsup, and about 5 × 107 bacteria in 0.02 ml of DMEMsup were added to each well containing macrophages. The microtiter plates were centrifuged at 250 × g for 5 min at room temperature in order to synchronize the infections. Cells were incubated for 20 min at 37°C in the presence of 5% CO2, free bacteria were removed by three washes with phosphate-buffered saline (PBS), and the zero-time samples were taken as described below. The washing solutions were collected, and extracellular bacteria were quantified by dilution in sterile PBS and plating on TSA. DMEMsup containing 50 μg of gentamicin per ml was added to the wells, and the cells were incubated at 37°C in the presence of 5% CO2. After 1 h, the DMEMsup containing 50 μg of gentamicin per ml was replaced with medium containing 25 μg of gentamicin per ml. Wells were sampled at zero time and 24 and 48 h after infection by aspirating the medium, lysing the macrophages with 0.5 ml of 0.5% Tween 20, and rinsing each well with 0.5 ml of PBS. Viable bacteria were quantified by dilution in sterile PBS and plating on TSA. All experiments were performed independently in triplicate at least three times, and the standard error for each time point was calculated.
Isolation and infection of mouse peritoneal macrophages.
As a previous study revealed that only low levels of NO were produced by proteose peptone-elicited murine peritoneal macrophages infected with B. abortus, we used a method that allowed us to obtain macrophages that produced both iNOS and NADPH oxidase (24). Elicited peritoneal macrophages were isolated from C57BL/6 mice by peritoneal lavage 4 days after injection of 5 mM sodium periodate, which stimulates both the phagocyte respiratory burst and inducible NO synthase (15). Cells were seeded into 96-well plates at a concentration of 3 × 105 cells/well and allowed to adhere to the plastic overnight. For activation, cells were incubated overnight in the presence of 20 U of murine recombinant interferon (IFN-γ; Endogen) per ml and 1 pg of S. enterica serovar Typhimurium lipopolysaccharide (LPS) (Sigma, St. Louis, Mo.) per ml. Cells were used for macrophage killing assays as described above for macrophage cell lines. For inhibition of iNOS, 250 μM NG-l-monomethyl arginine (MMLA) (Sigma) was maintained in the tissue culture medium throughout the assay. NO production by peritoneal macrophages was assayed by incubating macrophage supernatants with an equal volume of Griess reagent (Sigma) and measuring absorbance at 570 nm to determine the nitrite concentration.
Infection of mice.
All mice used in this study were derived from the C57BL/6 strain. C57BL/6 mice and C57BL/6-Cybbtm1 mice carrying a targeted knockout of the gene encoding gp91 (gp91phox) of NADPH oxidase (36) were obtained from Jackson Laboratory (Bar Harbor, Maine). C57BL/6 Ai-[KO]iNOS N5 mice carrying a targeted knockout of the Nos2 gene encoding iNOS were obtained from Taconic (Germantown, N.Y.) (26). Mice were kept in microisolator cages with sterile bedding and water and irradiated feed in a biosafety level 3 facility. For infection experiments, groups of five knockout mice and age-matched controls were inoculated intraperitoneally (i.p.) with 0.2 ml of PBS containing 5 × 105 to 10 × 105 CFU of B. abortus. At the appropriate times, mice were euthanized by CO2 asphyxiation, and the spleens and livers were collected aseptically at necropsy. A portion (one-quarter) of the spleen and the liver of each mouse were fixed in phosphate-buffered formalin and processed for sectioning and staining with hematoxylin and eosin. The remaining three-quarters of the spleen was homogenized in 3 ml of PBS, and serial dilutions of the homogenate were plated on TSA and TSA containing kanmycin for enumeration of CFU. The competitive index was calculated by dividing the mean ratio of mutant CFU to wild-type CFU recovered from spleens by the ratio of the mutant CFU to the wild-type CFU in the inoculum. The experimental groups each contained four or five knockout mice and four or five age-matched controls that were 6 to 10 weeks old. Hematoxylin- and eosin-stained sections of the spleen and liver from each mouse were coded and scored for pathological changes by a veterinary pathologist. All animal experiments were approved by the Texas A&M University Laboratory Animal Care and Use Committee and were conducted in accordance with institutional guidelines.
RESULTS
Intracellular survival of B. abortus in macrophages unable to produce superoxide.
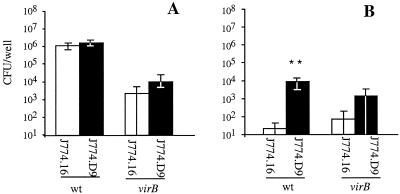
In order to determine whether the oxidative burst is required for control of B. abortus infection by macrophages, we compared the data for survival of B. abortus 2308 and its isogenic virB mutant in two clones of the J774 cell line that differ in the ability to produce an oxidative burst upon activation. J774.16 cells produce superoxide (O2−) upon activation with IFN-γ, while J774.D9 cells do not produce detectable levels of O2− due to a lack of expression of the cybb gene encoding the cytochrome b558 (gp91) subunit of NADPH oxidase (19). In the absence of IFN-γ activation, there was no significant difference (P > 0.05) in the numbers of B. abortus CFU (either mutant or wild type) recovered from J774.16 and J774.D9 cells at 48 h after infection (Fig. 1A). The numbers of virB mutant BA41 CFU recovered from both J774 clones at 48 h were approximately 1% the numbers of the wild-type CFU recovered, in agreement with previous reports (30, 39). The numbers of cell-associated CFU of B. abortus 2308 and the virB mutant determined at zero time were similar, suggesting that there were no differences in internalization of the two strains (data not shown).
FIG. 1.
Survival of B. abortus 2308 and isogenic virB mutant BA41 in J774.16 cells (open bars), which generate an oxidative burst upon activation, and J774.D9 cells (solid bars), which lack a complete NADPH oxidase. Cells were incubated for 24 h without IFN-γ (A) or with IFN-γ (B) and subsequently were infected with B. abortus 2308 or BA41. The numbers of intracellular CFU of B. abortus were determined 48 h postinoculation. Each bar indicates the mean ± standard deviation for a total of six data samples (from two independent experiments, each containing triplicate samples). Data were analyzed by using a Student's t test. Statistically significant differences between survival in J774.16 cells and survival in J774.D9 cells are indicated by asterisks (P < 0.05). The two J774 clones had equal numbers of cell-associated B. abortus CFU at zero time (data not shown). wt, wild type.
In cells activated with IFN-γ (Fig. 1B), significantly greater numbers of B. abortus 2308 CFU were recovered from J774.D9 cells than from J774.16 cells (P < 0.005), suggesting that production of superoxide by activated macrophages is a mechanism that limits intracellular growth of B. abortus. If the virB locus contributes to avoidance of oxidative killing in activated cells, we would expect the virB mutant to exhibit increased survival in cells unable to produce superoxide. Although a difference in the numbers of virB mutant BA41 CFU recovered from the two cell lines was observed at 48 h after infection (Fig. 1), this difference was not significant. Thus, these data did not suggest that the the virB locus protects B. abortus against oxidative killing by macrophages in vitro.
gp91phox gene knockout mice are able to control replication of both B. abortus wild type and the virB mutant to the same extent as C57BL/6 mice.
In order to determine whether NADPH oxidase may play a role in controlling B. abortus infection in vivo and whether the virB locus may play a role in evasion of this killing mechanism in the host, we performed mixed-infection experiments with C57BL/6 mice and with mice carrying a targeted knockout of the cybb gene encoding the gp91 cytochrome b subunit (gp91phox) of NADPH oxidase (36). Mixed infections were used in order to provide an internal control for the level of infection with wild-type B. abortus in each animal. Each mouse was injected i.p. with a mixed inoculum containing approximately 5 × 105 CFU of B. abortus 2308 and 5 × 105 CFU of BA41 (virB), and the CFU of each challenge strain were enumerated in spleen homogenates 14 and 60 days postinfection. If NADPH oxidase is required for control of B. abortus in vivo, the gp91phox knockout mice should harbor higher total numbers of B. abortus. If virB is required for evasion of oxidative killing in this model, the ratio of the CFU of the virB mutant to the CFU of the wild type would be expected to increase in the knockout mice relative to the ratio in C57BL/6 mice.
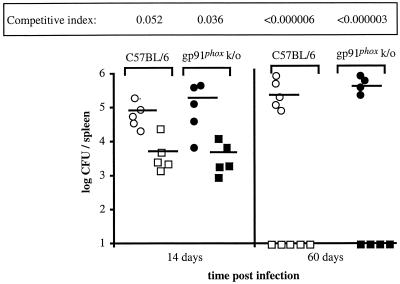
Figure 2 shows that at 2 weeks postinfection, although the total number of B. abortus CFU recovered from the spleens of gp91phox knockout mice was slightly higher than the total number of B. abortus CFU recovered from the spleens of C57BL/6 mice, the two groups did not differ significantly (P = 0.30), suggesting that NADPH oxidase is dispensable for control of B. abortus infection in vivo. Similarly, at 60 days postinoculation, we observed no significant difference between the two strains in terms of the number of wild-type or virB mutant bacteria recovered from the spleens. Furthermore, the ratios of virB mutant BA41 CFU to wild-type strain 2308 CFU also did not differ significantly at either time point, arguing against a role for the virB locus in evasion of this killing mechanism in mice. A control experiment in which C57BL/6 and gp91phox knockout mice were infected individually with B. abortus 2308 or BA41 (virB) resulted in numbers of wild-type and mutant bacteria in the spleens similar to the numbers obtained in the mixed-infection experiment. This result confirmed that wild-type B. abortus was not able to rescue the virB mutant in vivo by providing virB-mediated functions in trans (data not shown).
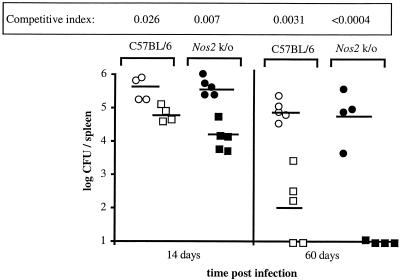
FIG. 2.
Effect of NADPH oxidase on growth of B. abortus 2308 and the virB mutant in mouse spleens. Groups of 10 C57BL/6 mice and 10 gp91phox knockout mice were inoculated i.p. with a mixture containing equal amounts of B. abortus 2308 and the virB mutant. The CFU of each strain were enumerated in the spleens of five mice from each group at 14 days and 60 days postinoculation. For each mouse, the results are indicated by one circle (log CFU of the wild type) and one square (log CFU of the virB mutant). The open circles indicate the log CFU of B. abortus 2308 recovered from the spleens of C57BL/6 mice, and the open squares indicate the log CFU of the virB mutant recovered from the spleens of C57BL/6 mice. The solid circles indicate the log CFU of B. abortus 2308 recovered from gp91phox knockout mice, and the solid squares indicate the log CFU of the virB mutant recovered from the spleens of gp91phox knockout mice. The means of the data are indicated by horizontal lines. The competitive index was calculated by dividing the mean ratio of mutant CFU to wild-type CFU recovered from spleens by the ratio of the mutant CFU to the wild-type CFU in the inoculum. k/o, knockout.
Inhibition of iNOS in activated peritoneal macrophages increases intracellular survival of the B. abortus wild type but not the virB mutant.
Like NADPH oxidase, iNOS (or NOS2) is a bactericidal enzyme whose activity increases in response to macrophage activation. We next sought to determine whether the virB locus mediates evasion of NO-mediated killing. The J774 cell lines used for previous experiments did not produce NO upon activation with IFN-γ and B. abortus infection (data not shown). Therefore, in order to determine whether NO produced by the macrophages is bactericidal, we measured the effect of inhibition of iNOS on survival of the B. abortus wild type and virB mutant BA41 in elicited peritoneal macrophages from C57BL/6 mice. Production of NO by macrophages was quantified by using the Griess reagent that measures nitrite, a reaction product of NO. Table 1 shows that periodate-elicited peritoneal macrophages produced only low levels of nitrite when they were infected with either the B. abortus wild type or the virB mutant and that these low levels were not reduced by treatment of cells with the iNOS inhibitor MMLA. These data document that B. abortus infection does not activate iNOS in these cells. However, activation of macrophages with 20 U of murine IFN-γ per ml and 1 pg of LPS per ml prior to infection increased the production of NO both by uninfected cells and by B. abortus-infected cells. NO production by activated macrophages could be inhibited by MMLA treatment, indicating that the NO is the product of iNOS activity (Table 1).
TABLE 1.
NO production by periodate-elicited murine peritoneal macrophages at 24 h postinfectiona
| Inoculum | Nitrite concn (μM/105 cells) in cell supernatants pretreated with:
|
|||
|---|---|---|---|---|
| None | MMLAb | IFN-γ + LPSc | IFN-γ + LPS + MMLA | |
| Uninfected | 3 ± 2 | 3 ± 2 | 102 ± 14 | 5 ± 0.3 |
| B. abortus 2308 | 11 ± 6 | 17 ± 10 | 570 ± 390 | 43 ± 13 |
| B. abortus virB mutant | 20 ± 5 | 12 ± 6 | 244 ± 104 | 37 ± 16 |
The nitrite concentration in 0.05 ml of cell supernatant was measured spectrophotometrically at 570 nm after incubation with Griess reagent. The values are averages ± standard deviations for triplicate wells.
MMLA (250 μM) was added to cells after infection with B. abortus.
Cells were activated 16 h before infection with 20 U of IFN-γ per ml and 1 pg of LPS per ml.
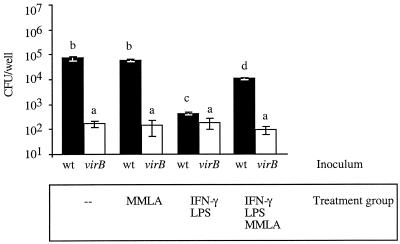
In nonactivated macrophages, inhibition of iNOS did not increase the number of either B. abortus 2308 or virB mutant BA41 CFU recovered from cells 24 h after inoculation (Fig. 3). However, in activated macrophages, inhibition of iNOS with MMLA led to a 10-fold reduction in the amount of NO produced and resulted in a 10-fold increase in the number of wild-type B. abortus CFU surviving intracellularly (compared to nontreated cells; P < 0.005) (Fig. 3). In contrast, the virB mutant was unable to grow within elicited peritoneal macrophages, irrespective of IFN-γ activation (Fig. 3). No difference in the number of cell-associated CFU was observed for different samples, suggesting that similar numbers of bacteria were internalized in all of the treatment groups (data not shown). These data indicate that NO generated by activated macrophages limits intracellular growth of B. abortus, but the finding that the virB mutant was not rescued by MMLA treatment of the macrophages suggests that the virB genes do not act in evasion of NO.
FIG. 3.
Survival of B. abortus 2308 and the isogenic virB mutant BA41 in elicited murine peritoneal macrophages. Macrophages were either not treated (-), treated with the iNOS inhibitor MMLA, activated with 20 U of murine IFN-γ per ml and 1 pg of LPS per ml, or activated and treated with MMLA. The solid bars indicate the number of intracellular CFU of B. abortus 2308 per well, and the open bars indicate the number of CFU of BA41 (virB) at 24 h postinoculation. Each bar indicates the mean ± standard deviation for triplicate samples from a single experiment. Statistical analysis was performed by using a Student's t test. The same letter above bars indicates that differences were not statistically significant (P > 0.05). Different letters above bars indicate that differences were statistically significant at P < 0.05. wt, wild type.
iNOS knockout mice control growth of a virB mutant better than C57BL/6 mice control growth of the mutant.
To determine whether the in vitro role of iNOS in limiting intracellular growth of B. abortus is relevant in the host, we compared the abilities of C57BL/6 mice and congenic Nos2 knockout mice to limit B. abortus infection. Mice were inoculated i.p. with a mixture containing approximately 5 × 105 CFU of B. abortus 2308 and 5 × 105 CFU of BA41 (virB), and the CFU of each challenge strain in the spleens were enumerated 14 and 60 days postinfection. If iNOS is required for control of B. abortus in vivo, the Nos2 knockout mice should harbor higher total numbers of B. abortus CFU in their spleens than the C57BL/6 mice harbor. If virB is required for evasion of NO in this model, the ratio of the number of CFU of the virB mutant to the number of CFU of the wild type is expected to increase in the Nos2 knockout mice relative to the ratio in the C57BL/6 mice.
At 14 days postinoculation, spleens from Nos2 knockout mice and C57BL/6 controls contained equivalent total numbers of B. abortus (P = 0.58). Similarly, at 60 days, no difference in total bacterial load was observed between the two groups of mice (P = 0.79), suggesting that the knockout mice are able to control the infection with B. abortus. Examination of histological sections of spleens and livers of Nos2 knockout mice and controls revealed similar pathological findings in the two mouse strains (data not shown).
Analysis of the mixed-infection data revealed that at 14 days postinoculation, the Nos2 knockout mice contained significantly less of the virB mutant than the wild-type controls contained, which was represented by a lower competitive index (P = 0.01) (Fig. 4). After 60 days, the two strains of mice were infected with equivalent numbers of the wild-type bacteria. A slight difference between the Nos2 knockout and C57BL/6 mice in the numbers of the virB mutant in the spleens was observed 60 days postinoculation. Three of the four Nos2 knockout mice had cleared the virB mutant, while four of the five C57BL/6 mice still harbored detectable numbers of the attenuated strain. However, the differences observed were not statistically significant (P = 0.06). These data suggest that while the Nos2 knockout mice and the control mice harbored similar numbers of wild-type B. abortus CFU, the Nos2 knockout mice were able to clear the attenuated virB strain more rapidly than the C57BL/6 controls. Taken together, these results indicate that iNOS activity is not required for control of B. abortus infection in the mouse and that the virB locus does not play a role in evasion of or resistance to NO.
FIG. 4.
Effect of iNOS on growth of B. abortus 2308 and the virB mutant in mouse spleens. Groups of 10 C57BL/6 mice and 10 Nos2 knockout mice were inoculated i.p. with a mixture containing equal amounts of B. abortus 2308 and the virB mutant. The CFU of each strain were enumerated in the spleens of five mice from each group at 14 days and 60 days postinoculation. For each mouse, the results are indicated by one circle (log CFU of the wild type) and one square (log CFU of the virB mutant). The open circles indicate log CFU of B. abortus 2308 recovered from the spleens of C57BL/6 mice, and the open squares indicate log CFU of the virB mutant recovered from the spleens of C57BL/6 mice. The solid circles indicate log CFU of B. abortus 2308 recovered from Nos2 knockout mice, and the solid squares indicate log CFU of the virB mutant recovered from the spleens of Nos2 knockout mice. The means of the data are indicated by horizontal lines. The competitive index was calculated by dividing the mean of mutant CFU recovered from spleens/wild-type CFU recovered from spleens by the ratio of the mutant to the wild type in the inoculum. k/o, knockout.
DISCUSSION
The function of the B. abortus virB locus is essential for intracellular survival, both in cultured cells and in the mouse model (17, 22, 30, 39). Disruption of the virB locus results in failure to traffic to a cellular compartment of HeLa cells that permits replication (5, 10). However, it is not clear how virB acts to enable replication of B. abortus in the macrophage, the cell type in which B. abortus is thought to persist in the host. Evidence obtained by other investigators suggests that B. abortus is able to inhibit phagosome-lysosome fusion and that phagosomes containing B. abortus do not contain NADPH oxidase (2, 18, 28). In order to elucidate the mechanism by which the virB genes enable B. abortus to survive in the macrophage, we investigated whether the virB locus enables B. abortus to evade killing by the macrophage NADPH oxidase and by iNOS.
To determine whether virB mediates evasion of NADPH oxidase, we examined the intracellular survival of B. abortus 2308 and an isogenic virB mutant in in vitro and in vivo NADPH oxidase deficiency models. The kinetics of bacterial replication and killing were examined in two J774 macrophage clones that differed in the ability to produce the oxidative burst. When the cells were not activated with IFN-γ prior to inoculation with B. abortus, cells of J774.16, the cell line capable of generating an oxidative burst, and J774.D9 cells, which are unable to generate superoxide, contained equivalent numbers of B. abortus 2308 cells and of virB mutant cells (Fig. 1). However after activation of these cells with IFN-γ, the J774.D9 cells contained 2 logs more of B. abortus 2308 and slightly more of the virB mutant than the clone J774.16 cells contained. This result suggests that in activated macrophages oxygen-dependent mechanisms are important for killing of B. abortus, but it did not provide evidence for virB-mediated evasion of NADPH oxidase. Similarly, when we performed competitive infections of gp91phox knockout mice and the inbred parent strain, we found no significant difference in the ratios of the virB mutant to the wild type recovered from the two mouse strains (Fig. 2). This result suggests that virB does not play a role in evasion of oxidative killing in vivo. Interestingly, the total numbers of B. abortus CFU recovered from the two mouse strains did not differ significantly, suggesting that in vivo NADPH oxidase is not required to control B. abortus replication. This result is in agreement with the findings of Ko et al. (25), which showed that reactive oxygen intermediates are not required for control of B. abortus infection in naïve mice.
Inhibition of iNOS in activated peritoneal macrophages increased survival of B. abortus 2308 in vitro, showing that NO limits bacterial replication in these cells. However, the virB mutant was unable to grow in these cells, independent of the activation state or NO production. In Nos2 knockout mice, B. abortus 2308 replicated to the same extent in the spleen as it replicated in the parent C57BL/6 strain. However, lower numbers of CFU of the virB mutant were recovered from the iNOS knockout mice than from the C57BL/6 mice, suggesting that the knockout mice are able to clear the attenuated mutant more rapidly. At 60 days postinfection, more of the iNOS knockout mice than of the C57BL/6 mice had cleared the attenuated virB mutant, although the difference between the numbers of CFU was not significant (P = 0.06) (Fig. 3). If the virB genes are involved in evasion of NO, we would expect to recover higher rather than lower numbers of CFU of the virB mutant from the knockout mice. Thus, these data show that iNOS is dispensable for control of B. abortus infection in vivo and argue against a role for the virB locus in evasion of nitrosative killing in vivo. Splitter and colleagues have found that high levels of NO production in a macrophage cell line correlate with increased survival after prolonged incubation, so taken together, our findings may indicate that NO production may in some way be advantageous to the persistence of B. abortus in vivo (41).
Several investigators have shown that in vitro, either opsonization of bacteria with specific antiserum or activation of cells with IFN-γ is required in order to detect generation of O2− and NO by macrophages when they are challenged with B. abortus or B. suis (16, 20, 21, 24). Based on these observations, it has been suggested that these defense mechanisms may play a role in acquired immunity (20). Although we did not observe differences in the numbers of B. abortus CFU in wild-type and knockout mice at 60 days postinfection, well after the onset of adaptive immunity, our data do not rule out the possibility that oxidative and nitrosative killing mechanisms may play a role in resistance to reinfection or infection in vaccinated hosts.
The lack of a requirement for both NADPH oxidase and iNOS in control of B. abortus infection contrasts with data obtained for acute S. enterica serovar Typhimurium infection, in which gp91phox knockout mice exhibit increased organ loads and succumb rapidly to infection with an attenuated isolate, while onset of mortality in iNOS knockout mice occurs approximately 1 week later (27). However, our findings resemble those obtained with a murine model of pulmonary tuberculosis, in which IFN-γ was also required for containment of bacterial infection but neither NADPH oxidase nor iNOS was a critical mediator controlling bacterial multiplication (6-8). Similarly, Ko et al. found that whereas IRF-1 knockout mice succumbed rapidly to B. abortus infection, both gp91phox and Nos2 knockout mice had only slightly higher organ loads of B. abortus than the parent mouse strains (25). Since both tuberculosis and brucellosis are chronic infections, the host may use a common IFN-γ-dependent mechanism for controlling these infections that is independent of NADPH oxidase and iNOS.
The J774.D9 cells activated with IFN-γ did not produce NO (data not shown), but they controlled replication of B. abortus significantly better than nonactivated macrophages controlled replication (Fig. 1). These data suggest that macrophage activation by IFN-γ may induce expression of killing mechanisms that are independent of iNOS and NADPH oxidase. In the case of Mycobacterium avium infection, it has been shown that activation of macrophages by IFN-γ increases acidification and alters maturation of the M. avium-containing vacuole (38). Similarly, treatment of S. enterica serovar Typhimurium-infected macrophages with IFN-γ has been shown to increase fusion of Salmonella-containing vacuoles with lysosomes (23). IFN-γ has also been shown to regulate intracellular cholesterol distribution, which has been shown to influence entry of B. suis into macrophages (29, 31). Like M. avium and other intracellular pathogens, B. abortus, B. suis, and Brucella melitensis have been shown to alter endocytic trafficking to establish a compartment favorable for intracellular replication. The virB locus has been shown to be required for modulation of intracellular trafficking in HeLa cells (5, 10). We have found that in nonactivated macrophages, the numbers of B. abortus wild-type CFU recovered are 1 to 2 logs higher than the numbers of virB mutant CFU recovered at 48 h (Fig. 1 A). However, after IFN-γ activation, the number of wild-type CFU is similar to the number of virB mutant CFU. Given the role of the virB genes in intracellular trafficking of Brucella spp. (5, 10), it is tempting to speculate that as in M. avium, IFN-γ activation may alter endocytic trafficking in a manner that prevents the action of the B. abortus VirB proteins from establishing a replicative niche in the macrophage. Further experiments will be required to understand the mechanism by which the B. abortus virB locus mediates intracellular replication of this organism in macrophages.
Acknowledgments
We thank N. Buchmeier and B. Bloom for providing the J774.16 and J774.D9 cell lines and A. Bäumler for critical reading of the manuscript.
This work was supported by PHS grant AI50553 to R.T.
Editor: D. L. Burns
REFERENCES
- 1.Alton, G. G., L. M. Jones, and D. E. Pietz. 1975. Laboratory techniques in brucellosis, 2nd ed. World Health Organization, Geneva, Switzerland. [PubMed]
- 2.Arenas, G. N., A. S. Staskevich, A. Aballay, and L. S. Mayorga. 2000. Intracellular trafficking of Brucella abortus in J774 macrophages. Infect. Immun. 68:4255-4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldwin, C. L., and A. J. Winter. 1994. Macrophages and Brucella. Immunol. Ser. 60:363-380. [PubMed] [Google Scholar]
- 4.Boschiroli, M. L., S. Ouahrani-Bettache, V. Foulongne, S. Michaux-Charachon, G. Bourg, A. Allardet-Servent, C. Cazevieille, J. P. Liautard, M. Ramuz, and D. O'Callaghan. 2002. The Brucella suis virB operon is induced intracellularly in macrophages. Proc. Natl. Acad. Sci. USA 99:1544-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Comerci, D. J., M. J. Martinez-Lorenzo, R. Sieira, J. P. Gorvel, and R. A. Ugalde. 2001. Essential role of the VirB machinery in the maturation of the Brucella abortus-containing vacuole. Cell Microbiol. 3:159-168. [DOI] [PubMed] [Google Scholar]
- 6.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper, A. M., J. E. Pearl, J. V. Brooks, S. Ehlers, and I. M. Orme. 2000. Expression of the nitric oxide synthase 2 gene is not essential for early control of Mycobacterium tuberculosis in the murine lung. Infect. Immun. 68:6879-6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper, A. M., B. H. Segal, A. A. Frank, S. M. Holland, and I. M. Orme. 2000. Transient loss of resistance to pulmonary tuberculosis in p47phox−/− mice. Infect. Immun. 68:1231-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damiani, G., C. Kiyotaki, W. Soeller, M. Sasada, J. Peisach, and B. R. Bloom. 1980. Macrophage variants in oxygen metabolism. J. Exp. Med. 152:808-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delrue, R. M., M. Martinez-Lorenzo, P. Lestrate, I. Danese, V. Bielarz, P. Mertens, X. De Bolle, A. Tibor, J. P. Gorvel, and J. J. Letesson. 2001. Identification of Brucella spp. genes involved in intracellular trafficking. Cell Microbiol. 3:487-497. [DOI] [PubMed] [Google Scholar]
- 11.DelVecchio, V. G., V. Kapatral, R. J. Redkar, G. Patra, C. Mujer, T. Los, N. Ivanova, I. Anderson, A. Bhattacharyya, A. Lykidis, G. Reznik, L. Jablonski, N. Larsen, M. D'Souza, A. Bernal, M. Mazur, E. Goltsman, E. Selkov, P. H. Elzer, S. Hagius, D. O'Callaghan, J. J. Letesson, R. Haselkorn, N. Kyrpides, and R. Overbeek. 2002. The genome sequence of the facultative intracellular pathogen Brucella melitensis. Proc. Natl. Acad. Sci. USA 99:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Detilleux, P. G., B. L. Deyoe, and N. F. Cheville. 1991. Effect of endocytic and metabolic inhibitors on the internalization and intracellular growth of Brucella abortus in Vero cells. Am. J. Vet. Res. 52:1658-1664. [PubMed] [Google Scholar]
- 13.Detilleux, P. G., B. L. Deyoe, and N. F. Cheville. 1990. Entry and intracellular localization of Brucella spp. in Vero cells: fluorescence and electron microscopy. Vet. Pathol. 27:317-328. [DOI] [PubMed] [Google Scholar]
- 14.Detilleux, P. G., B. L. Deyoe, and N. F. Cheville. 1990. Penetration and intracellular growth of Brucella abortus in nonphagocytic cells in vitro. Infect. Immun. 58:2320-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding, A. H., C. F. Nathan, and D. J. Stuehr. 1988. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J. Immunol. 141:2407-2412. [PubMed] [Google Scholar]
- 16.Eze, M. O., L. Yuan, R. M. Crawford, C. M. Paranavitana, T. L. Hadfield, A. K. Bhattacharjee, R. L. Warren, and D. L. Hoover. 2000. Effects of opsonization and gamma interferon on growth of Brucella melitensis 16M in mouse peritoneal macrophages in vitro. Infect. Immun. 68:257-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foulongne, V., G. Bourg, C. Cazevieille, S. Michaux-Charachon, and D. O'Callaghan. 2000. Identification of Brucella suis genes affecting intracellular survival in an in vitro human macrophage infection model by signature-tagged transposon mutagenesis. Infect. Immun. 68:1297-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gay, B., S. Sanchez-Teff, and R. Caravano. 1984. Ultrastructural localization of NADPH-oxidase activity in murine peritoneal macrophages during phagocytosis of Brucella. Correlation with the production of superoxide anions. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 45:147-155. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg, M., L. S. Belkowski, and B. R. Bloom. 1990. Regulation of macrophage function by interferon-gamma. Somatic cell genetic approaches in murine macrophage cell lines to mechanisms of growth inhibition, the oxidative burst, and expression of the chronic granulomatous disease gene. J. Clin. Investig. 85:563-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gross, A., S. Spiesser, A. Terraza, B. Rouot, E. Caron, and J. Dornand. 1998. Expression and bactericidal activity of nitric oxide synthase in Brucella suis-infected murine macrophages. Infect. Immun. 66:1309-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harmon, B. G., and L. G. Adams. 1987. Assessment of bovine mammary gland macrophage oxidative burst activity in a chemiluminescence assay. Am. J. Vet. Res. 48:119-125. [PubMed] [Google Scholar]
- 22.Hong, P. C., R. M. Tsolis, and T. A. Ficht. 2000. Identification of genes required for chronic persistence of Brucella abortus in mice. Infect. Immun. 68:4102-4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishibashi, Y., and T. Arai. 1990. Effect of gamma-interferon on phagosome-lysosome fusion in Salmonella typhimurium-infected murine macrophages. FEMS Microbiol. Immunol. 2:75-82. [DOI] [PubMed] [Google Scholar]
- 24.Jiang, X., B. Leonard, R. Benson, and C. L. Baldwin. 1993. Macrophage control of Brucella abortus: role of reactive oxygen intermediates and nitric oxide. Cell. Immunol. 151:309-319. [DOI] [PubMed] [Google Scholar]
- 25.Ko, J., A. Gendron-Fitzpatrick, and G. A. Splitter. 2002. Susceptibility of IFN regulatory factor 1- and IFN consensus sequence binding protein-deficient mice to brucellosis. J. Immunol. 168:2433-2440. [DOI] [PubMed] [Google Scholar]
- 26.MacMicking, J. D., C. Nathan, G. Hom, N. Chartrain, D. S. Fletcher, M. Trumbauer, K. Stevens, Q. W. Xie, K. Sokol, N. Hutchinson, et al. 1995. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell 81:641-650. [DOI] [PubMed] [Google Scholar]
- 27.Mastroeni, P., A. Vazquez-Torres, F. C. Fang, Y. Xu, S. Khan, C. E. Hormaeche, and G. Dougan. 2000. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. II. Effects on microbial proliferation and host survival in vivo. J. Exp. Med. 192:237-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naroeni, A., N. Jouy, S. Ouahrani-Bettache, J. P. Liautard, and F. Porte. 2001. Brucella suis-impaired specific recognition of phagosomes by lysosomes due to phagosomal membrane modifications. Infect. Immun. 69:486-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naroeni, A., and F. Porte. 2002. Role of cholesterol and the ganglioside GM1 in entry and short-term survival of Brucella suis in murine macrophages. Infect. Immun. 70:1640-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Callaghan, D., C. Cazevieille, A. Allardet-Servent, M. L. Boschiroli, G. Bourg, V. Foulongne, P. Frutos, Y. Kulakov, and M. Ramuz. 1999. A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Mol. Microbiol. 33:1210-1220. [DOI] [PubMed] [Google Scholar]
- 31.Panousis, C. G., and S. H. Zuckerman. 2000. Regulation of cholesterol distribution in macrophage-derived foam cells by interferon-gamma. J. Lipid Res. 41:75-83. [PubMed] [Google Scholar]
- 32.Pizarro-Cerda, J., M. Desjardins, E. Moreno, S. Akira, and J. P. Gorvel. 1999. Modulation of endocytosis in nuclear factor IL-6−/− macrophages is responsible for a high susceptibility to intracellular bacterial infection. J. Immunol. 162:3519-3526. [PubMed] [Google Scholar]
- 33.Pizarro-Cerda, J., S. Meresse, R. G. Parton, G. van der Goot, A. Sola-Landa, I. Lopez-Goni, E. Moreno, and J. P. Gorvel. 1998. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect. Immun. 66:5711-5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pizarro-Cerda, J., E. Moreno, and J. P. Gorvel. 2000. Invasion and intracellular trafficking of Brucella abortus in nonphagocytic cells. Microbes Infect. 2:829-835. [DOI] [PubMed] [Google Scholar]
- 35.Pizarro-Cerda, J., E. Moreno, V. Sanguedolce, J. L. Mege, and J. P. Gorvel. 1998. Virulent Brucella abortus prevents lysosome fusion and is distributed within autophagosome-like compartments. Infect. Immun. 66:2387-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pollock, J. D., D. A. Williams, M. A. Gifford, L. L. Li, X. Du, J. Fisherman, S. H. Orkin, C. M. Doerschuk, and M. C. Dinauer. 1995. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat. Genet. 9:202-209. [DOI] [PubMed] [Google Scholar]
- 37.Porte, F., J. P. Liautard, and S. Kohler. 1999. Early acidification of phagosomes containing Brucella suis is essential for intracellular survival in murine macrophages. Infect. Immun. 67:4041-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schaible, U. E., S. Sturgill-Koszycki, P. H. Schlesinger, and D. G. Russell. 1998. Cytokine activation leads to acidification and increases maturation of Mycobacterium avium-containing phagosomes in murine macrophages. J. Immunol. 160:1290-1296. [PubMed] [Google Scholar]
- 39.Sieira, R., D. J. Comerci, D. O. Sanchez, and R. A. Ugalde. 2000. A homologue of an operon required for DNA transfer in Agrobacterium is required in Brucella abortus for virulence and intracellular multiplication. J. Bacteriol. 182:4849-4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vazquez-Torres, A., Y. Xu, J. Jones-Carson, D. W. Holden, S. M. Lucia, M. C. Dinauer, P. Mastroeni, and F. C. Fang. 2000. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science 287:1655-1658. [DOI] [PubMed] [Google Scholar]
- 41.Wang, M., N. Qureshi, N. Soeurt, and G. Splitter. 2001. High levels of nitric oxide production decrease early but increase late survival of Brucella abortus in macrophages. Microb. Pathog. 31:221-230. [DOI] [PubMed] [Google Scholar]