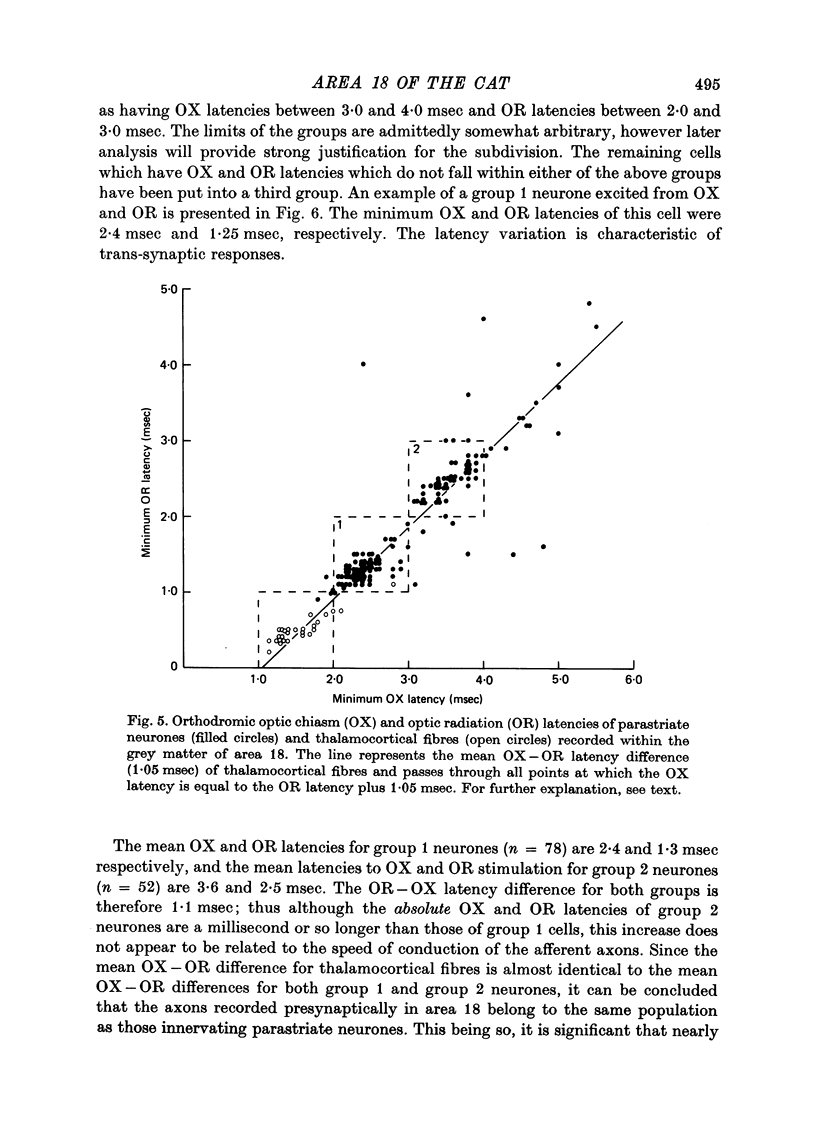
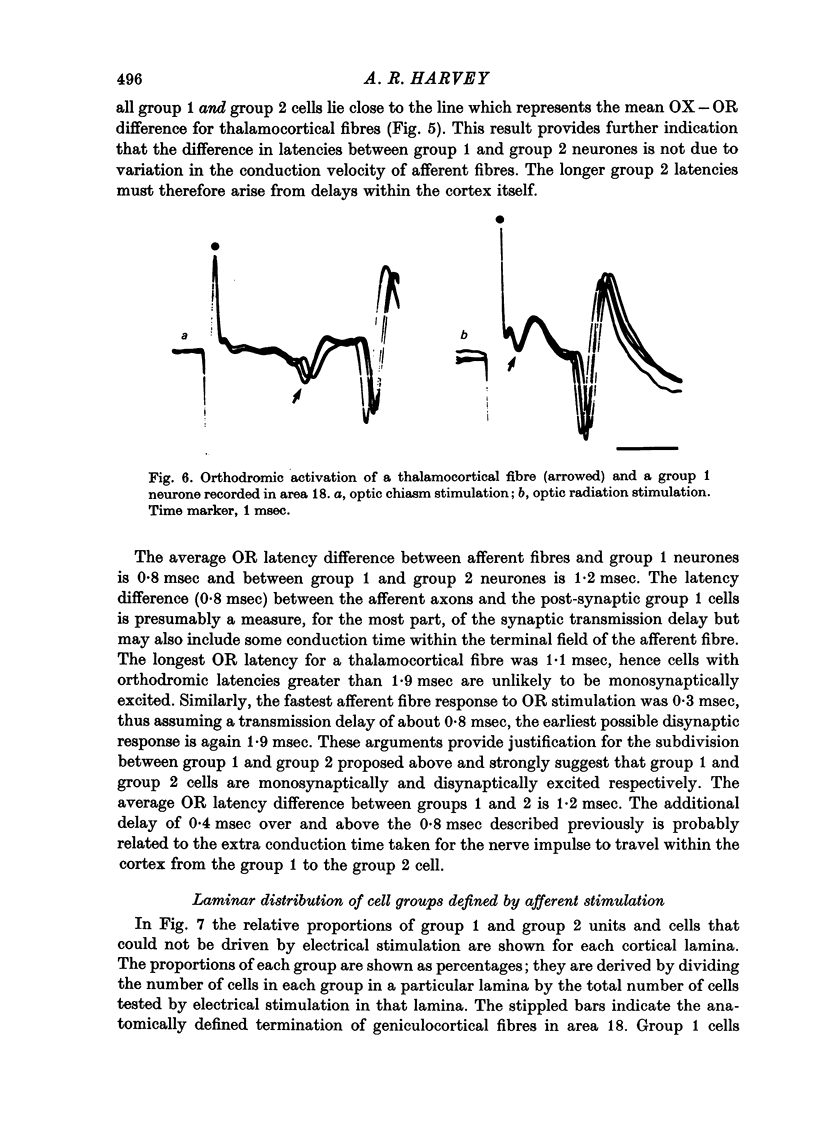
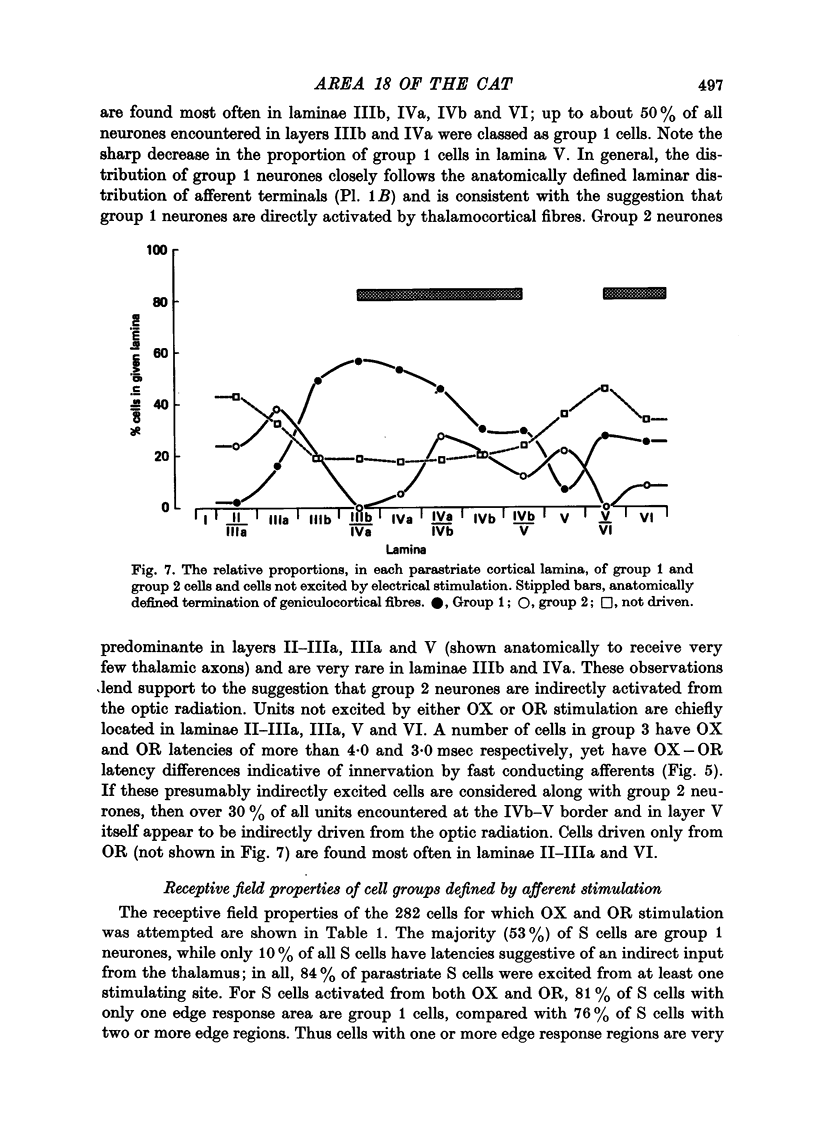
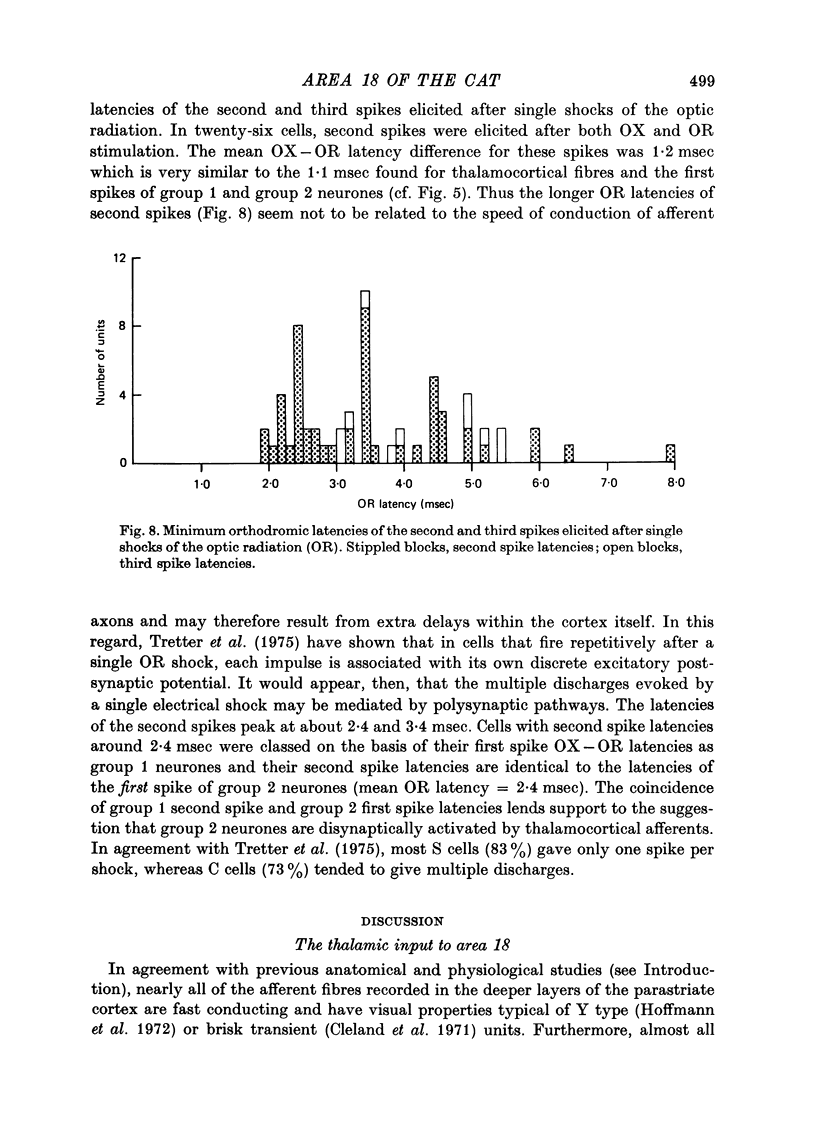
Abstract
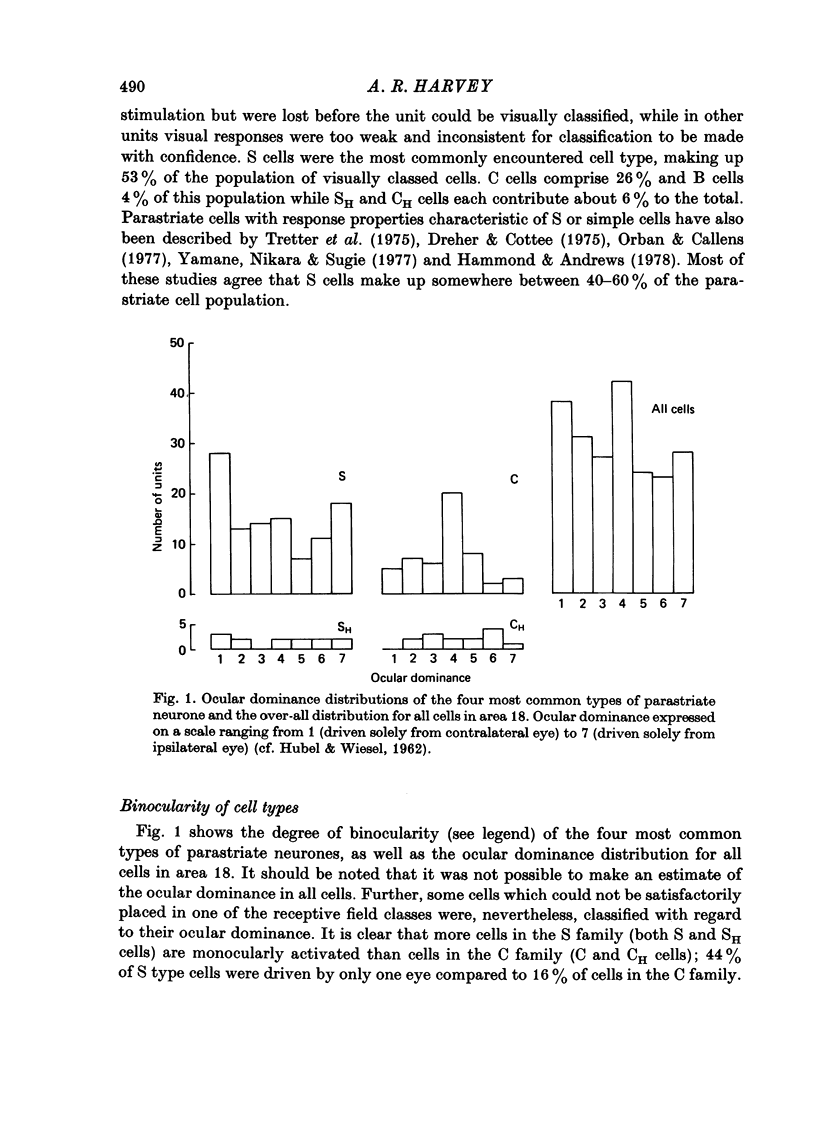
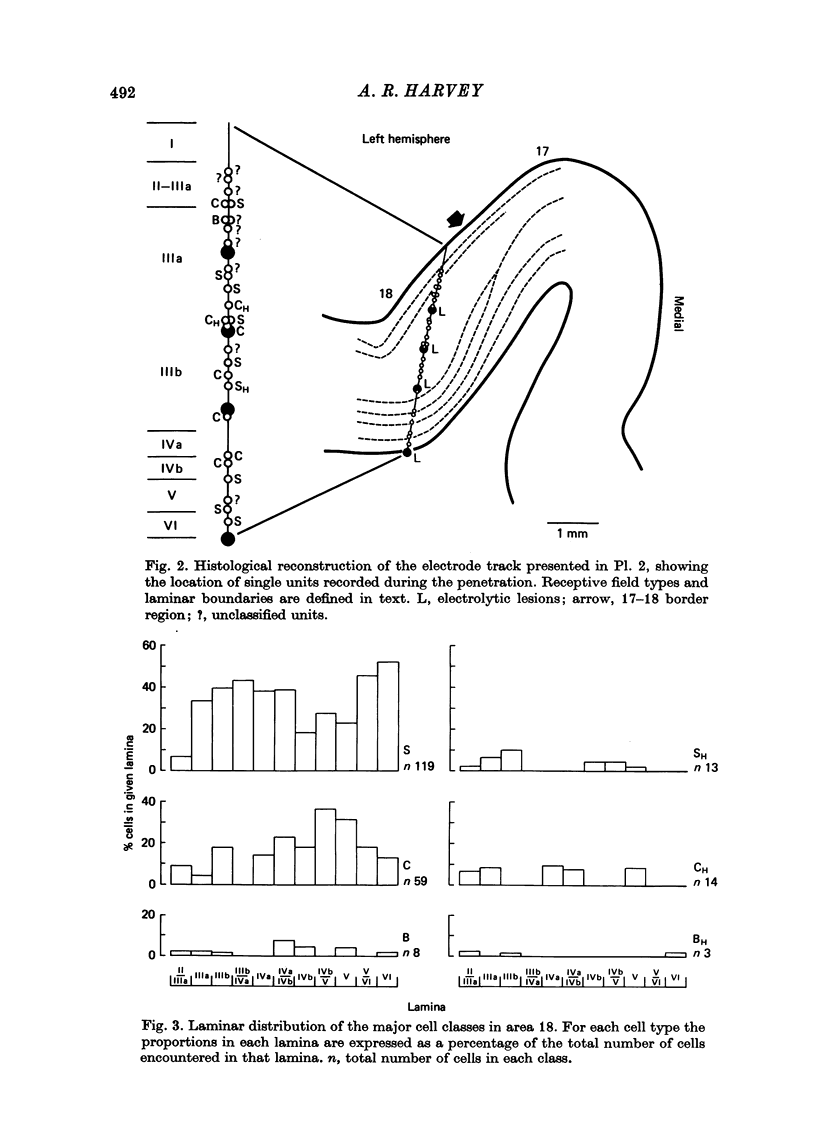
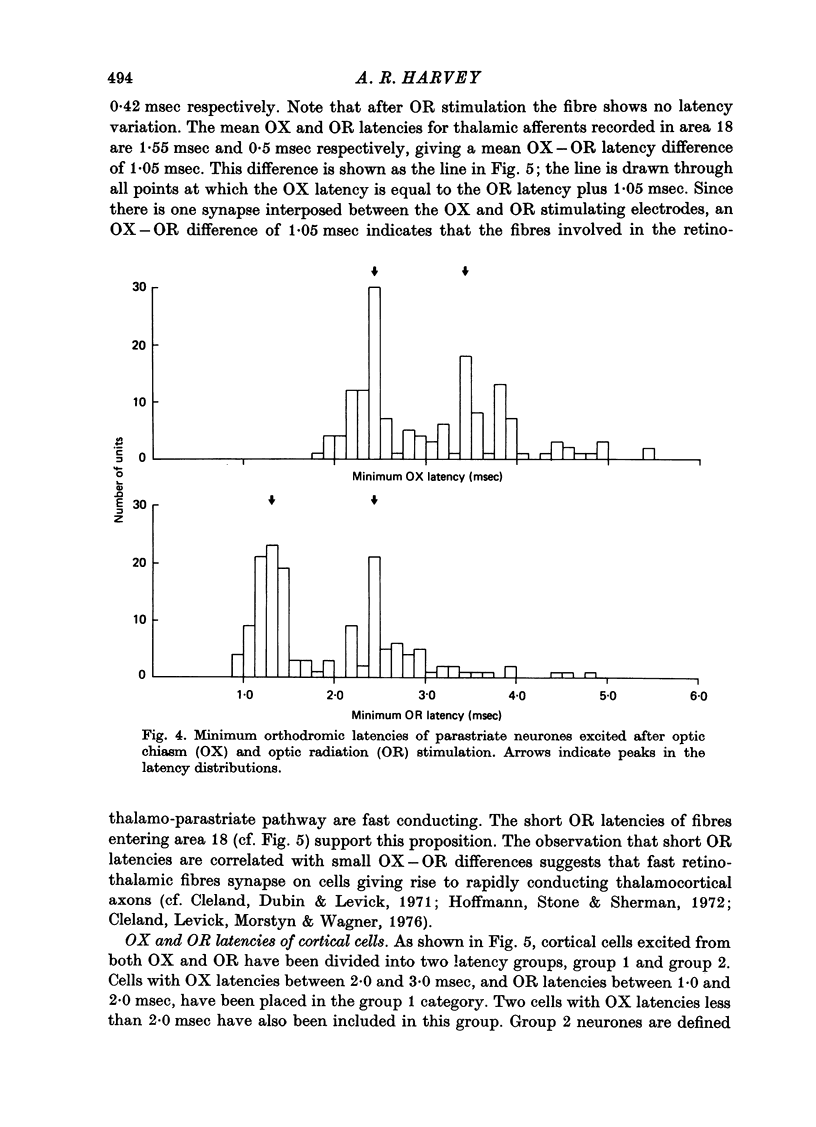
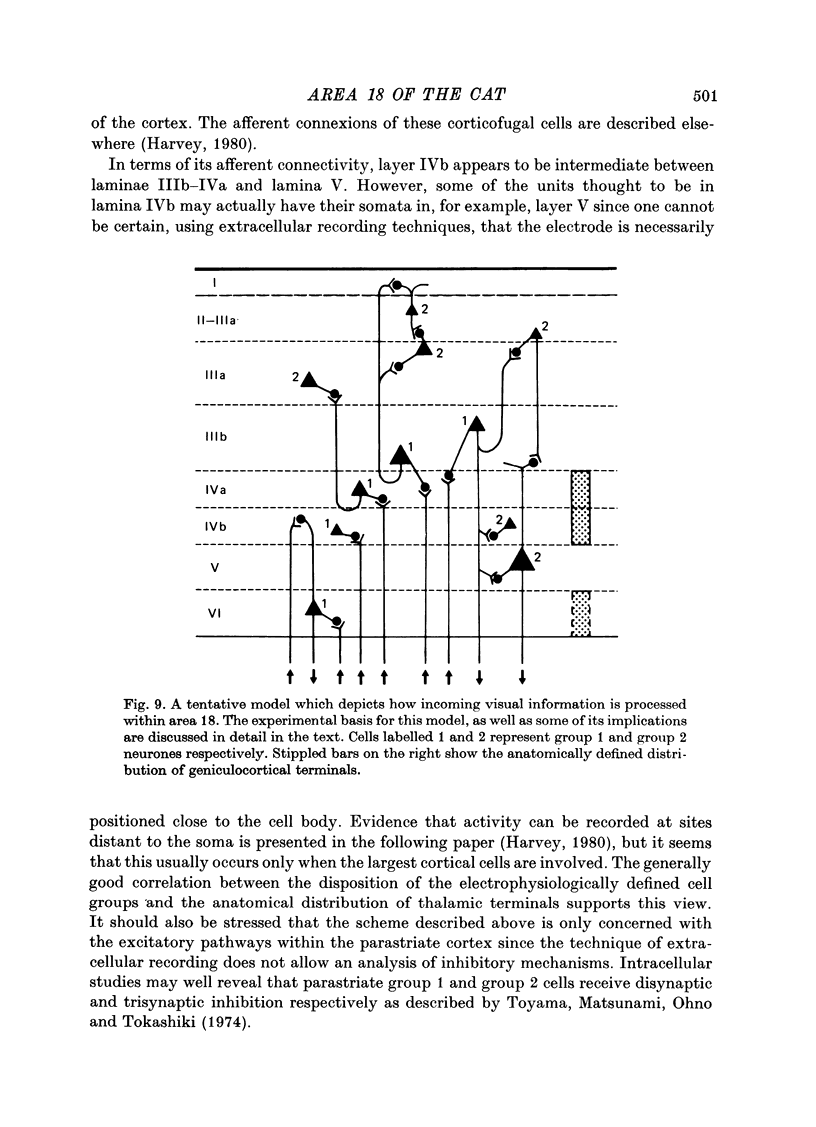
1. The receptive field properties, laminar distribution and afferent connectivity of cells in area 18 of the cat are described. 2. Testing with both moving and stationary stimuli revealed three main receptive field types which have been termed S, C and B, respectively (cf. Henry, 1977; Henry, Lund & Harvey, 1978). All three classes may show end-zone inhibition and units exhibiting this property have been designated SH, CH and BH. 3. S cells can be divided into spatially separate lights and/or dark edge response regions when tested with moving edges and usually have separate ON and/or OFF areas when tested with stationary flashing stimuli. They are the most commonly encountered cell type in area 18 and occur most frequently in laminae IIIb, IVa and VI. 4. Both C and B cells have spatially coincident light and dark edge response regions and give mixed ON and OFF discharges when tested with stationary flashing stimuli. Compared to B cells however, C cells have large receptive fields, they are broadly tuned for stimulus orientation and generally have a relatively high rate of spontaneous activity. C cells are more common than B cells and are encountered most often in laminae IVb and V. 5. Electrical stimulation of the optic chiasm (OX) and optic radiation (OR) was used to examine the afferent connectivity of parastriate neurons. Cells driven from both OX and OR have been divided into two main groups and it is argued that group 1 cells are directly, and group 2 cells are indirectly, excited by rapidly conducting afferent fibres. Group 1 cells are found most often in laminae IIIb, IVa, IVb and VI, and their distribution closely follows the anatomically defined laminar disposition of geniculocortical afferent terminals. Group 2 neurones predominate in laminae II-IIIa, IIIA and V. 6. The majority of S and SH cells are directly driven, whereas most C and CH cells have OX and OR latencies suggestive of indirect activation by thalamic afferents. 7. The intrinsic organization and possible functional role of area 18 is discussed in the light of these results.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albus K. Predominance of monocularly driven cells in the projection area of the central visual field in cat's striate cortex. Brain Res. 1975 May 23;89(2):341–347. doi: 10.1016/0006-8993(75)90725-8. [DOI] [PubMed] [Google Scholar]
- BISHOP P. O., BURKE W., DAVIS R. Single-unit recording from antidromically activated optic radiation neurones. J Physiol. 1962 Aug;162:432–450. doi: 10.1113/jphysiol.1962.sp006943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., KOZAK W., LEVICK W. R., VAKKUR G. J. The determination of the projection of the visual field on to the lateral geniculate nucleus in the cat. J Physiol. 1962 Oct;163:503–539. doi: 10.1113/jphysiol.1962.sp006991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Blakemore C., Pettigrew J. D. The neural mechanism of binocular depth discrimination. J Physiol. 1967 Nov;193(2):327–342. doi: 10.1113/jphysiol.1967.sp008360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. O., Henry G. H., Smith C. J. Binocular interaction fields of single units in the cat striate cortex. J Physiol. 1971 Jul;216(1):39–68. doi: 10.1113/jphysiol.1971.sp009508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. O., Henry G. H. Striate neurons: receptive field concepts. Invest Ophthalmol. 1972 May;11(5):346–354. [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971 Sep;217(2):473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R., Morstyn R., Wagner H. G. Lateral geniculate relay of slowly conducting retinal afferents to cat visual cortex. J Physiol. 1976 Feb;255(1):299–320. doi: 10.1113/jphysiol.1976.sp011281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt O. D., Nothdurft H. C. Representation of complex visual stimuli in the brain. Naturwissenschaften. 1978 Jun;65(6):307–318. doi: 10.1007/BF00368371. [DOI] [PubMed] [Google Scholar]
- Dreher B., Cottee L. J. Visual receptive-field properties of cells in area 18 of cat's cerebral cortex before and after acute lesions in area 17. J Neurophysiol. 1975 Jul;38(4):735–750. doi: 10.1152/jn.1975.38.4.735. [DOI] [PubMed] [Google Scholar]
- Dreher B., Sanderson K. J. Receptive field analysis: responses to moving visual contours by single lateral geniculate neurones in the cat. J Physiol. 1973 Oct;234(1):95–118. doi: 10.1113/jphysiol.1973.sp010336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher B., Sefton A. J. Properties of neurons in cat's dorsal lateral geniculate nucleus: a comparison between medial interlaminar and laminated parts of the nucleus. J Comp Neurol. 1979 Jan 1;183(1):47–64. doi: 10.1002/cne.901830105. [DOI] [PubMed] [Google Scholar]
- Dubin M. W., Cleland B. G. Organization of visual inputs to interneurons of lateral geniculate nucleus of the cat. J Neurophysiol. 1977 Mar;40(2):410–427. doi: 10.1152/jn.1977.40.2.410. [DOI] [PubMed] [Google Scholar]
- Garey L. J. A light and electron microscopic study of the visual cortex of the cat and monkey. Proc R Soc Lond B Biol Sci. 1971 Oct 12;179(1054):21–40. doi: 10.1098/rspb.1971.0079. [DOI] [PubMed] [Google Scholar]
- Garey L. J., Blakemore C. Monocular deprivation: morphological effects on different classes of neurons in the lateral geniculate nucleus. Science. 1977 Jan 28;195(4276):414–416. doi: 10.1126/science.831287. [DOI] [PubMed] [Google Scholar]
- Garey L. J., Powell T. P. An experimental study of the termination of the lateral geniculo-cortical pathway in the cat and monkey. Proc R Soc Lond B Biol Sci. 1971 Oct 12;179(1054):41–63. doi: 10.1098/rspb.1971.0080. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D., Kelly J. P. The projections of cells in different layers of the cat's visual cortex. J Comp Neurol. 1975 Sep;163(1):81–105. doi: 10.1002/cne.901630106. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS AND FUNCTIONAL ARCHITECTURE IN TWO NONSTRIATE VISUAL AREAS (18 AND 19) OF THE CAT. J Neurophysiol. 1965 Mar;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond P., Andrews D. P. Orientation tuning of cells in areas 17 and 18 of the cat's visual cortex. Exp Brain Res. 1978 Mar 15;31(3):341–351. doi: 10.1007/BF00237294. [DOI] [PubMed] [Google Scholar]
- Harvey A. R. A physiological analysis of subcortical and commissural projections of areas 17 and 18 of the cat. J Physiol. 1980 May;302:507–534. doi: 10.1113/jphysiol.1980.sp013258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry G. H., Harvey A. R., Lund J. S. The afferent connections and laminar distribution of cells in the cat striate cortex. J Comp Neurol. 1979 Oct 15;187(4):725–744. doi: 10.1002/cne.901870406. [DOI] [PubMed] [Google Scholar]
- Henry G. H., Lund J. S., Harvey A. R. Cells of the striate cortex projecting to the Clare-Bishop area of the cat. Brain Res. 1978 Jul 28;151(1):154–158. doi: 10.1016/0006-8993(78)90958-7. [DOI] [PubMed] [Google Scholar]
- Henry G. H. Receptive field classes of cells in the striate cortex of the cat. Brain Res. 1977 Sep 9;133(1):1–28. doi: 10.1016/0006-8993(77)90045-2. [DOI] [PubMed] [Google Scholar]
- Hoffmann K. P., Stone J., Sherman S. M. Relay of receptive-field properties in dorsal lateral geniculate nucleus of the cat. J Neurophysiol. 1972 Jul;35(4):518–531. doi: 10.1152/jn.1972.35.4.518. [DOI] [PubMed] [Google Scholar]
- Holländer H., Vanegas H. The projection from the lateral geniculate nucleus onto the visual cortex in the cat. A quantitative study with horseradish-peroxidase. J Comp Neurol. 1977 Jun 1;173(3):519–536. doi: 10.1002/cne.901730308. [DOI] [PubMed] [Google Scholar]
- Kato H., Bishop P. O., Orban G. A. Hypercomplex and simple/complex cell classifications in cat striate cortex. J Neurophysiol. 1978 Sep;41(5):1071–1095. doi: 10.1152/jn.1978.41.5.1071. [DOI] [PubMed] [Google Scholar]
- Kennedy H., Baleydier C. Direct projections from thalamic intralaminar nuclei to extra-striate visual cortex in the cat traced with horseradish peroxidase. Exp Brain Res. 1977 May 23;28(1-2):133–139. doi: 10.1007/BF00237091. [DOI] [PubMed] [Google Scholar]
- Kratz K. E., Webb S. V., Sherman S. M. Studies of the cat's medial interlaminar nucleus: a subdivision of the dorsal lateral geniculate nucleus. J Comp Neurol. 1978 Oct 1;181(3):601–614. doi: 10.1002/cne.901810308. [DOI] [PubMed] [Google Scholar]
- LeVay S., Ferster D. Relay cell classes in the lateral geniculate nucleus of the cat and the effects of visual deprivation. J Comp Neurol. 1977 Apr 15;172(4):563–584. doi: 10.1002/cne.901720402. [DOI] [PubMed] [Google Scholar]
- LeVay S., Gilbert C. D. Laminar patterns of geniculocortical projection in the cat. Brain Res. 1976 Aug 20;113(1):1–19. doi: 10.1016/0006-8993(76)90002-0. [DOI] [PubMed] [Google Scholar]
- Levick W. R. Another tungsten microelectrode. Med Biol Eng. 1972 Jul;10(4):510–515. doi: 10.1007/BF02474199. [DOI] [PubMed] [Google Scholar]
- Levick W. R., Cleland B. G., Dubin M. W. Lateral geniculate neurons of cat: retinal inputs and physiology. Invest Ophthalmol. 1972 May;11(5):302–311. [PubMed] [Google Scholar]
- Maciewicz R. J. Thalamic afferents to areas 17, 18 and 19 of cat cortex traced with horseradish peroxidase. Brain Res. 1975 Feb 7;84(2):308–312. doi: 10.1016/0006-8993(75)90985-3. [DOI] [PubMed] [Google Scholar]
- Movshon J. A., Thompson I. D., Tolhurst D. J. Spatial and temporal contrast sensitivity of neurones in areas 17 and 18 of the cat's visual cortex. J Physiol. 1978 Oct;283:101–120. doi: 10.1113/jphysiol.1978.sp012490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimi K., Sprague J. M. Thalamo-cortical organization of the visual system in the cat. J Comp Neurol. 1970 Feb;138(2):219–250. doi: 10.1002/cne.901380208. [DOI] [PubMed] [Google Scholar]
- OTSUKA R., HASSLER R. [On the structure and segmentation of the cortical center of vision in the cat]. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1962;203:212–234. doi: 10.1007/BF00352744. [DOI] [PubMed] [Google Scholar]
- Orban G. A. Area 18 of the cat: the first step in processing visual movement information. Perception. 1977;6(5):501–511. doi: 10.1068/p060501. [DOI] [PubMed] [Google Scholar]
- Orban G. A., Callens M. Receptive field types of area 18 neurones in the cat. Exp Brain Res. 1977 Oct 24;30(1):107–123. doi: 10.1007/BF00237862. [DOI] [PubMed] [Google Scholar]
- Palmer L. A., Rosenquist A. C. Visual receptive fields of single striate corical units projecting to the superior colliculus in the cat. Brain Res. 1974 Feb 15;67(1):27–42. doi: 10.1016/0006-8993(74)90295-9. [DOI] [PubMed] [Google Scholar]
- Rose D. Responses of single units in cat visual cortex to moving bars of light as a function of bar length. J Physiol. 1977 Sep;271(1):1–23. doi: 10.1113/jphysiol.1977.sp011987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenquist A. C., Edwards S. B., Palmer L. A. An autoradiographic study of the projections of the dorsal lateral geniculate nucleus and the posterior nucleus in the cat. Brain Res. 1974 Nov 8;80(1):71–93. doi: 10.1016/0006-8993(74)90724-0. [DOI] [PubMed] [Google Scholar]
- Singer W., Bedworth N. Inhibitory interaction between X and Y units in the cat lateral geniculate nucleus. Brain Res. 1973 Jan 30;49(2):291–307. doi: 10.1016/0006-8993(73)90424-1. [DOI] [PubMed] [Google Scholar]
- Stone J., Dreher B. Projection of X- and Y-cells of the cat's lateral geniculate nucleus to areas 17 and 18 of visual cortex. J Neurophysiol. 1973 May;36(3):551–567. doi: 10.1152/jn.1973.36.3.551. [DOI] [PubMed] [Google Scholar]
- Tolhurst D. J. Separate channels for the analysis of the shape and the movement of moving visual stimulus. J Physiol. 1973 Jun;231(3):385–402. doi: 10.1113/jphysiol.1973.sp010239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama K., Matsunami K., Ono T., Tokashiki S. An intracellular study of neuronal organization in the visual cortex. Exp Brain Res. 1974;21(1):45–66. doi: 10.1007/BF00234257. [DOI] [PubMed] [Google Scholar]
- Toyama K., Matsunami K. Synaptic action of specific visual inpulses upon cat's parastriate cortex. Brain Res. 1968 Sep;10(3):473–476. doi: 10.1016/0006-8993(68)90220-5. [DOI] [PubMed] [Google Scholar]
- Tretter F., Cynader M., Singer W. Cat parastriate cortex: a primary or secondary visual area. J Neurophysiol. 1975 Sep;38(5):1099–1113. doi: 10.1152/jn.1975.38.5.1099. [DOI] [PubMed] [Google Scholar]
- Venes J. L., Collins W. F., Taub A. Nitrous oxide: an anesthetic for experiments in cats. Am J Physiol. 1971 Jun;220(6):2028–2031. doi: 10.1152/ajplegacy.1971.220.6.2028. [DOI] [PubMed] [Google Scholar]
- Yamane S., Nikara T., Sugie N. Sustained and transient cortical neurons in area 18 of the cat. Experientia. 1977 Apr 15;33(4):477–479. doi: 10.1007/BF01922221. [DOI] [PubMed] [Google Scholar]