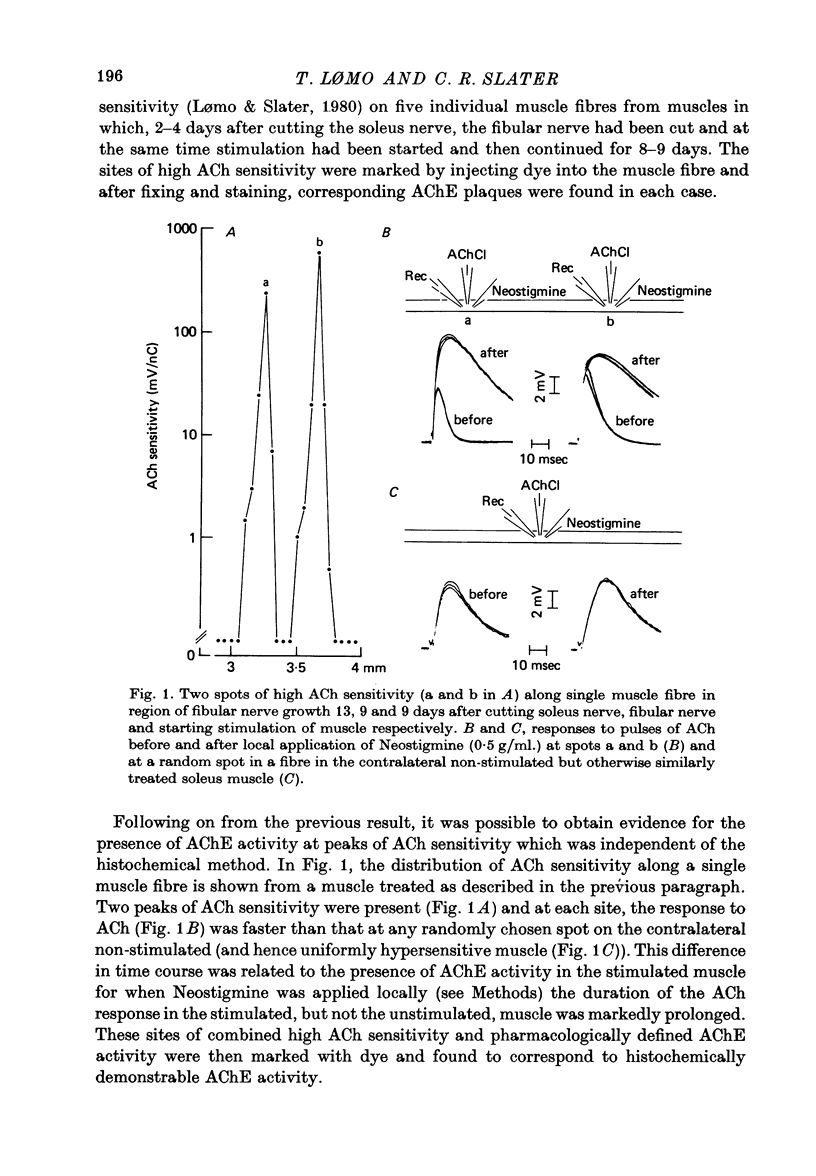
Abstract
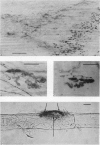
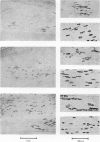
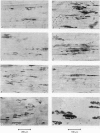
1. The development of AChE at ectopic neuromuscular junctions forming between a transplanted foreign nerve (the superficial fibular nerve) and the denervated soleus muscle has been studied in adult rats. 2. Junctional AChE activity began to appear in the vicinity of the fibular nerve sprouts 6-7 days after section of the soleus nerve and 3-4 days after the onset of transmission. 3. No histochemically detectable AChE appeared when the fibular nerve was cut 0-4 days after the soleus nerve had been cut. 4. Direct electrical stimulation of the denervated soleus muscle caused plaques of true AChE, as determined by inhibitor studies, to appear in muscles where the fibular nerve had been cut 2-4 days after the soleus nerve but not in muscles where the two nerves had been cut at the same time. The plaques appeared only in the vicinity of fibular nerve sprouts and coincided with newly formed but stable peaks of ACh sensitivity. Local application of Neostigmine prolonged and increased the depolarising response evoked by pulses of ACh at these sites. 5. In muscles where the fibular nerve was intact the AChE plaques changed gradually over a few weeks from an immature appearance to a mature appearance characteristic of normal end-plates. In stimulated muscles where the fibular nerve had been cut the plaques stained intensely but remained morphologically immature. 6. We conclude (1) that muscle activity is important for the appearance of AChE at developing neuromuscular junctions and (2) that AChE accumulates only at sites on the muscle surface where the nerve fibres have left a 'trace' upon contact with the muscle fibres. These traces form quickly and persist after nerve-muscle interaction of as little as 2 days. The muscle appears as a major source of junctional AChE since stimulation of the muscle induces intense AChE activity in muscles where the nerve has degenerated.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELSSON J., THESLEFF S. A study of supersensitivity in denervated mammalian skeletal muscle. J Physiol. 1959 Jun 23;147(1):178–193. doi: 10.1113/jphysiol.1959.sp006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley G. A., Heaton J. A quantitative study of cholinesterase in myoneural junctions from rat and guinea-pig extraocular muscles. J Physiol. 1968 Dec;199(3):743–749. doi: 10.1113/jphysiol.1968.sp008676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler I. J., Drachman D. B., Goldberg A. M. The effect of disuse on cholinergic enzymes. J Physiol. 1978 Jan;274:593–600. doi: 10.1113/jphysiol.1978.sp012168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey B., Younkin S. G. Effect of nerve stump length on cholinesterase in denervated rat diaphragm. Exp Neurol. 1978 Mar;59(1):168–175. doi: 10.1016/0014-4886(78)90210-8. [DOI] [PubMed] [Google Scholar]
- Di Giamberardino L., Couraud J. Y. Rapid accumulation of high molecular weight acetylcholinesterase in transected sciatic nerve. Nature. 1978 Jan 12;271(5641):170–172. doi: 10.1038/271170a0. [DOI] [PubMed] [Google Scholar]
- Duchen L. W. Changes in motor innervation and cholinesterase localization induced by botulinum toxin in skeletal muscle of the mouse: differences between fast and slow muscles. J Neurol Neurosurg Psychiatry. 1970 Feb;33(1):40–54. doi: 10.1136/jnnp.33.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdandez H. L., Inestrosa N. C. Role of axoplasmic transport in neurotrophic regulation of muscle end plate acetylcholinesterase. Nature. 1976 Jul 1;262(5563):55–56. doi: 10.1038/262055a0. [DOI] [PubMed] [Google Scholar]
- GUTH L., ALBERS R. W., BROWN W. C. QUANTITATIVE CHANGES IN CHOLINESTERASE ACTIVITY OF DENERVATED MUSCLE FIBERS AND SOLE PLATES. Exp Neurol. 1964 Sep;10:236–250. doi: 10.1016/0014-4886(64)90065-2. [DOI] [PubMed] [Google Scholar]
- Giacobini G., Filogamo G., Weber M., Boquet P., Changeux J. P. Effects of a snake alpha-neurotoxin on the development of innervated skeletal muscles in chick embryo. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1708–1712. doi: 10.1073/pnas.70.6.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth L., Brown W. C., Watson P. K. Studies on the role of nerve impulses and acetylcholine release in the regulation of the cholinesterase activity of muscle. Exp Neurol. 1967 Aug;18(4):443–452. doi: 10.1016/0014-4886(67)90061-1. [DOI] [PubMed] [Google Scholar]
- Guth L., Zalewski A. A., Brown W. C. Quantitative changes in cholinesterase activity of denervated sole plates following implantation of nerve into muscle. Exp Neurol. 1966 Oct;16(2):136–147. doi: 10.1016/0014-4886(66)90093-8. [DOI] [PubMed] [Google Scholar]
- Hall Z. W., Reiness C. G. Electrical stimulation of denervated muscles reduces incorporation of methionine into the ACh receptor. Nature. 1977 Aug 18;268(5621):655–657. doi: 10.1038/268655a0. [DOI] [PubMed] [Google Scholar]
- JOHNS T. R., THESLEFF S. Effects of motor inactivation on the chemical sensitivity of skeletal muscle. Acta Physiol Scand. 1961 Feb-Mar;51:136–141. doi: 10.1111/j.1748-1716.1961.tb02121.x. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomo T., Rosenthal J. Control of ACh sensitivity by muscle activity in the rat. J Physiol. 1972 Mar;221(2):493–513. doi: 10.1113/jphysiol.1972.sp009764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomo T., Westgaard R. H. Further studies on the control of ACh sensitivity by muscle activity in the rat. J Physiol. 1975 Nov;252(3):603–626. doi: 10.1113/jphysiol.1975.sp011161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lwebuga-Mukasa J. S., Lappi S., Taylor P. Molecular forms of acetylcholinesterase from Torpedo californica: their relationship to synaptic membranes. Biochemistry. 1976 Apr 6;15(7):1425–1434. doi: 10.1021/bi00652a012. [DOI] [PubMed] [Google Scholar]
- Lømo T., Slater C. R. Acetylcholine sensitivity of developing ectopic nerve-muscle junctions in adult rat soleus muscles. J Physiol. 1980 Jun;303:173–189. doi: 10.1113/jphysiol.1980.sp013279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lømo T., Slater C. R. Control of acetylcholine sensitivity and synapse formation by muscle activity. J Physiol. 1978 Feb;275:391–402. doi: 10.1113/jphysiol.1978.sp012196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan U. J., Sanes J. R., Marshall L. M. Cholinesterase is associated with the basal lamina at the neuromuscular junction. Nature. 1978 Jan 12;271(5641):172–174. doi: 10.1038/271172a0. [DOI] [PubMed] [Google Scholar]
- Salpeter M. M., Rogers A. W., Kasprzak H., McHenry F. A. Acetylcholinesterase in the fast extraocular muscle of the mouse by light and electron microscope autoradiography. J Cell Biol. 1978 Jul;78(1):274–285. doi: 10.1083/jcb.78.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skau K. A., Brimijoin S. Release of acetylcholinesterase from rat hemidiaphragm preparations stimulated through the phrenic nerve. Nature. 1978 Sep 21;275(5677):224–226. doi: 10.1038/275224a0. [DOI] [PubMed] [Google Scholar]
- Vigny M., Koenig J., Rieger F. The motor end-plate specific form of acetylcholinesterase: appearance during embryogenesis and re-innervation of rat muscle. J Neurochem. 1976 Dec;27(6):1347–1353. doi: 10.1111/j.1471-4159.1976.tb02614.x. [DOI] [PubMed] [Google Scholar]
- Waerhaug O., Korneliussen H. Morphological types of motor nerve terminals in rat hindlimb muscles, possibly innervating different muscle fiber types. Z Anat Entwicklungsgesch. 1974;144(3):237–247. doi: 10.1007/BF00522809. [DOI] [PubMed] [Google Scholar]
- Waerhaug O., Korneliussen H., Sommerschild H. Morphology of motor nerve terminals on rat soleus muscle fibers reinnervated by the original and by a "foreign" nerve. Anat Embryol (Berl) 1977 Aug 9;151(1):1–15. doi: 10.1007/BF00315293. [DOI] [PubMed] [Google Scholar]
- Wake K. Formation of myoneural and myotendinous junctions in the chick embryo. Role of acetylcholinesterase-rich granules in the developing muscle fibers. Cell Tissue Res. 1976 Oct 13;173(3):383–400. doi: 10.1007/BF00220326. [DOI] [PubMed] [Google Scholar]
- Weinberg C. B., Hall Z. W. Junctional form of acetylcholinesterase restored at nerve-free endplates. Dev Biol. 1979 Feb;68(2):631–635. doi: 10.1016/0012-1606(79)90233-1. [DOI] [PubMed] [Google Scholar]
- Wilson B. W., Nieberg P. S., Walker C. R., Linkhart T. A., Fry D. M. Production and release of acetylcholinesterase by cultured chick embryo muscle. Dev Biol. 1973 Aug;33(2):285–299. doi: 10.1016/0012-1606(73)90138-3. [DOI] [PubMed] [Google Scholar]
- Younkin S. G., Brett R. S., Davey B., Younkin L. Substances moved by axonal transport and released by nerve stimulation have an innervation-like effect on muscle. Science. 1978 Jun 16;200(4347):1292–1295. doi: 10.1126/science.78522. [DOI] [PubMed] [Google Scholar]
- Zigmond R. E., Ben-Ari Y. Electrical stimulation of preganglionic nerve increases tyrosine hydroxylase activity in sympathetic ganglia. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3078–3080. doi: 10.1073/pnas.74.7.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]