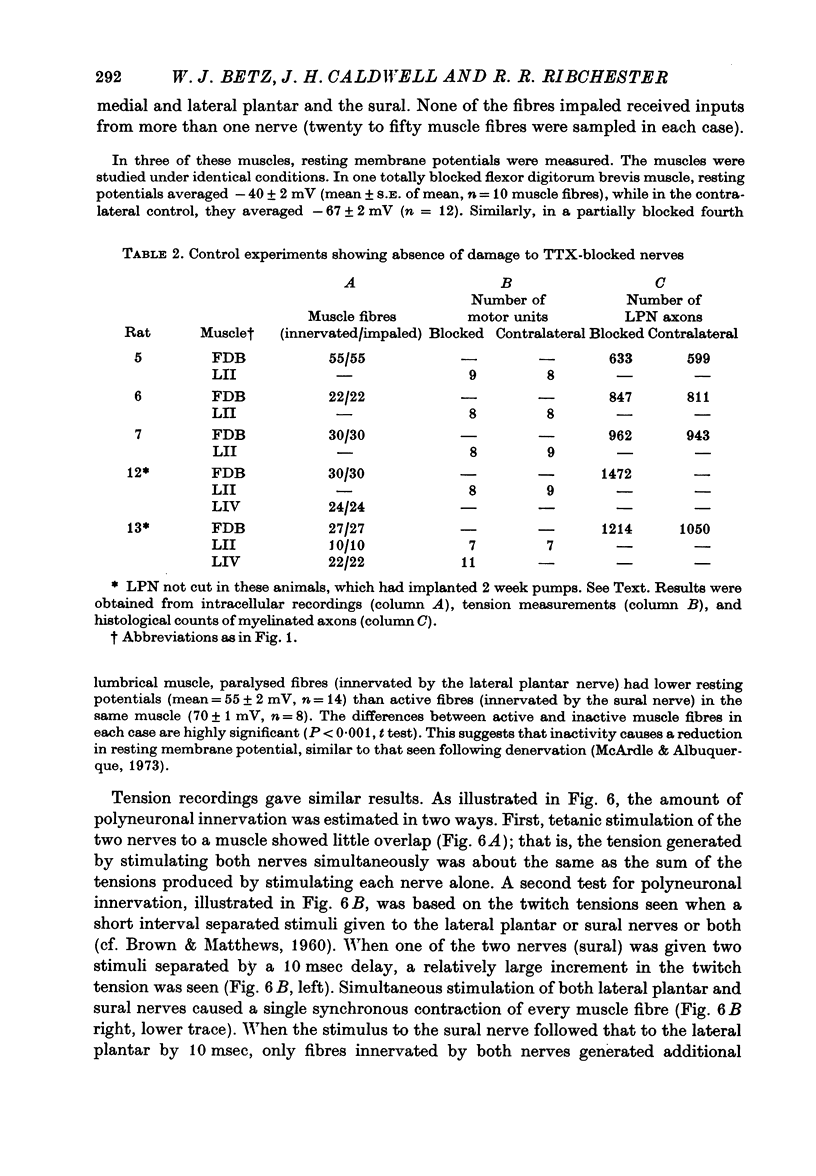
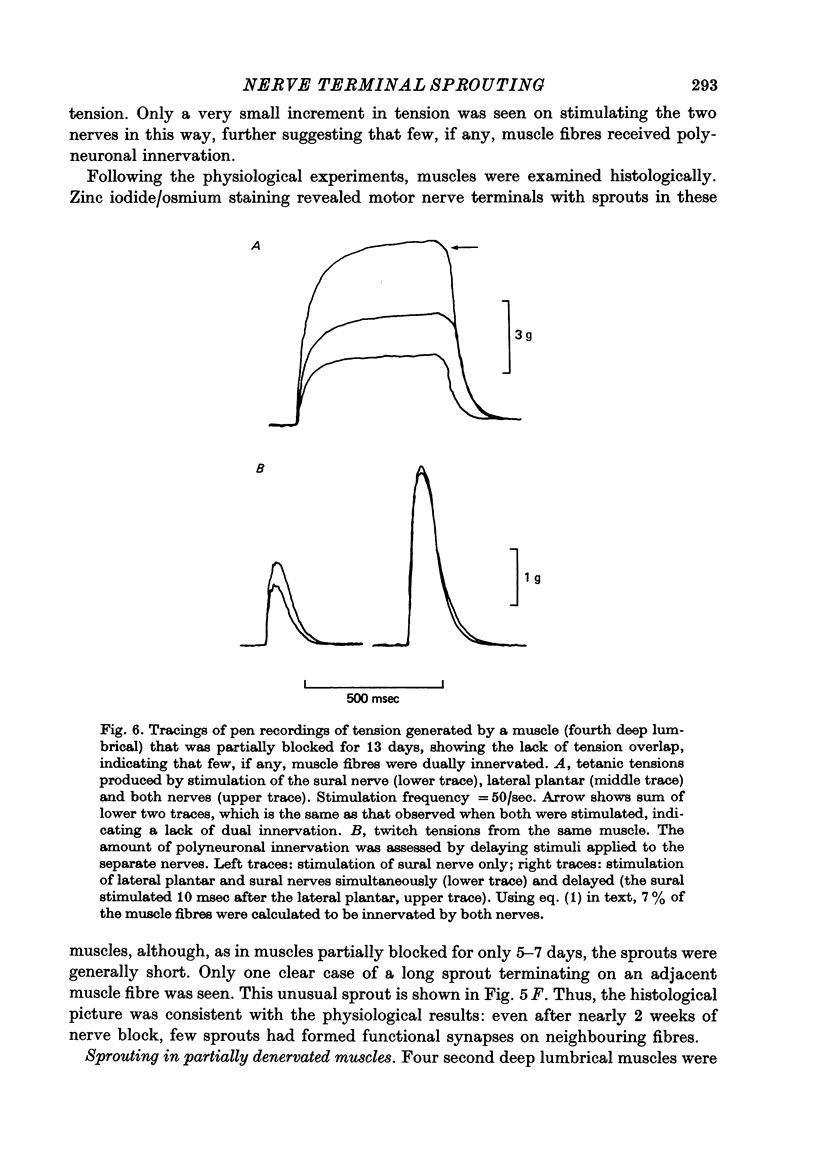
Abstract
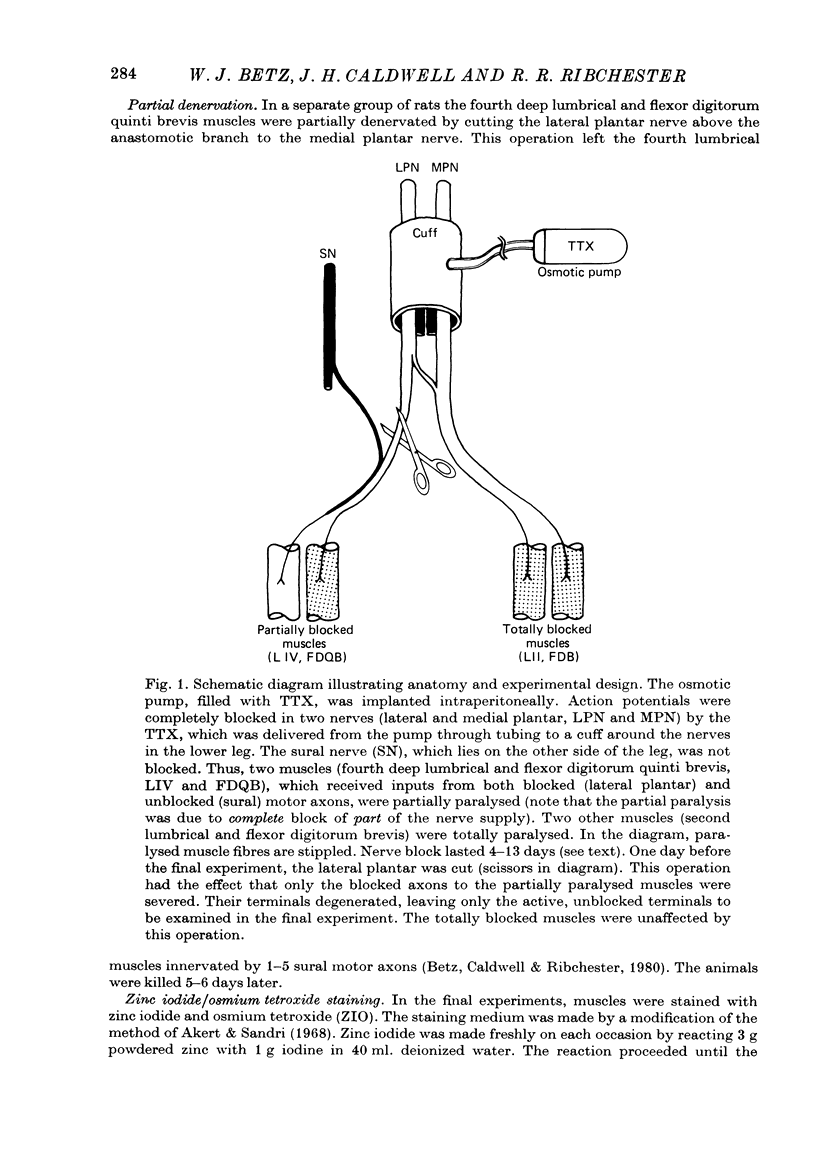
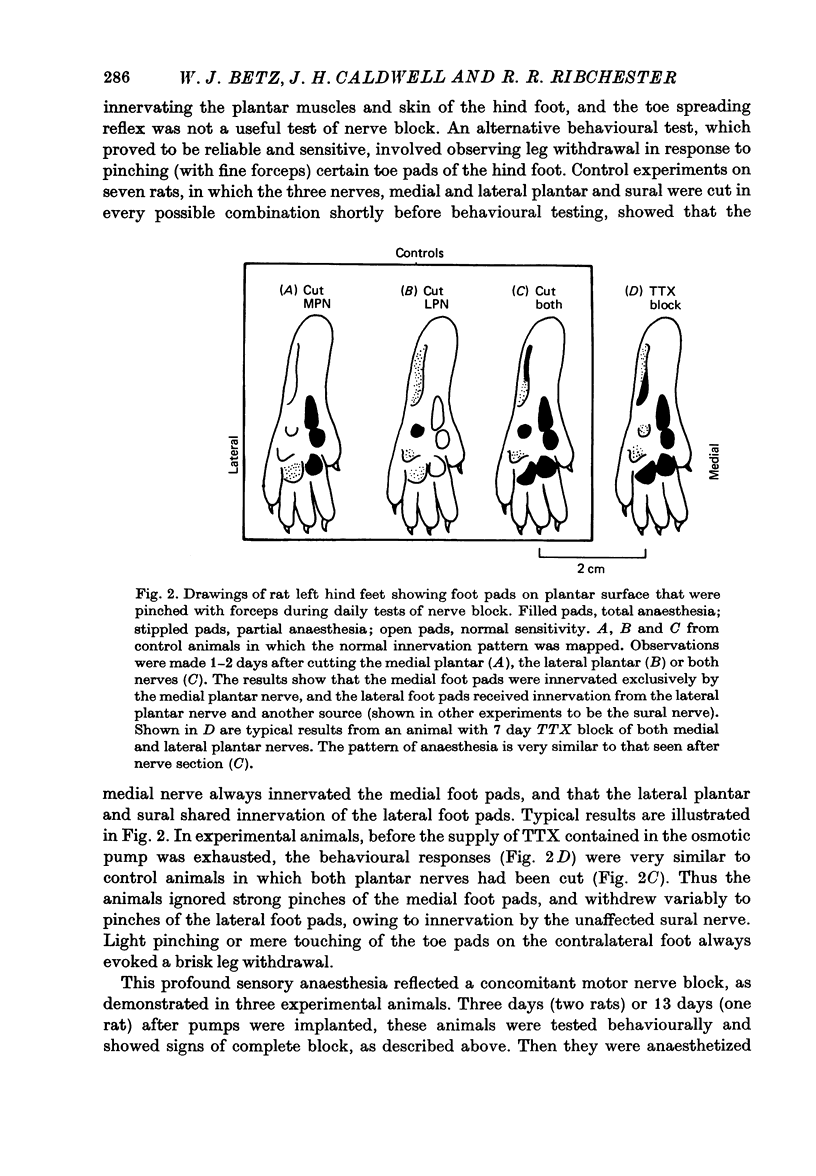
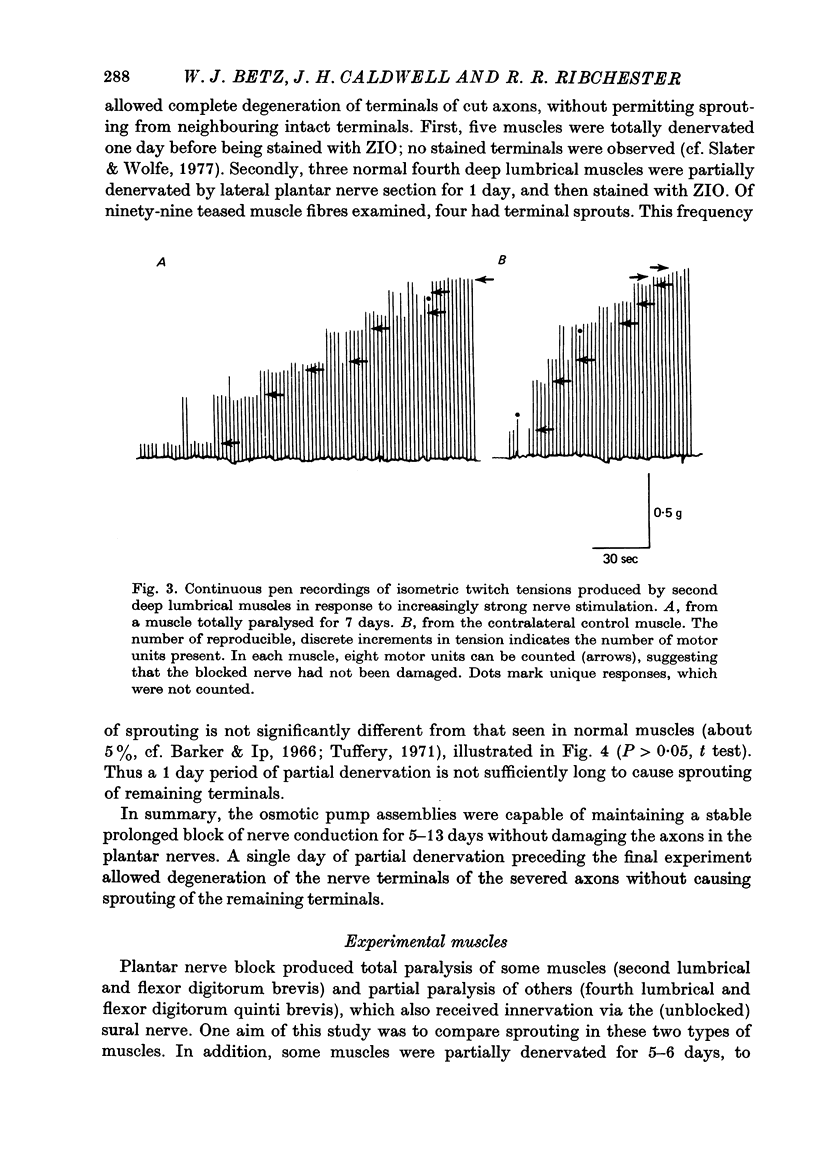
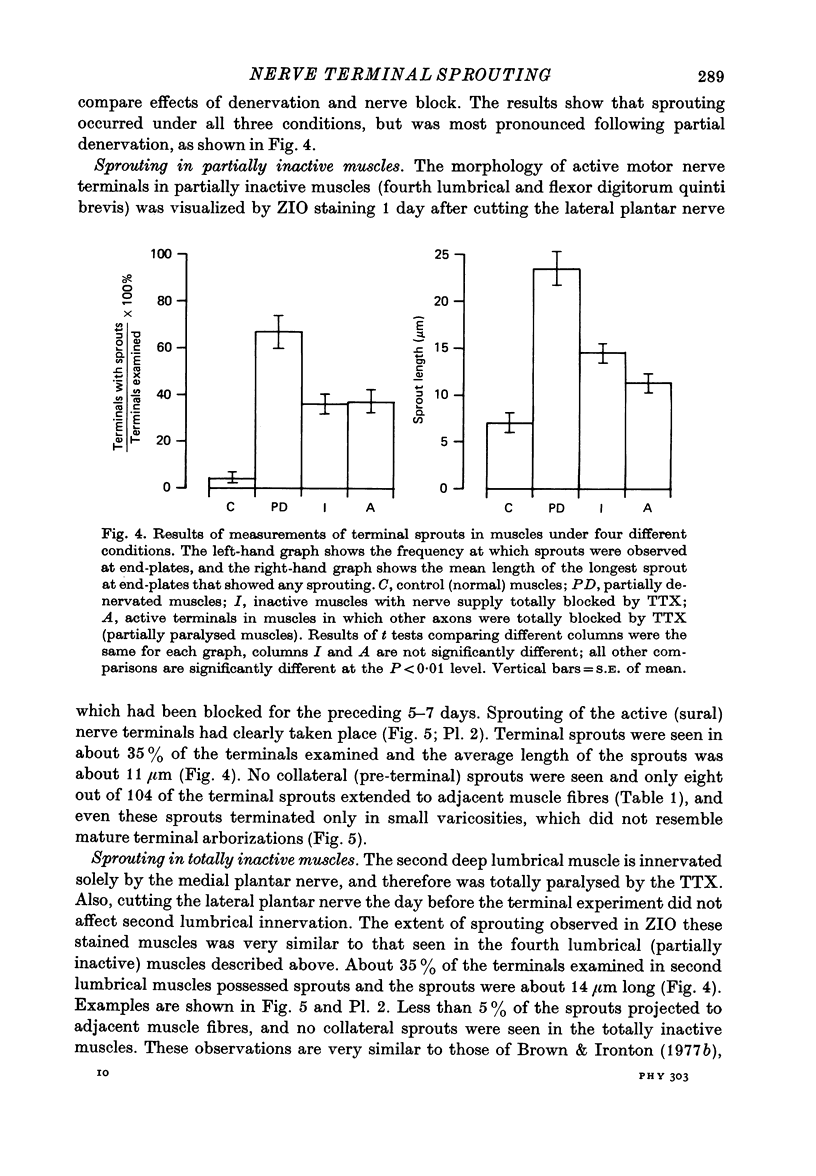
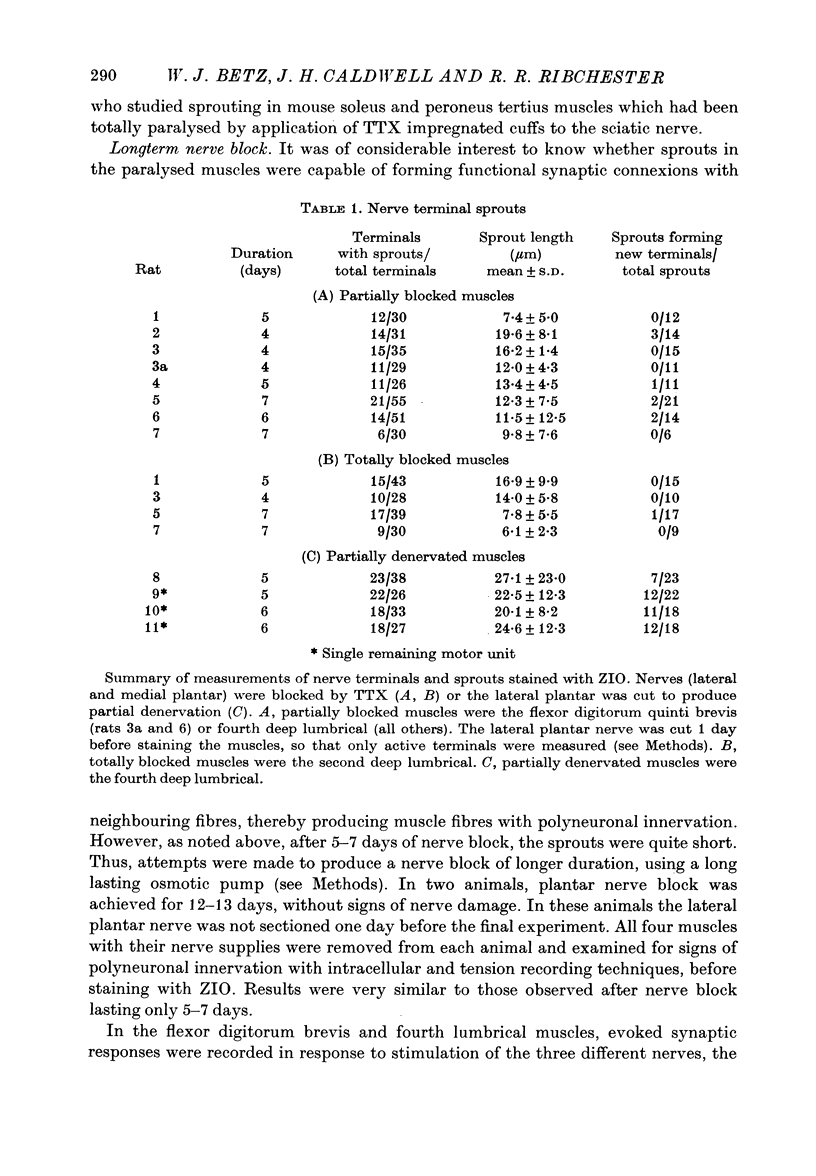
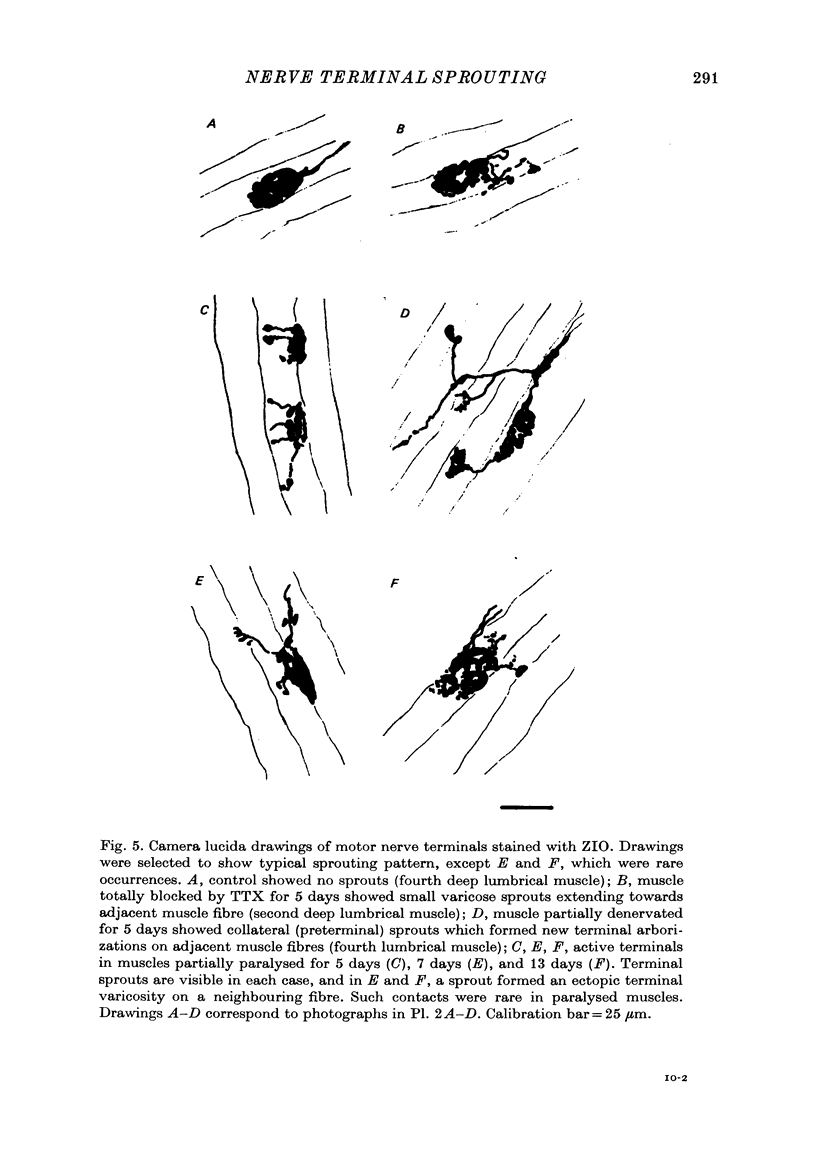
1. Certain muscles in the hind foot of rats were partially paralysed by applying tetrodotoxin to part of their motor innervation. In these muscles motor nerve sprouting occurred from the terminals of the unblocked axons. The extent of sprouting was compared with that seen in totally paralysed and in partially denervated muscles. 2. Action potentials were blocked in the medial and lateral plantar nerves of adult rats for 5-13 days by continuous superfusion with a solution containing tetrodotoxin. The drug was delivered through a tube and nerve cuff from an osmotic pump placed intraperitoneally. Control experiments showed that nerve block was complete and that signs of nerve damage were absent in the animals included in the study. 3. Two muscles (the second lumbrical and flexor digitorum brevis), which received innervation only from the medial plantar nerve, were totally paralysed by the nerve block. Two different muscles (the fourth lumbrical and flexor digitorum quinti brevis) were only partially paralysed, since they received their innervation from the lateral plantar nerve and, in addition, from the sural nerve which was not blocked. One day before the final experiment, the lateral plantar nerve was cut, and its terminals degenerated. Thus in the partially paralysed muscles only the unblocked terminals from the sural nerve remained. These terminals were observed after staining with zinc iodide and osmium tetroxide. Similarly, terminals from the medial plantar nerve were examined in the totally blocked muscles from the same animal. 4. In other experiments, muscles were partially denervated by cutting the lateral plantar nerve in order to compare effects of nerve block and nerve section. 5. Sprouting occurred under all three conditions. Active terminals in the muscles partially paralysed for 5-7 days sprouted to the same extent as terminals in muscles totally blocked during the same period: about 35% of the terminals had sprouts, and their average length was about 13 micron. Sprouting was more pronounced in partially denervated muscles: about 65% of the terminals had sprouts and they averaged 24 micron in length. Collateral (preterminal) sprouts were seen only after partial denervation. 6. Physiological and histological observations suggested that sprouts in paralysed muscles, unlike those in partially denervated muscles, seldom if ever made new synapses on neighbouring muscle fibres, even after 12-13 days of nerve block. 7. The results show that inactive muscle fibres cause active nerve terminals on neighbouring fibres to sprout, perhaps by releasing a diffusible, sprout-promoting factor, which is part of the stimulus for motor nerve sprouting in partially denervated muscles.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akert K., Sandri C. An electron-microscopic study of zinc iodide-osmium impregnation of neurons. I. Staining of synaptic vesicles at cholinergic junctions. Brain Res. 1968 Feb;7(2):286–295. doi: 10.1016/0006-8993(68)90104-2. [DOI] [PubMed] [Google Scholar]
- BROWN M. C., MATTHEWS P. B. An investigation into the possible existence of polyneuronal innervation of individual skeletal muscle fibres in certain hind-limb muscles of the cat. J Physiol. 1960 Jun;151:436–457. doi: 10.1113/jphysiol.1960.sp006450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D., Ip M. C. Sprouting and degeneration of mammalian motor axons in normal and de-afferentated skeletal muscle. Proc R Soc Lond B Biol Sci. 1966 Jan 18;163(993):538–554. doi: 10.1098/rspb.1966.0008. [DOI] [PubMed] [Google Scholar]
- Betz W. J., Caldwell J. H., Ribchester R. R. The effects of partial denervation at birth on the development of muscle fibres and motor units in rat lumbrical muscle. J Physiol. 1980 Jun;303:265–279. doi: 10.1113/jphysiol.1980.sp013284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W. J., Caldwell J. H., Ribchester R. R. The size of motor units during post-natal development of rat lumbrical muscle. J Physiol. 1979 Dec;297(0):463–478. doi: 10.1113/jphysiol.1979.sp013051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Goodwin G. M., Ironton R. Prevention of motor nerve sprouting in botulinum toxin poisoned mouse soleus muscles by direct stimulation of the muscle [proceedings]. J Physiol. 1977 May;267(1):42P–43P. [PubMed] [Google Scholar]
- Brown M. C., Holland R. L., Ironton R. Degenerating nerve products affect innervated muscle fibres. Nature. 1978 Oct 19;275(5681):652–654. doi: 10.1038/275652a0. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Ironton R. Motor neurone sprouting induced by prolonged tetrodotoxin block of nerve action potentials. Nature. 1977 Feb 3;265(5593):459–461. doi: 10.1038/265459a0. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Ironton R. Sprouting and regression of neuromuscular synapses in partially denervated mammalian muscles. J Physiol. 1978 May;278:325–348. doi: 10.1113/jphysiol.1978.sp012307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangiano A., Lutzemberger L. Partial denervation affects both denervated and innervated fibers in the mammalian skeletal muscle. Science. 1977 Apr 29;196(4289):542–545. doi: 10.1126/science.850797. [DOI] [PubMed] [Google Scholar]
- Devor M., Schonfeld D., Seltzer Z., Wall P. D. Two modes of cutaneous reinnervation following peripheral nerve injury. J Comp Neurol. 1979 May 1;185(1):211–220. doi: 10.1002/cne.901850113. [DOI] [PubMed] [Google Scholar]
- Diamond J., Cooper E., Turner C., Macintyre L. Trophic regulation of nerve sprouting. Science. 1976 Jul 30;193(4251):371–377. doi: 10.1126/science.935873. [DOI] [PubMed] [Google Scholar]
- Duchen L. W. Changes in motor innervation and cholinesterase localization induced by botulinum toxin in skeletal muscle of the mouse: differences between fast and slow muscles. J Neurol Neurosurg Psychiatry. 1970 Feb;33(1):40–54. doi: 10.1136/jnnp.33.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen L. W., Tonge D. A. The effects of implantation of an extra nerve on axonal sprouting usually induced by botulinum toxin in skeletal muscle of the mouse. J Anat. 1977 Sep;124(Pt 1):205–215. [PMC free article] [PubMed] [Google Scholar]
- EDDS M. V., Jr Collateral nerve regeneration. Q Rev Biol. 1953 Sep;28(3):260–276. doi: 10.1086/399699. [DOI] [PubMed] [Google Scholar]
- HOFFMAN H. Local re-innervation in partially denervated muscle; a histophysiological study. Aust J Exp Biol Med Sci. 1950 Jul;28(4):383–397. doi: 10.1038/icb.1950.39. [DOI] [PubMed] [Google Scholar]
- Jansen J. K., Van Essen D. C. Re-innervation of rat skeleton muscle in the presence of alpha-bungarotoxin. J Physiol. 1975 Sep;250(3):651–667. doi: 10.1113/jphysiol.1975.sp011075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie P. A., Collier B., Tenenhouse A. Role of skeletal muscle activity in the control of muscle acetylcholine sensitivity. Exp Neurol. 1977 Jan;54(1):148–171. doi: 10.1016/0014-4886(77)90242-4. [DOI] [PubMed] [Google Scholar]
- McArdle J. J., Albuquerque E. X. A study of the reinnervation of fast and slow mammalian muscles. J Gen Physiol. 1973 Jan;61(1):1–23. doi: 10.1085/jgp.61.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestronk A., Drachman D. B. Motor nerve sprouting and acetylcholine receptors. Science. 1978 Mar 17;199(4334):1223–1225. doi: 10.1126/science.204007. [DOI] [PubMed] [Google Scholar]
- Redfern P. A. Neuromuscular transmission in new-born rats. J Physiol. 1970 Aug;209(3):701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper S., Ko C. P. Impulse blockade in frog cardiac ganglion does not resemble partial denervation in changing synaptic organization. Science. 1978 Oct 6;202(4363):66–68. doi: 10.1126/science.308697. [DOI] [PubMed] [Google Scholar]
- Rotshenker S. Sprouting of intact motor neurons induced by neuronal lesion in the absence of denervated muscle fibers and degenerating axons. Brain Res. 1978 Oct 27;155(2):354–356. doi: 10.1016/0006-8993(78)91029-6. [DOI] [PubMed] [Google Scholar]
- Sanes J. R., Marshall L. M., McMahan U. J. Reinnervation of muscle fiber basal lamina after removal of myofibers. Differentiation of regenerating axons at original synaptic sites. J Cell Biol. 1978 Jul;78(1):176–198. doi: 10.1083/jcb.78.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater C. R., Wolfe A. F. Abnormal reinnervation of muscles in dystrophic mice [proceedings]. J Physiol. 1978 Feb;275:73P–74P. [PubMed] [Google Scholar]
- Thompson W., Kuffler D. P., Jansen J. K. The effect of prolonged, reversible block of nerve impulses on the elimination of polyneuronal innervation of new-born rat skeletal muscle fibers. Neuroscience. 1979;4(2):271–281. doi: 10.1016/0306-4522(79)90088-5. [DOI] [PubMed] [Google Scholar]
- Thompson W. Reinnervation of partially denervated rat soleus muscle. Acta Physiol Scand. 1978 May;103(1):81–91. doi: 10.1111/j.1748-1716.1978.tb06193.x. [DOI] [PubMed] [Google Scholar]
- Tiedt T. N., Albuquerque E. X., Guth L. Degenerating nerve fiber products do not alter physiological properties of adjacent innervated skeletal muscle fibers. Science. 1977 Nov 25;198(4319):839–841. doi: 10.1126/science.918666. [DOI] [PubMed] [Google Scholar]
- Tuffery A. R. Growth and degeneration of motor end-plates in normal cat hind limb muscles. J Anat. 1971 Nov;110(Pt 2):221–247. [PMC free article] [PubMed] [Google Scholar]