Abstract
The role of interleukin-12 (IL-12) has been clearly established in the resistance of C57BL/6 (B6) mice to Leishmania major infection, but its involvement in the control of Leishmania mexicana infection remains to be determined. Here, we show the following. (i) L. mexicana, in contrast to L. major, induces the development of nonhealing lesions in B6 mice. (ii) Cells expressing IL-12p40, gamma interferon (IFN-γ), NOS2, and CD40L are numerous in the footpad lesion and/or the draining popliteal lymph node of animals infected for up to 14 weeks with L. mexicana. (iii) B6 mice, either IL-12p40 deficient or treated with IL-12p40-neutralizing antibodies, display a dramatic enhancement of primary and secondary lesions leading to death 10 weeks after inoculation with L. mexicana. (iv) Splenocytes harvested 4 and 8 weeks after infection of IL-12p40−/− B6 mice with L. mexicana are unable to produce IFN-γ, but secrete IL-4, IL-10, and IL-18. Thus, the early control of L. mexicana infection by B6 mice is independent of IL-12, whereas IL-12 and Th1 responses play a key role in controlling the late stages of L. mexicana infection. However, they fail to resolve lesions, in contrast to L. major infection, emphasizing the different outcomes induced by these two Leishmania species in B6 mice.
The various clinical forms of human leishmaniases have been partially reproduced in different inbred strains of mice. For example, CBA, C3H, C57BL/6 (B6), and B10.D2 mice infected with Leishmania major develop cutaneous lesions that spontaneously resolve, whereas BALB/c, SWR/J, DBA/2, and A/J mice harbor nonhealing primary cutaneous lesions prone to dissemination (6). Such experimental models have allowed definition of some of the immunological factors involved in resistance and susceptibility to L. major infection (29).
Interleukin-12 (IL-12), a heterodimeric cytokine composed of p35 and p40 subunits, through its binding to IL-12 receptor (IL-12R) (23) and WSX-1 (40), plays a key role in the induction and the long-term maintenance of the T-cell-dependent resistance of B6 mice to L. major infection (20, 27, 32, 34, 38). Macrophages and dendritic cells produce high levels of IL-12 when infected in vitro with L. major amastigotes (37). Gamma interferon (IFN-γ) produced by Th1 cells mediates protection by activating the NOS2 gene in macrophages and the subsequent production of the leishmanicidal NO. This cascade depends on CD40-CD40L interaction (8, 18), as well as other factors (26), and each element is essential to lesion resolution in resistant mice.
Although Leishmania mexicana induces cutaneous or diffuse lesions in American patients (3, 11, 35), the immunological mechanisms determining susceptibility and resistance to this Leishmania species have been less studied. In vitro experiments suggest that neither macrophages nor dendritic cells produce IL-12 when infected with L. mexicana amastigotes (5, 39), and susceptibility to L. mexicana seems to be due to a prostaglandin-dependent inability to produce IL-12 (28, 30).
To extend our previous studies of this infection model (1, 2), we investigated the role of IL-12 in B6 mice infected with L. mexicana. The courses of L. mexicana and L. major infections were compared in wild-type (WT), IL-12p40-deficient, and B6 mice treated with IL-12p40-neutralizing antibodies. We also analyzed the density of cells producing IL-12p40, IFN-γ, and NOS2 and expressing CD40L in the footpad lesion and/or the draining lymph node (LN) of L. mexicana-infected WT animals, and the capacity of splenocytes of IL-12p40−/− B6 mice infected with L. mexicana to secrete IFN-γ, IL-4, IL-10, and IL-18. The data indicate that IL-12 is not required to control the early phase of infection, but is critical for long-term stabilization of L. mexicana lesions, although insufficient to totally cure the disease, in contrast with L. major infection.
MATERIALS AND METHODS
Parasites.
Promastigotes of L. mexicana (strain MHOM/BZ/82/BEL21) and L. major (strain WHOM/IR/−173) were cultured in RPMI medium (Life Technologies, Gaithersburg, Md.), supplemented with 10% fetal calf serum (FCS; Life Technologies), penicillin G (100 U/ml), and streptomycin (100 μg/ml). Parasites in the stationary phase, harvested after 8 to 10 days of culture, were centrifuged (2,500 × g, 10 min, 4°C) and then washed three times in RPMI before being counted and used for inoculation of animals. L. mexicana promastigotes lysed by 10 cycles of freezing in liquid nitrogen and thawing in a water bath at 37°C were also used for cell stimulation studies.
Mice and Leishmania infection.
C57BL/6 male mice were purchased from Banting & Kingman Universal, Ltd. (Hull, United Kingdom). Male and female IL-12p40−/− C57BL/6 mice were prepared as previously described (13). In another experiment, each week, C57BL/6 mice received intraperitoneally (i.p.) anti-IL-12p40 monoclonal antibody (MAb; clone C17.8, rat immunoglobulin G2a [IgG2a]; kindly given by V. Flamand, Free University of Brussels [ULB], Brussels, Belgium) or unrelated rat IgG2a (clone IR418, kindly provided by H. Bazin, UCL, Brussels, Belgium). The maintenance and care of mice complied with the guidelines of the ULB Ethics Committee for the Humane Use of Laboratory Animals. Mice (8 to 12 weeks old) were infected subcutaneously, as reported previously (1, 2), in the rear left hind footpad with 107 stationary-phase promastigotes of L. mexicana or L. major in a final volume of 25 μl (in RPMI medium). The controlateral right footpad received an identical volume of RPMI medium without parasites as an internal control.
Lesion monitoring, tissue processing, and quantification of L. mexicana amastigotes.
The thickness of infected and uninfected footpads was regularly measured with a Vernier calliper, and the difference between both measurements corresponded to lesion size. Mice were also regularly examined to detect cutaneous ulcers and secondary lesions.
At selected time points, some infected mice were killed by ether inhalation. Footpad lesions cut tangentially to the bone ground and popliteal homolateral draining LN were collected for histological and/or immunohistochemical studies.
The parasite burdens in footpad lesions of IL-12p40−/− and WT C57BL/6 infected animals were determined after tissue homogenization by staining released amastigotes with acridine orange (Sigma, Brussels, Belgium), as described previously (2).
Histological and immunohistochemical studies.
Footpad tissues and LN were either snap-frozen in liquid nitrogen or fixed in 10% formalin, immediately after the sacrifice of animals. Tissue sections (5 μm) of fixed material embedded in paraffin were stained with hematoxylin-eosin-safranin to study their microarchitecture by light microscopic examination.
All immunohistochemical procedures used for in situ analysis of IL-12p40, CD40L, IFN-γ, and NOS2 in frozen tissues have been described in detail elsewhere (9). Briefly, 6-μm frozen sections were thaw mounted on glass slides and kept overnight at room temperature in a box with humidified atmosphere. The next day, sections were air dried at room temperature and fixed for 10 min in fresh acetone containing 0.02% hydrogen peroxide to inhibit endogenous peroxidase activity. Air-dried sections were washed once with phosphate-buffered saline (PBS)-0.05% Tween 20 and incubated overnight at 4°C with the antibody (rat anti-IL-12p40 C15.6 labeled with biotin from Pharmingen; mouse anti-IFN-γ DB-1-biotin from U-Cytech, Utrecht, The Netherlands; rabbit anti-NOS2-biotin from Calbiochem; hamster anti-CD40L MR-1 labeled with alkaline phosphatase (AP) from R. J. Noelle, Lebanon, N.H.) at the previously determined optimal dilution in PBS-0.1% bovine serum albumin. Sections incubated with the AP-labeled antibody were washed with PBS and revealed with Fast Blue BB Base giving dark blue precipitates. Horseradish peroxidase-conjugated streptavidin was applied for 1 h at room temperature on sections previously incubated with biotin-labeled antibodies, and aminoethylcarbazole was used as a chromogen, giving a bright red translucent precipitate. Enzymatic reactions were stopped by washing in PBS and slides were mounted with glycerol-gelatine. Immunohistochemical stains were evaluated by light microscopy, and positive cells were counted in the whole-section area to determine their number per square millimeter by using the Image analysis Vidas system (Zeiss, Weesp, The Netherlands)
Cell stimulation and quantification of cytokines.
Single-splenocyte suspensions of IL-12p40−/− and WT mice were treated for 1 min with a lysis buffer (9 parts 0.16 M ammonium chloride and 1 part 0.17 M Tris-HCl [pH 7.5]). The erythrocyte-free cells were then washed twice in RPMI 1640 medium (containing 5% FCS), and their viability was checked by the trypan blue exclusion test (viable cells, >95%), before being resuspended and distributed in 24-well plates (Nunc, Roskilde, Denmark; 3 × 106 cells per 800 μl per well). Cell stimulations were performed with lysed L. mexicana promastigotes (106 parasites per well). After incubation at 37°C for 48 h in a 5% CO2-humidified atmosphere, supernatants of unstimulated or stimulated cells were collected by centrifugation at 280 × g for 10 min and kept frozen at −70°C for subsequent determination of cytokine levels.
Cytokine quantification was performed by enzyme-linked immunosorbent assay (ELISA) in cell supernatants by using commercially available kits (IL-18, M1800; IFN-γ, IL-4, and IL-12, duo set; IL-10, Intertest 10x [all from Genzyme Diagnostics, Cambridge, Mass.]). The IL-12 assay detected monomeric and dimeric p40, as well as p70 subunits. All ELISAs were performed according to the manufacturer's instructions, with a detection limit of 5 pg/ml for each of them.
RESULTS
L. mexicana, in contrast to L. major, induces nonhealing cutaneous lesions in B6 mice.
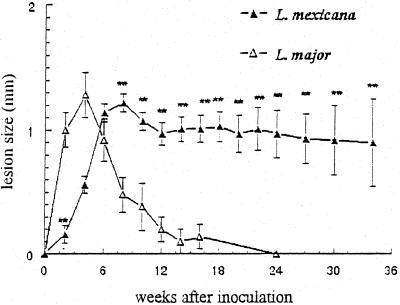
As shown in Fig. 1, lesions in B6 mice inoculated with L. mexicana and L. major displayed similar maximal sizes (of about 1.4 mm in diameter), at weeks postinfection (wpi) 8 and 4, respectively. The L. major lesions resolved progressively from wpi 4 and were totally cured by wpi 24. In contrast, the lesions induced by L. mexicana never resolved, and their size, although decreasing in some mice, remained nearly constant for the entire observation period. Data on amastigote counts in footpad lesions and draining LN of L. mexicana-infected animals are reported elsewhere (2)
FIG. 1.
Size of primary footpad lesions in B6 mice infected with L. mexicana or L. major. Results are expressed as means ± standard errors obtained from 18 animals infected with L. mexicana and 13 animals infected with L. major. ∗∗, P < 0.001 (Student's t test for comparison of L. mexicana and L. major infections).
Lesions and/or LN of B6 mice infected with L. mexicana contain cells expressing IL-12p40, CD40L, IFN-γ, and NOS2.
Immunostaining of footpad sections with the MAb C15.6 showed some large cells containing intracellular IL-12p40 throughout the dermis and near the vessels of B6 mice 24 weeks after infection with L. mexicana. Such cells were not detected in footpad tissues collected before infection or at wpi 14 (data not shown). Popliteal LN of infected mice harbored numerous IL-12p40-containing cells on wpi 14 and 24, which are rarely found in uninfected animals (Table 1). Positive cells in infected mice were found either isolated or in clusters of two to three cells, mainly in the medulla and occasionally in the cortex, but not in the subcapsular sinus of LN. L. mexicana infection also strongly increased the frequencies of CD40L-, IFN-γ-, and NOS2-expressing cells by 6- to 12-fold in LN of B6 mice at wpi 14 and 24 compared to uninfected mice (Table 1).
TABLE 1.
Number of cells expressing IL-12p40, CD40L, IFN-γ, and NOS2 in LN draining footpad lesions in B6 mice infected with L. mexicana
| wpi | No. of positive cells/mm2 in LNa
|
|||
|---|---|---|---|---|
| IL-12p40 | CD40L | IFN-γ | NOS2 | |
| 0 | 0.1 ± 0.1 | 1.9 ± 1.7 | 0.0 ± 0.0 | 3.3 ± 0.6 |
| 14 | 5.2 ± 0.9* | 15.0 ± 2.7* | 9.4 ± 2.1* | 30.0 ± 10.7* |
| 24 | 7.2 ± 0.7* | 12.9 ± 2.4* | 11.3 ± 3.1* | 38.8 ± 7.6* |
Results are expressed as means ± standard errors obtained from four animals, with four sections being examined per mouse.
P < 0.05 (Student's t test, comparing data from infected animals [wpi 14 and 24] to uninfected ones [wpi 0]).
IL-12 is involved in the late control of L. mexicana infection in B6 mice.
The role of IL-12 produced in response to L. mexicana infection was investigated in two types of experiments, by using B6 mice either deficient in IL-12 production by targeted inactivation of IL-12p40 genes or treated with anti-IL-12p40 MAb (clone C17.8). Control mice were WT B6 mice or B6 mice receiving the isotype-matched control antibody IR418, respectively. C17.8 or IR418 antibodies were injected intraperitoneally (i.p.) (1 mg in 0.1 ml of PBS) 1 h before parasite inoculation and weekly for 10 weeks. All mice were inoculated with 107 L. mexicana promastigotes. In addition, as positive controls, IL-12p40−/− or WT mice were similarly infected with L. major.
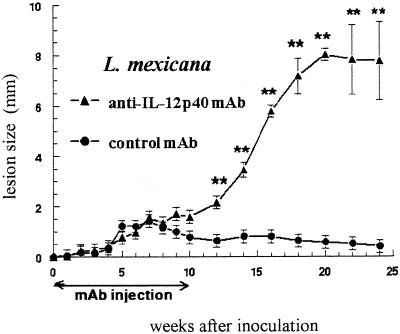
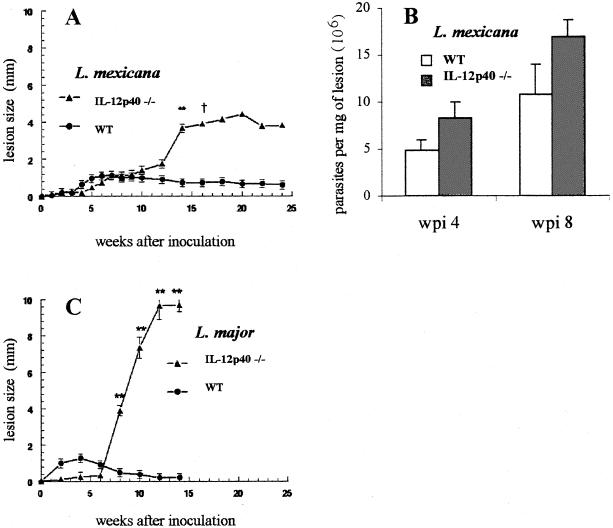
As shown in Fig. 2 and 3A, during the first 10 wpi with L. mexicana, the lesion sizes remained similar in both C17.8 antibody-treated and IL-12p40−/− B6 mice and their respective IL-12-sufficient controls. Thereafter, IL-12p40-deficient mice exhibited a dramatic increase in lesion sizes, comparable to that previously observed in BALB/c mice inoculated with the same strain of parasite (1). Moreover, all of the IL-12p40-deficient animals displayed multiple secondary cutaneous lesions that were never observed in control B6 mice, which instead showed stabilized or slightly reduced lesion sizes from wpi 10 onward. While all control mice survived L. mexicana infection, all IL-12p40−/− mice and three out of the five mice receiving the anti-IL-12p40 antibody died between wpi 22 and 25. As expected, the IL-12p40−/− mice infected with L. major showed a dramatic increase in lesion sizes from wpi 6 (Fig. 3C), and all mice developed ulcerative lesions leading to mutilations and died at wpi 15, while all WT animals survived.
FIG. 2.
Size of primary footpad lesions in B6 mice receiving anti-IL-12p40 antibodies and infected with L. mexicana. Results are expressed as means ± standard errors (n = 5 in both animal groups). ∗∗, P < 0.001 (Student's t test for comparisons between the two groups).
FIG. 3.
Size of primary footpad lesions in IL-12p40−/− B6 mice infected with L. mexicana (A) or L. major (C) and parasite burdens in lesions of L. mexicana-infected animals (B). Results are expressed as means ± standard errors. Four IL-12p40−/− mice and seven WT mice were inoculated with L. mexicana or L. major, and two animals of each group were used for determination of parasite burdens. ∗∗, P < 0.001 (Student's t test for comparison of IL-12p40−/− and WT mice). †, results from wpi 16 onward are from one mouse.
The early control of L. mexicana infection in IL-12p40−/− B6 mice is associated with Th2 cytokine release in the presence of IL-18.
Some IL-12p40−/− and WT animals infected with L. mexicana were killed on wpi 4 or 8 to collect footpad lesions for parasite quantification and spleen cells for cytokine analysis. As shown in Fig. 3B, footpad parasite burdens remained roughly similar in IL-12p40−/− and WT mice at wpi 4 and 8 (n = 2, P > 0.05). As shown in Table 2, the analysis of culture supernatants of splenocytes, incubated for 48 h in the presence or absence of parasite lysate, confirmed the IL-12p40 depletion in gene-deleted mice. The IFN-γ response to L. mexicana was also undetectable, whereas the secretion of IL-4 and IL-10 was higher than that in cells from WT mice similarly infected. Interestingly, despite the absence of IFN-γ, IL-18, an IFN-γ-amplifying factor (22), was present at comparable levels in all samples from both groups of mice (Table 2).
TABLE 2.
Cytokine production by splenocytes from IL-12p40−/− and WT B6 mice infected with L. mexicana
| Mice | wpi | Stimulusa | Cytokine production (pg/106 cells)b
|
||||
|---|---|---|---|---|---|---|---|
| IL-12p40 | IFN-γ | IL-18 | IL-4 | IL-10 | |||
| IL-12p40−/− | 4 | − | <5 | <5 | 203 ± 31 | <5 | <5 |
| 4 | L. mexicana | <5 | <5 | 389 ± 53 | 32 ± 10 | 20 ± 2* | |
| 8 | − | <5 | <5 | 118 ± 4 | <5 | 8 ± 1 | |
| 8 | L. mexicana | <5 | <5 | 206 ± 18 | 23 ± 2 | 86 ± 26* | |
| WT | 4 | − | 148 ± 32 | NDc | 161 ± 8 | <5 | <5 |
| 4 | L. mexicana | 177 ± 71 | ND | 275 ± 82 | <5 | 10 ± 1 | |
| 8 | − | 213 ± 4 | <5 | 131 ± 14 | <5 | <5 | |
| 8 | L. mexicana | 450 ± 21 | 558 ± 100 | 173 ± 26 | <5 | 36 ± 1 | |
Cells were incubated for 48 h in the presence (L. mexicana) or absence (−) of parasite lysate.
Results are expressed as means ± standard errors obtained from three mice per group. *, P < 0.05 (Student's t test, comparing data from IL-12p40−/− and WT C57BL/6 mice).
ND, not determined.
DISCUSSION
This study demonstrates that IL-12 is critical for long-term stabilization of L. mexicana lesions, but insufficient to totally cure the infection, in contrast with L. major infection. Interestingly, the early control of L. mexicana infection appears to be independent of IL-12. Indeed, L. mexicana-infected B6 mice depleted of IL-12 reversed their disease phenotype from a localized and stabilized lesion to an uncontrolled, disseminated, and lethal form of leishmaniasis, similar to that in susceptible BALB/c mice (1). The activation of the IL-12-IFN-γ-NOS2 cascade, leading to the release of leishmanicidal NO, likely contributes to the control of this late phase of L. mexicana infection, as indicated by the increased frequencies of LN cells expressing these mediators at wpi 14 and 24. This is consistent with previous reports on the capacity of macrophages infected in vitro with L. mexicana to release NO (39) and with the NO production by cells from L. mexicana-infected mice (28). Moreover, the abrogation of IFN-γ production in L. mexicana-infected IL-12 p40−/− mice, which bear exacerbated lesions, suggests a causal relationship between these mediators and lesion stabilization. The concomitant expression of CD40L suggests that CD40-CD40L interactions are necessary to trigger IL-12 production in mice infected with L. mexicana, as previously reported for the closely related parasite Leishmania amazonensis (33). The present results obtained with L. mexicana agree with those previously reported for L. major, confirming that IL-12, IFN-γ, and NOS2 are elements essential to maintain long-term control of Leishmania replication (20, 27).
However, besides these similarities in the events occurring during the late phases of both infection models, L. mexicana lesions never heal and parasites persist (2), in contrast with the complete cure that occurs in L. major infection. Different mechanisms could account for this late persistence of L. mexicana-containing lesions. First, the stimulation and maintenance of IL-12 production and/or the responsiveness to IL-12 might be different between these two parasite species. Indeed, lesions of L. major-infected resistant mice treated with anti-IL-12 antibodies resolve once the treatment is terminated (15), unlike our observations with L. mexicana infection. The latter species, in addition to a reduced capacity to stimulate IL-12 production (30), might also downregulate the expression of the IL-12R β2 chain, as previously observed in infection of resistant mice with L. amazonensis (17). Second, the Fas/FasL-dependent cytotoxicity of IFN-γ-activated Th1 cells against infected macrophages might be less efficient against L. mexicana than against L. major (10, 16). Third, although not yet explored, a possible higher release of antagonistic factors of IL-12 and IFN-γ production, such as IL-10 (19) or IL-12p40 monomer or dimer (14), might reduce the Th1 response in L. mexicana infection. Fourth, another possibility relates to a reduced rate of lymphocyte apoptosis within the L. mexicana lesion through tumor necrosis factor (TNF)-TNF receptor (TNFR) and Fas-FasL interactions (12, 36). Whether or not similar mechanisms may operate in L. mexicana-infected mice remains to be addressed.
Another interesting result from our experiments with IL-12p40-depleted mice is the absence of marked growth of lesions in the first weeks after inoculation of L. mexicana or L. major. Moreover, in this early step of infection, cells from L. mexicana-infected IL-12p40−/− mice secreted only type 2 cytokines, such as IL-4 and IL-10, whereas WT mice produced type 1 cytokines, such as IL-12p40 and IFN-γ. This suggests the involvement of IL-12-independent immunological mechanisms, mediated by CD8 or NK cells, able to control the first phase of L. mexicana infection. However, involvement of CD8 cytotoxic T cells is unlikely, since it has been previously reported that L. mexicana antigens are preferentially presented in association with MHC class II molecules (4), and class I-deficient mice display L. mexicana lesions similar to those of WT mice (25). Moreover, immunohistochemical studies from our laboratory indicated that CD8 T cells are very scarce in L. mexicana cutaneous lesions (F. Aguilar Torrentera, unpublished results). A more likely mechanism could be the activation of cytotoxic NK cells, which might target L. mexicana-infected macrophages. Indeed, SCID mice, lacking mature T and B cells, but with normal NK cells, also display a latency period before lesion growth when infected with L. mexicana (31). Activation of NK cells might be induced by the simultaneous presence of IL-10 and IL-18, since the combination of both cytokines has been shown to be a potent activator of NK proliferation and cytotoxic activity (7). Cells from IL-12p40−/− B6 mice released larger amounts of IL-10 upon stimulation with parasite lysate at wpi 4 and 8 in comparison to WT animals, and significant levels of IL-18 were detected in these culture supernatants. IL-18 is known to play a role in the early control of L. major infection (21, 24). Whether such a mechanism also occurs in L. mexicana-infected WT B6 mice, in addition to or instead of the classical IL-12-IFN-γ pathway, remains to be determined.
In conclusion, our results show that the early control of L. mexicana infection by B6 mice is independent of IL-12, whereas IL-12 and Th1 responses have a key role in the late control of L. mexicana infection, although it is insufficient to resolve lesions, as in L. major infection. Such results emphasize marked differences between both pathogens in driving the immune response of B6-resistant mice, as previously reported for susceptible BALB/c animals (1).
Acknowledgments
We thank D. Le Ray (Institute of Tropical Medicine, Antwerp, Belgium) and N. Glaichenhaus (CNRS, Valbonne, France) for giving us the MHOM/BZ/82/BEL21 and WHOM/IR/−173 strains of L. mexicana and L. major, respectively. We are indebted to C. Truyens for critical review of the manuscript and help with the management of references and A. Wathelet, P. Delblandre, K. Mjidi, and A. Ben Messaoud for diligent technical assistance.
This work was supported by grants from the Belgian Ministry of Scientific Policy (“Action de Recherche Concertée”) and ULB. F. Aguilar-Torrentera belongs to the scientific staff of the “Escuela Nacional de Ciencias Biologicas” at the “Instituto Politecnico Nacional” in Mexico. To perform her Ph.D. studies at ULB, she was supported successively by fellowships from the “Comision de Operacion y Fomento de Actividades Academicos” (COFAA) of the “Instituto Politecnico Nacional” (Mexico) and ULB (Brussels).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Aguilar Torrentera, F., N. Glaichenhaus, J. D. Laman, and Y. Carlier. 2001. T-cell responses to immunodominant LACK antigen do not play a critical role in determining susceptibility of BALB/c mice to Leishmania mexicana. Infect. Immun. 69:617-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguilar Torrentera, F., M. A. Lambot, J. D. Laman, M. Van Meurs, R. Kiss, J. C. Noel, and Y. Carlier. 2002. Parasitic load and histopathology of cutaneous lesions, lymph node, spleen and liver from BALB/c and C57Bl/6 mice infected with Leishmania mexicana. Am. J. Trop. Med. 66:275-281. [DOI] [PubMed] [Google Scholar]
- 3.Andrade-Narvaez, F. J., E. Simmonds-Diaz, S. Rico-Aguilar, M. Andrade-Narveez, A. Palomo-Cetina, S. B. Canto-Lara, M. R. Garcia-Miss, M. Madera-Sevilla, and N. Albertos-Alpuche. 1990. Incidence of localized cutaneous leishmaniasis (Chiclero's ulcer) in Mexico. Trans. R. Soc. Trop. Med. Hyg. 84:219-220. [DOI] [PubMed] [Google Scholar]
- 4.Antoine, J. C., E. Prina, T. Lang, and N. Courret. 1998. The biogenesis and properties of the parasitophorous vacuoles that harbour Leishmania in murine macrophages. Trends Microbiol. 6:392-401. [DOI] [PubMed] [Google Scholar]
- 5.Bennett, C. L., A. Misslitz, L. Colledge, T. Aebischer, and C. C. Blackburn. 2001. Silent infection of bone marrow-derived dendritic cells by Leishmania mexicana amastigotes. Eur. J. Immunol. 31:876-883. [DOI] [PubMed] [Google Scholar]
- 6.Bradley, D. J. 1987. Genetics of susceptibility and resistance in the vertebrate host, p. 551-581. In W. Peters and R. Killick-Kendrick (ed.), The leishmaniases in biology and medicine. Academic Press, London, United Kingdom.
- 7.Cai, G., R. A. Kastelein, and C. A. Hunter. 1999. IL-10 enhances NK cell proliferation, cytotoxicity and production of IFN-gamma when combined with IL-18. Eur. J. Immunol. 29:2658-2665. [DOI] [PubMed] [Google Scholar]
- 8.Campbell, K. A., P. J. Ovendale, M. K. Kennedy, W. C. Fanslow, S. G. Reed, and C. R. Maliszewski. 1996. CD40 ligand is required for protective cell-mediated immunity to Leishmania major. Immunity 4:283-289. [DOI] [PubMed] [Google Scholar]
- 9.Claassen, E., and S. H. M. Jeurissen. 1997. A step by step guide to in situ immune response analysis of lymphoid tissues by immunohistochemical methods p. 1-12. In D. M. Weir, L. A. Herzenberg, L. Herzenberg, and C. Blackwell (ed.), Handbook of experimental immunology. Blackwell Scientific Publications, Oxford, United Kingdom.
- 10.Conceicao-Silva, F., M. Hahne, M. Schroter, J. Louis, and J. Tschopp. 1998. The resolution of lesions induced by Leishmania major in mice requires a functional Fas (APO-1, CD95) pathway of cytotoxicity. Eur. J. Immunol. 28:237-245. [DOI] [PubMed] [Google Scholar]
- 11.Convit, J., M. Ulrich, C. T. Fernandez, F. J. Tapia, G. Caceres-Dittmar, M. Castes, and A. J. Rondon. 1993. The clinical and immunological spectrum of American cutaneous leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 87:444-448. [DOI] [PubMed] [Google Scholar]
- 12.Desbarats, J., J. E. Stone, L. Lin, Z. F. Zakeri, G. S. Davis, L. M. Pfeiffer, R. G. Titus, and M. K. Newell. 2000. Rapid early onset lymphocyte cell death in mice resistant, but not susceptible to Leishmania major infection. Apoptosis 5:189-196. [DOI] [PubMed] [Google Scholar]
- 13.Galbiati, F., L. Rogge, and L. Adorini. 2000. IL-12 receptor regulation in IL-12-deficient BALB/c and C57BL/6 mice. Eur. J. Immunol. 30:29-37. [DOI] [PubMed] [Google Scholar]
- 14.Gately, M. K., L. M. Renzetti, J. Magram, A. S. Stern, L. Adorini, U. Gubler, and D. H. Presky. 1998. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu. Rev. Immunol. 16:495-521. [DOI] [PubMed] [Google Scholar]
- 15.Hondowicz, B. D., A. Y. Park, M. M. Elloso, and P. Scott. 2000. Maintenance of IL-12-responsive CD4+ T cells during a Th2 response in Leishmania major-infected mice. Eur. J. Immunol. 30:2007-2014. [DOI] [PubMed] [Google Scholar]
- 16.Huang, F. P., D. Xu, E. O. Esfandiari, W. Sands, X. Q. Wei, and F. Y. Liew. 1998. Mice defective in Fas are highly susceptible to Leishmania major infection despite elevated IL-12 synthesis, strong Th1 responses, and enhanced nitric oxide production. J. Immunol. 160:4143-4147. [PubMed] [Google Scholar]
- 17.Jones, D. E., L. U. Buxbaum, and P. Scott. 2000. IL-4-independent inhibition of IL-12 responsiveness during Leishmania amazonensis infection. J. Immunol. 165:364-372. [DOI] [PubMed] [Google Scholar]
- 18.Kamanaka, M., P. Yu, T. Yasui, K. Yoshida, T. Kawabe, T. Horii, T. Kishimoto, and H. Kikutani. 1996. Protective role of CD40 in Leishmania major infection at two distinct phases of cell-mediated immunity. Immunity 4:275-281. [DOI] [PubMed] [Google Scholar]
- 19.Ma, X., and G. Trinchieri. 2001. Regulation of interleukin-12 production in antigen-presenting cells. Adv. Immunol. 79:55-92. [DOI] [PubMed] [Google Scholar]
- 20.Mattner, F., J. Magram, J. Ferrante, P. Launois, K. Di Padova, R. Behin, M. K. Gately, J. A. Louis, and G. Alber. 1996. Genetically resistant mice lacking interleukin-12 are susceptible to infection with Leishmania major and mount a polarized Th2 cell response. Eur. J. Immunol. 26:1553-1559. [DOI] [PubMed] [Google Scholar]
- 21.Monteforte, G. M., K. Takeda, M. Rodriguez-Sosa, S. Akira, J. R. David, and A. R. Satoskar. 2000. Genetically resistant mice lacking IL-18 gene develop Th1 response and control cutaneous Leishmania major infection. J. Immunol. 164:5890-5893. [DOI] [PubMed] [Google Scholar]
- 22.Nakahira, M., H. J. Ahn, W. R. Park, P. Gao, M. Tomura, C. S. Park, T. Hamaoka, T. Ohta, M. Kurimoto, and H. Fujiwara. 2002. Synergy of IL-12 and IL-18 for IFN-gamma gene expression: IL-12-induced STAT4 contributes to IFN-gamma promoter activation by up-regulating the binding activity of IL-18-induced activator protein 1. J. Immunol. 168:1146-1153. [DOI] [PubMed] [Google Scholar]
- 23.Nishikomori, R., S. Gurunathan, K. Nishikomori, and W. Strober. 2001. BALB/c mice bearing a transgenic IL-12 receptor beta 2 gene exhibit a nonhealing phenotype to Leishmania major infection despite intact IL-12 signaling. J. Immunol. 166:6776-6783. [DOI] [PubMed] [Google Scholar]
- 24.Ohkusu, K., T. Yoshimoto, K. Takeda, T. Ogura, S.-I. Kashiwamura, Y. Iwakura, S. Akira, H. Okamura, and K. Nakanishi. 2000. Potentiality of interleukin-18 as a useful reagent for treatment and prevention of Leishmania major infection. Infect. Immun. 68:2449-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Overath, P., and D. Harbecke. 1993. Course of Leishmania infection in beta 2-microglobulin-deficient mice. Immunol. Lett. 37:13-17. [DOI] [PubMed] [Google Scholar]
- 26.Padigel, U. M., P. J. Perrin, and J. P. Farrell. 2001. The development of a Th1-type response and resistance to Leishmania major infection in the absence of CD40-CD40L costimulation. J. Immunol. 167:5874-5879. [DOI] [PubMed] [Google Scholar]
- 27.Park, A. Y., B. D. Hondowicz, and P. Scott. 2000. IL-12 is required to maintain a Th1 response during Leishmania major infection. J. Immunol. 165:896-902. [DOI] [PubMed] [Google Scholar]
- 28.Perez-Santos, J. L., and P. Talamas-Rohana. 2001. In vitro indomethacin administration upregulates interleukin-12 production and polarizes the immune response towards a Th1 type in susceptible BALB/c mice infected with Leishmania mexicana. Parasite Immunol. 23:599-606. [DOI] [PubMed] [Google Scholar]
- 29.Reiner, S. L., and R. M. Locksley. 1995. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13:151-177. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez-Sosa, M., G. M. Monteforte, and A. R. Satoskar. 2001. Susceptibility to Leishmania mexicana infection is due to the inability to produce IL-12 rather than lack of IL-12 responsiveness. Immunol. Cell Biol. 79:320-322. [DOI] [PubMed] [Google Scholar]
- 31.Satoskar, A., F. Brombacher, W. J. Dai, I. McInnes, F. Y. Liew, J. Alexander, and W. Walker. 1997. SCID mice reconstituted with IL-4-deficient lymphocytes, but not immunocompetent lymphocytes, are resistant to cutaneous leishmaniasis. J. Immunol. 159:5005-5013. [PubMed] [Google Scholar]
- 32.Scharton-Kersten, T., L. C. Afonso, M. Wysocka, G. Trinchieri, and P. Scott. 1995. IL-12 is required for natural killer cell activation and subsequent T helper 1 cell development in experimental leishmaniasis. J. Immunol. 154:5320-5330. [PubMed] [Google Scholar]
- 33.Soong, L., J. C. Xu, I. S. Grewal, P. Kima, J. Sun, B. J. Longley, N. H. Ruddle, D. McMahon-Pratt, and R. A. Flavell. 1996. Disruption of CD40-CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity 4:263-273. [DOI] [PubMed] [Google Scholar]
- 34.Swihart, K., U. Fruth, N. Messmer, K. Hug, R. Behin, S. Huang, G. Del Giudice, M. Aguet, and J. A. Louis. 1995. Mice from a genetically resistant background lacking the interferon gamma receptor are susceptible to infection with Leishmania major but mount a polarized T helper cell 1-type CD4+ T cell response. J. Exp. Med 181:961-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Velasco, O., S. J. Savarino, B. C. Walton, A. A. Gam, and F. A. Neva. 1989. Diffuse cutaneous leishmaniasis in Mexico. Am. J. Trop. Med. Hyg. 41:280-288. [PubMed] [Google Scholar]
- 36.Vieira, L. Q., M. Goldschmidt, M. Nashleanas, K. Pfeffer, T. Mak, and P. Scott. 1996. Mice lacking the TNF receptor p55 fail to resolve lesions caused by infection with Leishmania major, but control parasite replication. J. Immunol. 157:827-835. [PubMed] [Google Scholar]
- 37.von Stebut, E., Y. Belkaid, T. Jakob, D. L. Sacks, and M. C. Udey. 1998. Uptake of Leishmania major amastigotes results in activation and interleukin 12 release from murine skin-derived dendritic cells: implications for the initiation of anti-Leishmania immunity. J. Exp. Med. 188:1547-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei, X. Q., I. G. Charles, A. Smith, J. Ure, G. J. Feng, F. P. Huang, D. Xu, W. Muller, S. Moncada, and F. Y. Liew. 1995. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature 375:408-411. [DOI] [PubMed] [Google Scholar]
- 39.Weinheber, N., M. Wolfram, D. Harbecke, and T. Aebischer. 1998. Phagocytosis of Leishmania mexicana amastigotes by macrophages leads to a sustained suppression of IL-12 production. Eur. J. Immunol. 28:2467-2477. [DOI] [PubMed] [Google Scholar]
- 40.Yoshida, H., S. Hamano, G. Senaldi, T. Covey, R. Faggioni, S. Mu, M. Xia, A. C. Wakeham, H. Nishina, J. Potter, C. J. Saris, and T. W. Mak. 2001. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity 15:569-578. [DOI] [PubMed] [Google Scholar]