Abstract
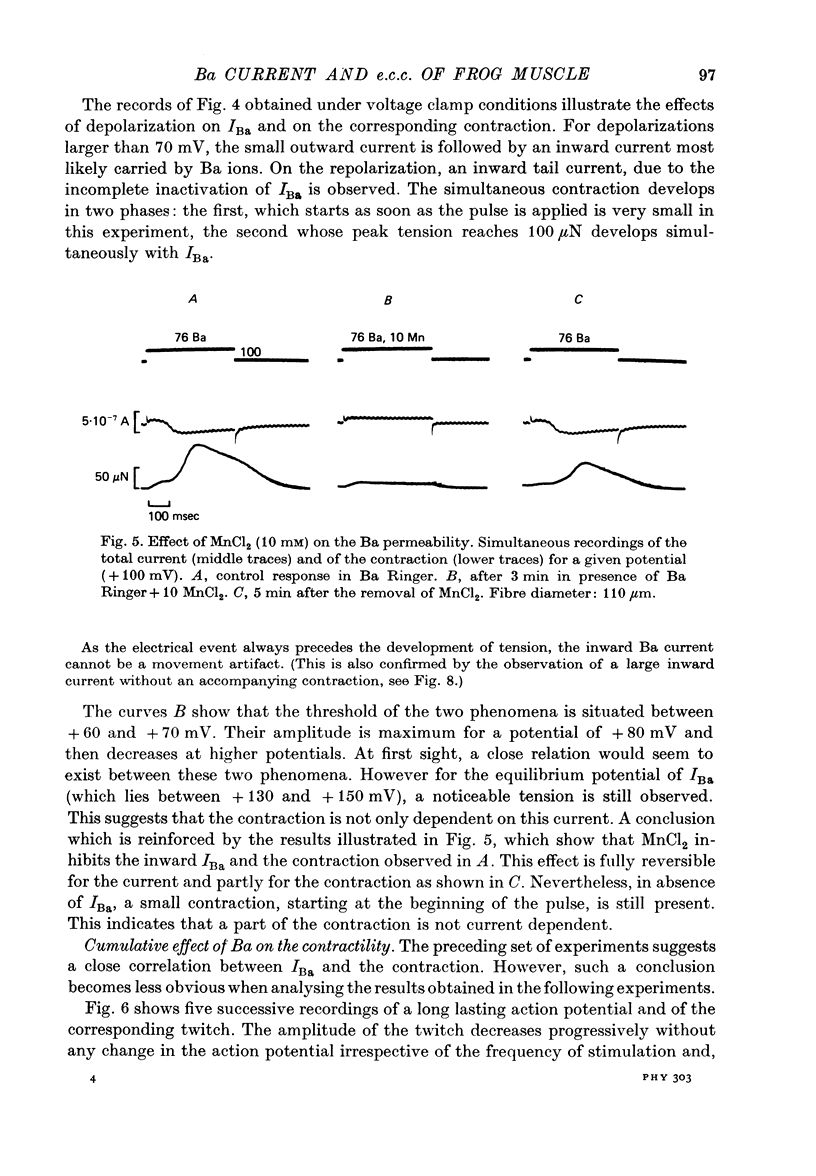
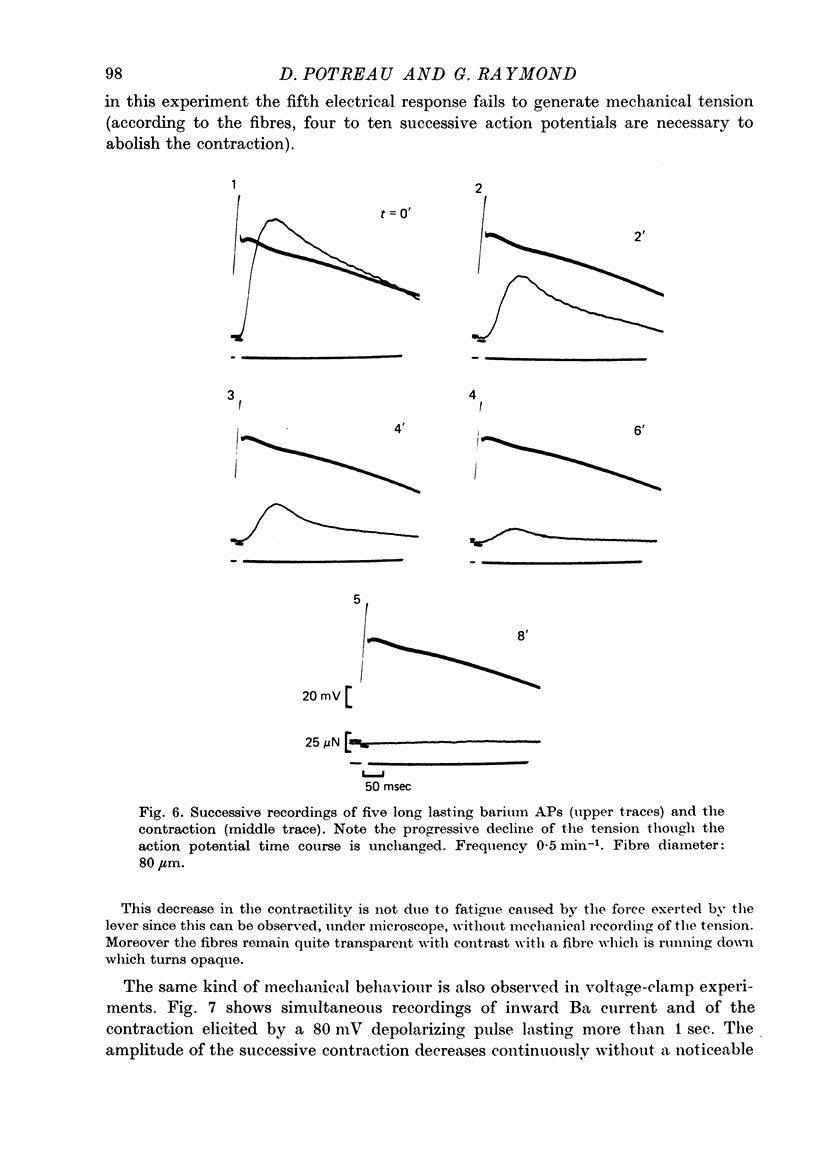
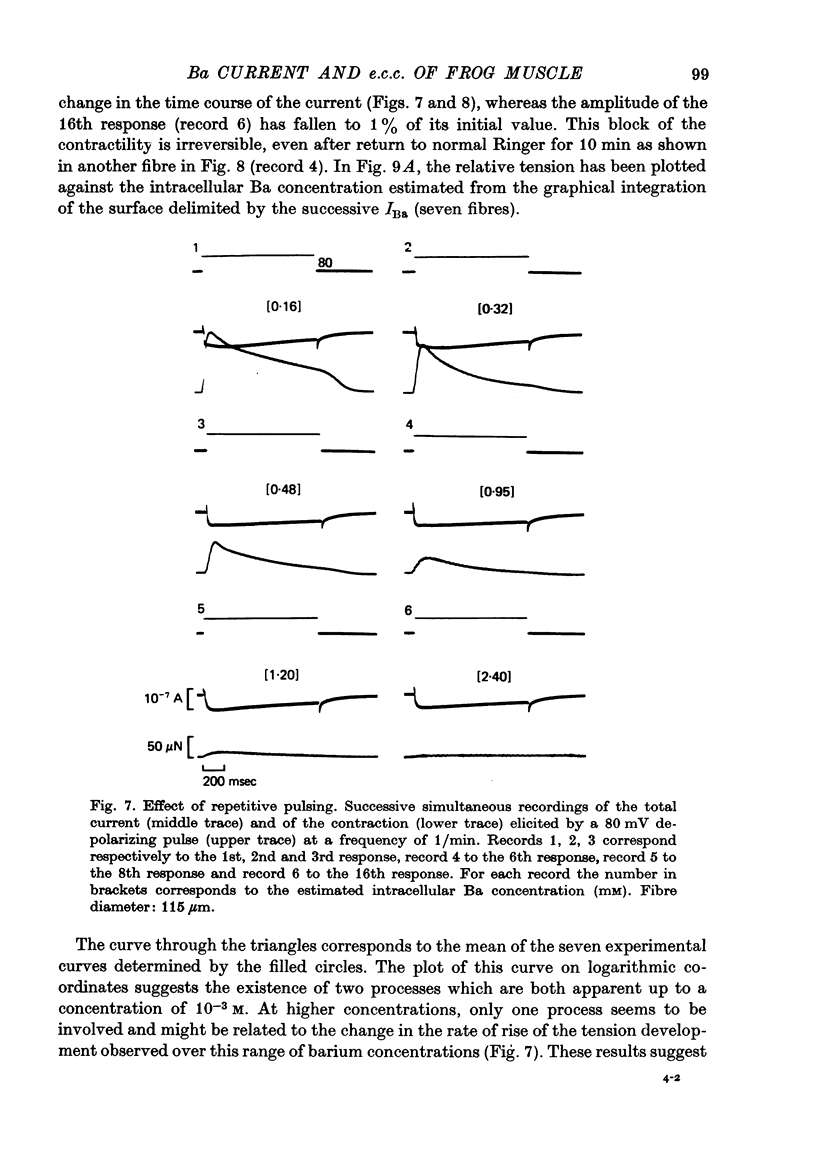
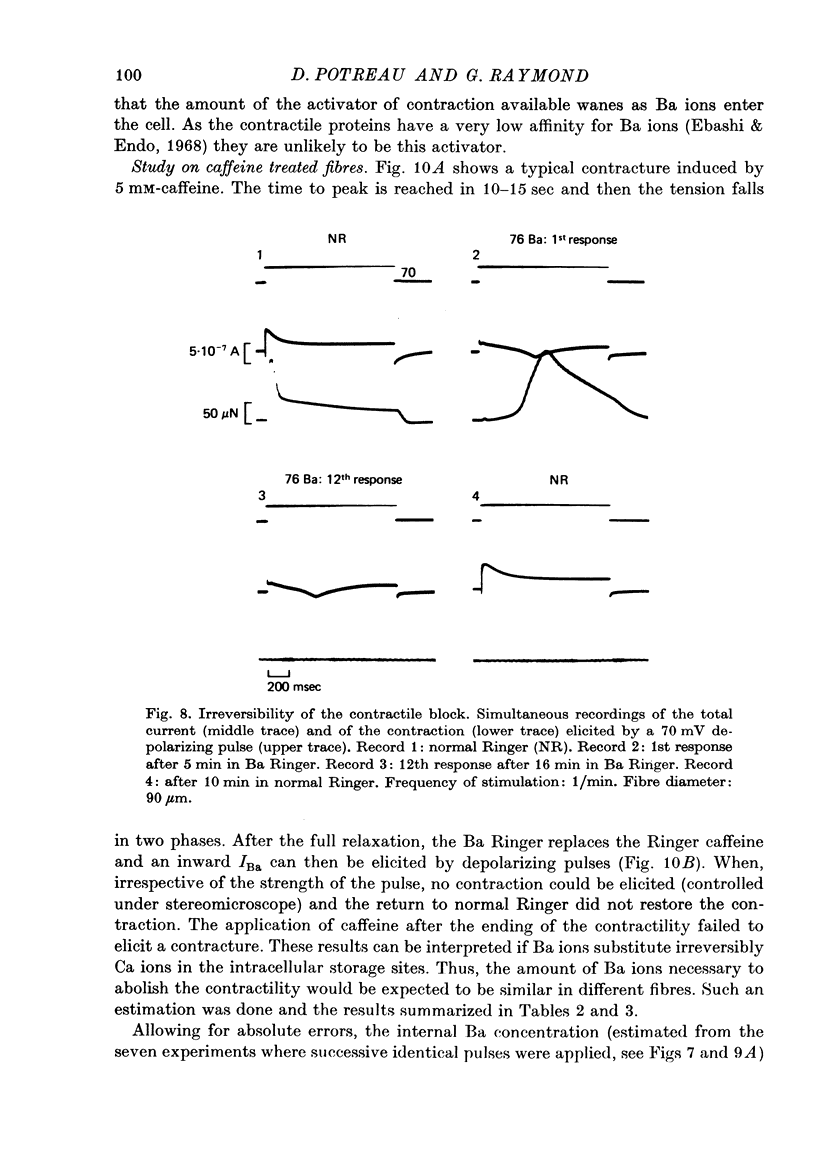
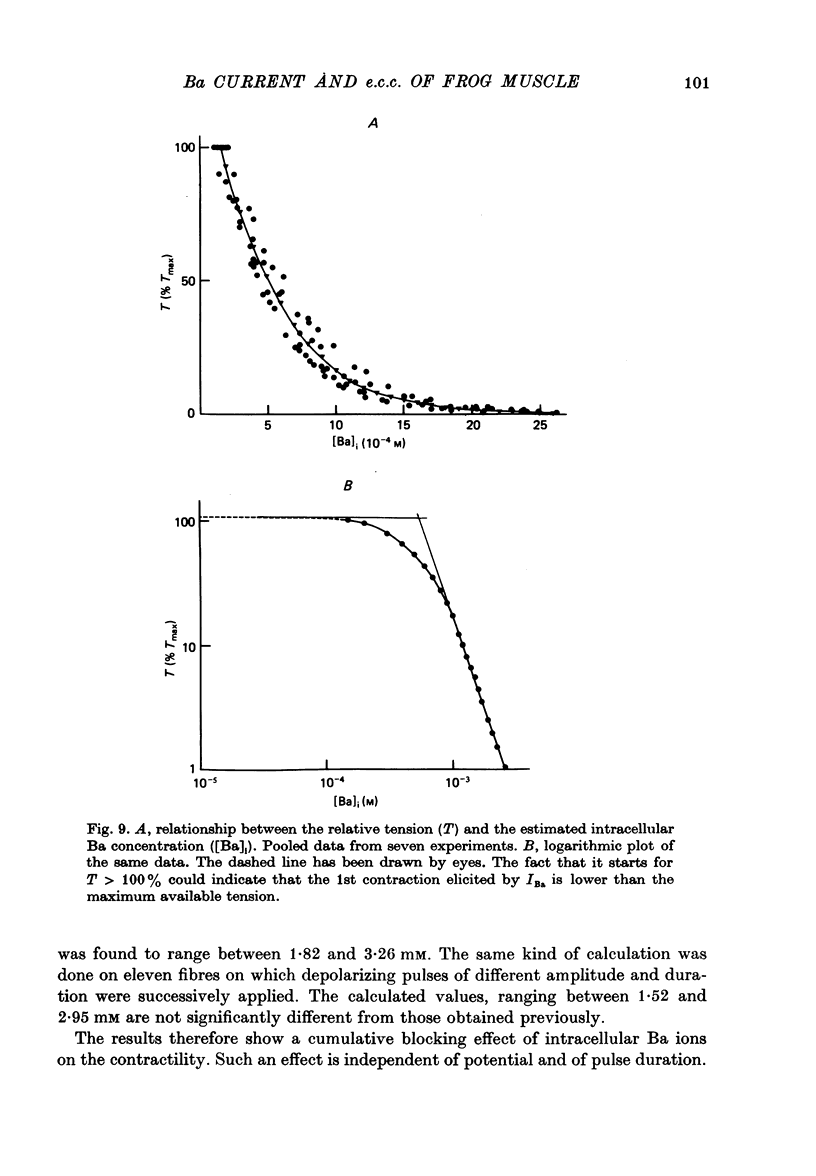
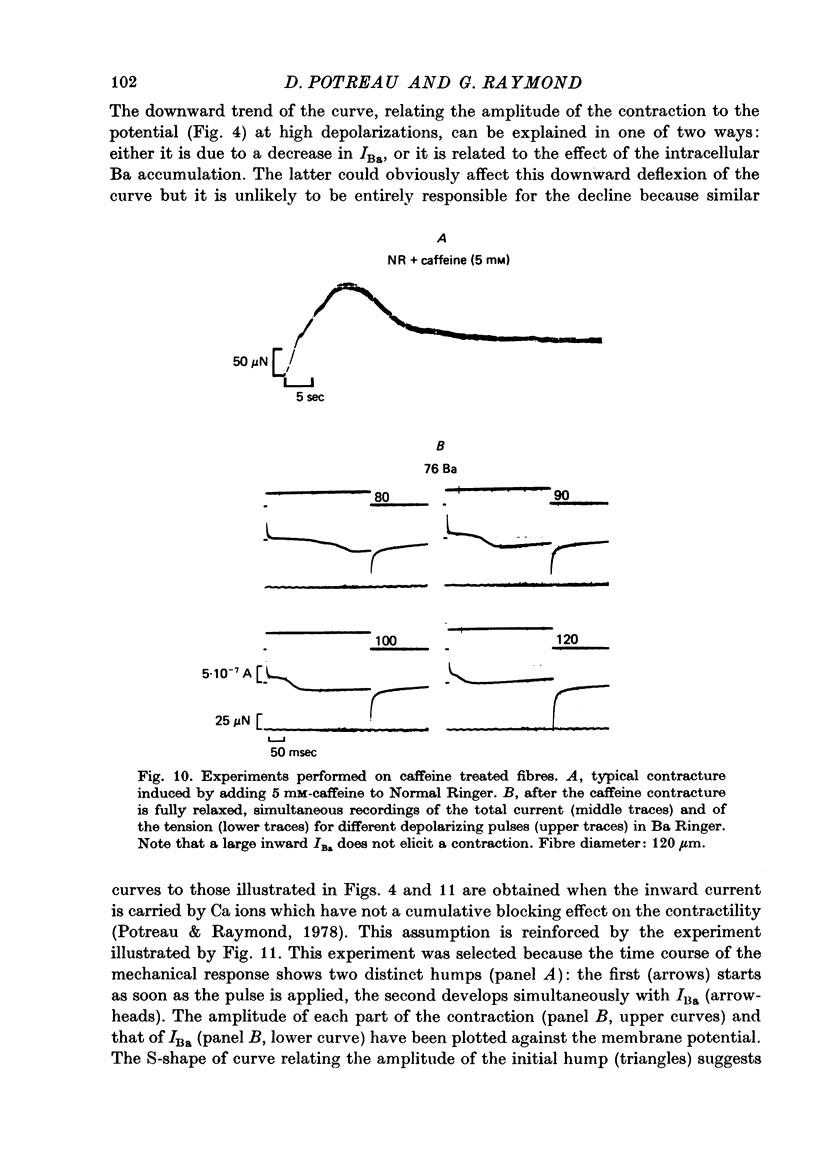
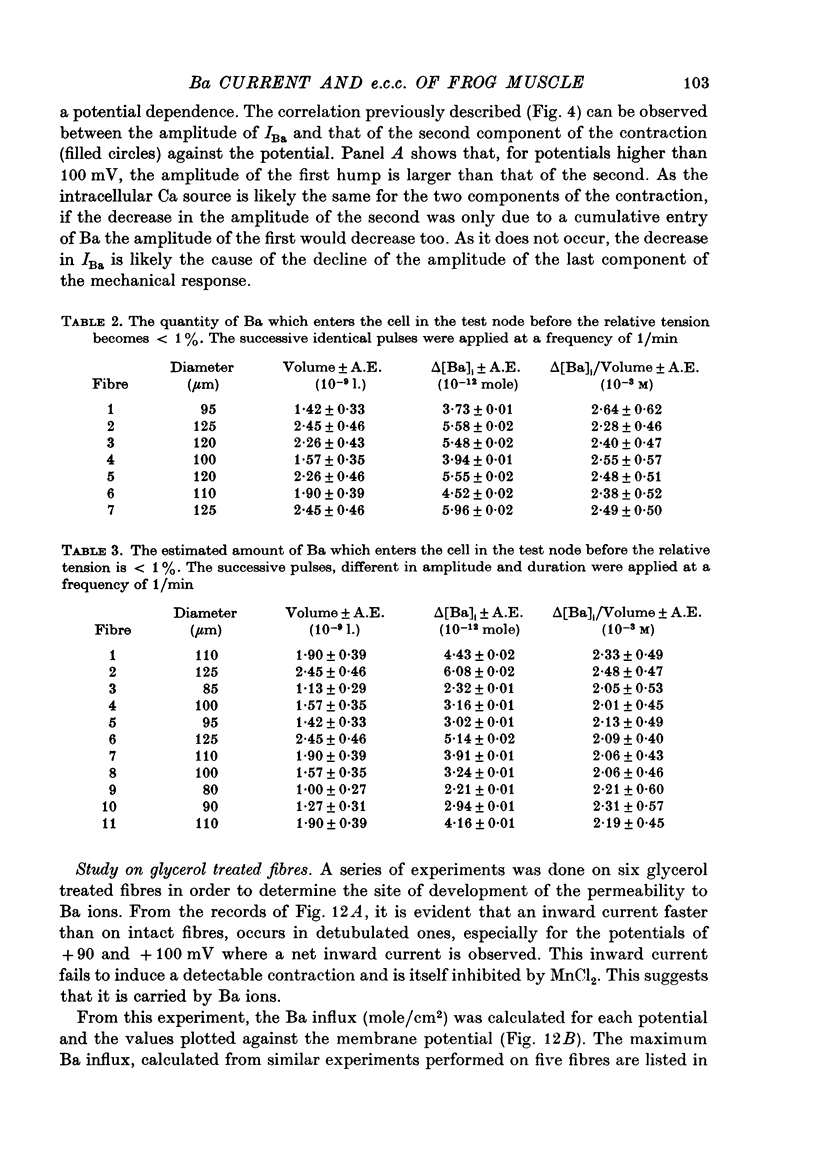
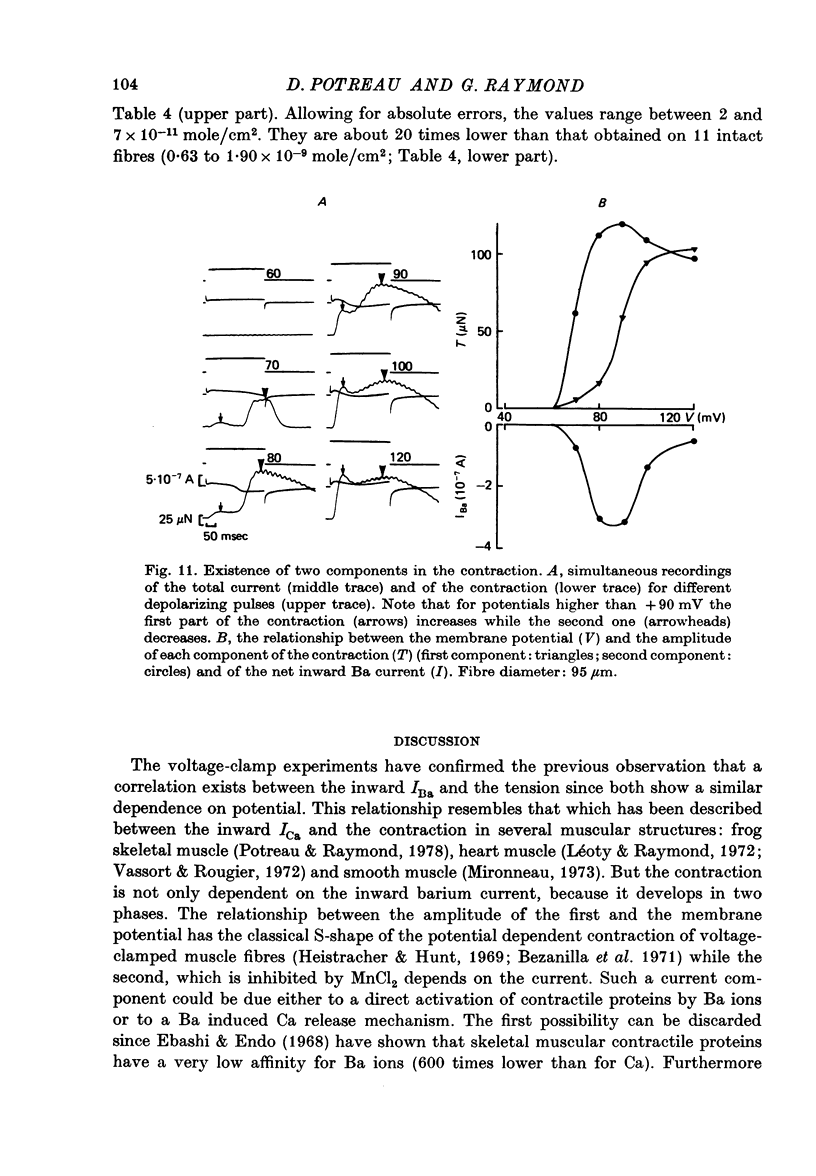
1. Excitation-contraction coupling process in isolated frog muscle fibres, under conditions which allow the development of a Ba permeability, has been investigated by the simultaneous recording of electrical and mechanical activity. 2. The sustained contraction elicited by a long lasting Ba action potential depends on two mechanisms. The first is potential dependent, the second which is inhibited by MnCl2 (10 mM), depends on the inward flux of Ba ions. 3. The relationship observed between the inward IBa and the peak tension resembles that which has been observed between ICa and the contraction on other muscular structures. 4. The relative tension progressively declines as the intracellular Ba concentration increases and the contractility ends after a series of depolarizing pulses (or Ba action potentials). This indicates that the Ba ions which enter the cell release Ca ions and replace them in the intracellular storage sites. 5. Following a pretreatment with caffeine, the inward IBa fails to induce a contraction. Moreover a muscle which has been loaded with barium until the contraction ceases, does not develop a contracture in presence of caffeine. These results show that the Ba induced Ca release is located at the level of the sarcoplasmic reticulum. 6. Calculations show that the amount of Ba ions necessary to abolish the contractility corresponds to the maximum ability of the sarcoplasmic reticulum for Ca binding. 7. Almost all the inward flux of Ba ions and the contraction are abolished by glycerol-treatment which suggests that the coupling occurs at the T-system level. The results are discussed in regard to the technical limitations of the voltage-clamp method.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Chandler W. K., Hodgkin A. L. Slow changes in potassium permeability in skeletal muscle. J Physiol. 1970 Jul;208(3):645–668. doi: 10.1113/jphysiol.1970.sp009140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. The kinetics of mechanical activation in frog muscle. J Physiol. 1969 Sep;204(1):207–230. doi: 10.1113/jphysiol.1969.sp008909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Peachey L. D. Reconstruction of the action potential of frog sartorius muscle. J Physiol. 1973 Nov;235(1):103–131. doi: 10.1113/jphysiol.1973.sp010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard C., Cardinaux J. C., Potreau D. Proceedings: Long-duration responses and slow inward current obtained from isolated skeletal fibres with barium ions. J Physiol. 1976 Mar;256(1):18P–19P. [PubMed] [Google Scholar]
- COSTANTIN L. L., FRANZINI-ARMSTRONG C., PODOLSKY R. J. LOCALIZATION OF CALCIUM-ACCUMULATING STRUCTURES IN STRIATED MUSCLE FIBERS. Science. 1965 Jan 8;147(3654):158–160. doi: 10.1126/science.147.3654.158. [DOI] [PubMed] [Google Scholar]
- Caillé J., Ildefonse M., Rougier O. Existence of a sodium current in the tubular membrane of frog twitch muscle fibre; its possible role in the activation of contraction. Pflugers Arch. 1978 May 18;374(2):167–177. doi: 10.1007/BF00581298. [DOI] [PubMed] [Google Scholar]
- Chapman R. A., Léoty C. The time-dependent and dose-dependent effects of caffeine on the contraction of the ferret heart. J Physiol. 1976 Apr;256(2):287–314. doi: 10.1113/jphysiol.1976.sp011326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Edwards C., Lorković H., Weber A. The effect of the replacement of calcium by strontium on excitation-contraction coupling in frog skeletal muscle. J Physiol. 1966 Oct;186(2):295–306. doi: 10.1113/jphysiol.1966.sp008035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. S., Howell J. N., Vaughan P. C. The maintenance of resting potentials in glycerol-treated muscle fibres. J Physiol. 1971 May;215(1):95–102. doi: 10.1113/jphysiol.1971.sp009459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Endo M., Tanaka M., Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970 Oct 3;228(5266):34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- FATT P., GINSBORG B. L. The ionic requirements for the production of action potentials in crustacean muscle fibres. J Physiol. 1958 Aug 6;142(3):516–543. doi: 10.1113/jphysiol.1958.sp006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREYGANG W. H., Jr, GOLDSTEIN D. A., HELLAM D. C. THE AFTER-POTENTIAL THAT FOLLOWS TRAINS OF IMPULSES IN FROG MUSCLE FIBERS. J Gen Physiol. 1964 May;47:929–952. doi: 10.1085/jgp.47.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Podolsky R. J. Calcium uptake and force development by skinned muscle fibres in EGTA buffered solutions. J Physiol. 1972 May;223(1):1–19. doi: 10.1113/jphysiol.1972.sp009830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Podolsky R. J. Regenerative calcium release within muscle cells. Science. 1970 Jan 2;167(3914):58–59. doi: 10.1126/science.167.3914.58. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. Potassium contractures in single muscle fibres. J Physiol. 1960 Sep;153:386–403. doi: 10.1113/jphysiol.1960.sp006541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Fukuda J., Eaton D. C. Membrane currents carried by Ca, Sr, and Ba in barnacle muscle fiber during voltage clamp. J Gen Physiol. 1974 May;63(5):564–578. doi: 10.1085/jgp.63.5.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heistracher P., Hunt C. C. The relation of membrane changes ot contraction in twitch muscle fibres. J Physiol. 1969 May;201(3):589–611. doi: 10.1113/jphysiol.1969.sp008774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Campbell D. T. An improved vaseline gap voltage clamp for skeletal muscle fibers. J Gen Physiol. 1976 Mar;67(3):265–293. doi: 10.1085/jgp.67.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUETTGAU H. C. THE ACTION OF CALCIUM IONS ON POTASSIUM CONTRACTURES OF SINGLE MUSCLE FIBRES. J Physiol. 1963 Oct;168:679–697. doi: 10.1113/jphysiol.1963.sp007215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léoty C., Alix J. Some technical improvements for the voltage clamp with the double sucrose gap. Pflugers Arch. 1976 Sep 3;365(1):95–97. doi: 10.1007/BF00583633. [DOI] [PubMed] [Google Scholar]
- Léoty C., Raymond G. Mechanical activity and ionic currents in frog atrial trabeculae. Pflugers Arch. 1972;334(2):114–128. doi: 10.1007/BF00586785. [DOI] [PubMed] [Google Scholar]
- Lüttgau H. C., Oetliker H. The action of caffeine on the activation of the contractile mechanism in straited muscle fibres. J Physiol. 1968 Jan;194(1):51–74. doi: 10.1113/jphysiol.1968.sp008394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironneau J. Excitation-contraction coupling in voltage clamped uterine smooth muscle. J Physiol. 1973 Aug;233(1):127–141. doi: 10.1113/jphysiol.1973.sp010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S., Bastian J. Double sucrose-gap method applied to single muscle fiber of Xenopus laevis. J Gen Physiol. 1974 Feb;63(2):235–256. doi: 10.1085/jgp.63.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriarca P., Carafoli E. A comparative study of the intracellular divalent Ca movements in white and red muscle. Experientia. 1969 Jun 15;25(6):598–599. doi: 10.1007/BF01896534. [DOI] [PubMed] [Google Scholar]
- Potreau D., Raymond G. Slow inward calcium current and contraction on frog single muscle fibres [proceedings]. J Physiol. 1978 Sep;282:17P–18P. [PubMed] [Google Scholar]
- Raymond G., Potreau D. Barium ions and excitation-contraction coupling of frog single muscle fibres under controlled current and voltage. J Physiol (Paris) 1977 Oct;73(5):617–631. [PubMed] [Google Scholar]
- Reuter H. Divalent cations as charge carriers in excitable membranes. Prog Biophys Mol Biol. 1973;26:1–43. doi: 10.1016/0079-6107(73)90016-3. [DOI] [PubMed] [Google Scholar]
- Rougier O., Vassort G., Stämpfli R. Voltage clamp experiments on frog atrial heart muscle fibres with the sucrose gap technique. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;301(2):91–108. doi: 10.1007/BF00362729. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V., Devine C. E., Peters P. D., Hall T. A. Electron microscopy and electron probe analysis of mitochondrial cation accumulation in smooth muscle. J Cell Biol. 1974 Jun;61(3):723–742. doi: 10.1083/jcb.61.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperelakis N., Schneider M. F., Harris E. J. Decreased K+ conductance produced by Ba++ in frog sartorius fibers. J Gen Physiol. 1967 Jul;50(6):1565–1583. doi: 10.1085/jgp.50.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield P. R. A calcium dependent inward current in frog skeletal muscle fibres. Pflugers Arch. 1977 Apr 25;368(3):267–270. doi: 10.1007/BF00585206. [DOI] [PubMed] [Google Scholar]
- Thorpe W. R., Seeman P. The site of action of caffeine and procaine in skeletal muscle. J Pharmacol Exp Ther. 1971 Nov;179(2):324–330. [PubMed] [Google Scholar]
- Vassort G., Rougier O. Membrane potential and slow inward current dependence of frog cardiac mechanical activity. Pflugers Arch. 1972;331(3):191–203. doi: 10.1007/BF00589126. [DOI] [PubMed] [Google Scholar]
- Weber A. The mechanism of the action of caffeine on sarcoplasmic reticulum. J Gen Physiol. 1968 Nov;52(5):760–772. doi: 10.1085/jgp.52.5.760. [DOI] [PMC free article] [PubMed] [Google Scholar]


