Abstract
Human macrophage chemoattractant protein 1 (MCP-1) is a potent mediator of macrophage migration and therefore plays an essential role in early events of inflammation. In endothelial cells, at least three independent pathways regulate MCP-1 expression by NF-κB and AP-1. Orientia tsutsugamushi causes vasculitis in humans by replicating inside macrophages and endothelial cells. In the present study, we investigated the cis-acting and trans-acting elements involved in O. tsutsugamushi-induced MCP-1 gene expression in human umbilical vein endothelial cells (HUVEC). Although NF-κB activation was observed in HUVEC infected with O. tsutsugamushi, inhibition of NF-κB activation did not affect the MCP-1 expression. However, treatment of HUVEC with extracellular signal-regulated kinase (ERK) kinase inhibitor or p38 mitogen-activated protein kinase (MAPK) inhibitor suppressed expression of MCP-1 mRNA concomitant with downregulation of activator protein 1 (AP-1) activation. Deletion of triphorbol acetate response elements (TRE) at position −69 to −63 of MCP-1 gene abolished inducible promoter activity. Deletion of TRE at position −69 to −63−96 to −90 or deletion of NF-κB-binding site at position −69 to −63−88 to −79 did not affect the inducibility of promoter. Site-directed mutagenesis of the NF-κB binding sites at positions −2640 to −2632, −2612 to −2603 in the enhancer region, or the AP-1 biding site at position −2276 to −2270 decreased the inducible activity of the promoter. Taken together, AP-1 activation by both the ERK pathway and the p38 MAPK pathway as well as their binding to TRE at position −69 to −63 in proximal promoter and TRE at position −2276 to −2270 in enhancer region is altogether essential in induction of MCP-1 mRNA in HUVEC infected with O. tsutsugamushi. Although NF-κB activation is not essential per se, the κB site in the enhancer region is important in MCP-1 induction of HUVEC. This discrepancy in the involvement of the NF-κB may be due to the function of chromatin structures and other transcription cofactors in the regulation of MCP-1 gene expression in response to O. tsutsugamushi infectioin.
Vascular endothelial cells respond to environment and cellular stimuli with profound changes of adhesive and inflammatory phenotypes (4, 12). This process of endothelial cell activation results in release of inflammatory and chemotactic cytokines and enhanced leukocyte recruitment via expression of adhesion molecules (12). During infection, the activated endothelial cells also play a pivotal role in the increased leukocyte-endothelial cell interaction and initiation of host inflammatory responses (4). The recruitment of subsets of leukocytes into inflamed tissues is an important process for the appropriate immune responses and host defense. In particular, the macrophages in pathological foci play a central role in the inflammatory responses through their ability to produce various mediators. Although the mechanism of monocyte infiltration is not yet fully understood, macrophage chemoattractant protein 1 (MCP-1) is thought to play a significant role (34). MCP-1 is a member of the CC subfamily of the chemokine family and attracts both monocytes and lymphocytes (2, 35, 55). Various types of cells such as endothelial cells and macrophages are known to produce MCP-1 in response to diverse stimuli, including proinflammatory cytokines and pathological microorganisms (11, 17, 18, 41, 55). In addition, high levels of MCP-1 mRNA were found in pathological foci of atherosclerosis, glomerulonephritis, and rheumatoid arthritis (48, 59, 65). The patterns of stimulus-induced MCP-1 mRNA expression, however, vary with each cell type or stimulus. Modulation of MCP-1 synthesis may provide a means to suppress cellular recruitment in these pathological conditions, as well as a variety of other inflammatory diseases.
Multiple signaling mechanisms have been reported to be involved in the intracellular activation of MCP-1 gene expression in vascular endothelial cells by a various stimuli (27, 31, 43, 52). Diverse signal transduction pathways, including activation of phosphatidylinositol-3-OH kinase, Akt/protein kinase B (43), phospholipase C (27), p60src-Ras (31), protein kinase C, and tyrosine kinases (52), are involved in the expression of the MCP-1 gene in response to a variety of extracellular stimuli such as cytokines, mitogens, and shear stress. Some of the pathways are probably activated concomitantly or selectively according to the stimulating agents (27, 52). Subsequently, signals from these stimuli may be transmitted to the nucleus through the activation of mitogen-activated protein (MAP) kinases (46) and transcription factors such as NF-κB or AP-1 (21, 25, 63, 64). The involvement of MAP kinase pathways in the regulation of the MCP-1 expression has been reported in endothelial cells in response to various stimulating agents (6, 28, 31, 39, 51). Additionally, the MAP kinase pathways were also selectively activated according to the stimuli (15, 28, 39). The activated MAP kinase pathways have been suggested to be closely involved in the transcriptional regulations mediated by transcription factors, Activator protein 1 (AP-1) (6, 38, 64) or NF-κB (5, 63). The promoter region of the human MCP-1 gene has been shown to contain putative consensus binding sites for a variety of transcription factors (54). The triphorbol acetate response elements (TRE), which is recognized by the AP-1 transcription heterodimer c-Jun and c-Fos and/or NF-κB sites upstream of the transcription start site are involved in the regulation of MCP-1 gene induction in various cells types in responses to diverse extracellular stimuli (37, 51, 54). It has been suggested that the differential activation and binding of inducible transcription factors such as AP-1 and NF-κB to the promoter regions of chemokine genes provides a critical regulatory mechanism by which the chemokine can be selectively expressed in a cell type-specific and stimulus-specific manner (47).
Although a number of molecular studies have identified the signal transduction pathways and the cis-regulatory elements and trans-acting factors in the expression of MCP-1 gene in endothelial cells, the intracellular signaling pathways that couple endothelial activation from receptor levels to the level of MCP-1 expression are so far only poorly understood. Especially, little is known about the role of the different MAP kinase cascades and the subsequent activation of transcription factors involved in the transcriptional regulation of MCP-1 in response to infectious agents.
Orientia tsutsugamushi is the causative agent of scrub typhus, which is characterized by eschar, rashes, and other inflamed organs where dense collections of mononuclear cells, including lymphocytes and macrophages, are found around the vasculatures (1). The extent of infiltrating leukocytes around the blood vessels is closely related with the clinical manifestation of scrub typhus (1, 9, 62). Previously, we have reported that subsets of chemokine genes were produced in macrophages and endothelial cells infected with O. tsutsugamushi (10, 11). Among the chemokine genes expressed, mononuclear cell-specific chemokine MCP-1 was highly up-regulated in both types of cells infected with O. tsutsugamushi. Furthermore, we have suggested that the induction of MCP-1 might be mediated by an NF-κB-independent mechanism and the activation of AP-1 is possibly involved in the expression of the chemokine genes in human transformed microvascular endothelial cell (HMEC) line HMEC-1 infected with O. tsutsugamushi (10). In this work, we have analyzed the functional role of the two transcription factors, AP-1 and NF-κB, in MCP-1 transcription in human umbilical vein endothelial cells (HUVEC) infected with O. tsutsugamushi. By using the inhibitors which block the activation of NF-κB or inhibit the activation of AP-1 through blocking the specific MAP kinases, the involvement of the signal transduction pathways in the induction of the MCP-1 gene was investigated. In addition, we have performed the functional analysis of the putative binding sites for AP-1 or NF-κB located in the proximal promoter region or enhancer region of MCP-1 gene by using a transient-transfection approach in HUVEC.
MATERIALS AND METHODS
Cell culture.
HUVEC were obtained by collagenase digestion of umbilical veins as previously described (26). The isolated HUVEC were cultured in M199 medium (Life Technologies, Grand Island, N.Y.), containing 20% fetal bovine serum, 2 mM l-glutamine, endothelial cell growth supplement (30 μg/ml; Sigma Chemical Co., St. Louis, Mo.), penicillin (100 U/ml), and streptomycin (100 μg/ml). Endothelial cells were identified by cobblestone appearance and specific staining for Von Willebrand factor (DAKO Co., Carpinteria, Calif.). HUVEC from between passages 3 and 6 were used for the experiments.
Preparation of O. tsutsugamushi inoculum.
O. tsutsugamushi Karp (American Type Culture Collection, Manassas, Va.) was propagated in monolayers of L929 cells as described previously (11). The titer of infectivity of the inoculum was determined as described previously (11). A total of 1.4 × 107 infected-cell counting units of O. tsutsugamushi was used to infect endothelial cells for the preparation of total RNA and nuclear extract. The L929 cell lysate was prepared as described above and was used in infection of endothelial cells for the control experiments.
Stimulation of cells.
Endothelial cells were seeded onto 100-mm-diameter dishes (Becton Dickinson Labware, Franklin Lakes, N.J.) for the preparation of total RNA or for the preparation of nuclear extract. Total RNA was isolated at 0, 0.5, 1, 3, 6, 12, and 24 h after infection of O. tsutsugamushi. Nuclear protein extracts were prepared at 0, 0.25, 0.5, 1, 4, and 8 h after infection. In the inhibition assays, endothelial cells were preincubated with 50 μM pyrrolidinedithiocarbamate (PDTC) (Sigma Chemical Co.), 100 μM N-tosyl-l-phenylalanine chloromethyl ketone (TPCK) (Sigma Chemical Co.), and various concentrations of SB 203580 (Sigma Chemical Co.) and PD 098059 (Sigma Chemical Co.) for 1 h before O. tsutsugamushi was inoculated. The inhibitors were maintained during the course of inhibition assays.
RNase protection assay.
Total RNA was prepared with an RNeasy kit (Qiagen GmbH, Hilden, Germany) as specified by the manufacturer and was quantified spectrophotometrically. Detection and semiquantification of various human chemokine mRNAs was performed with a multiprobe RNase protection assay system from Pharmingen (San Diego, Calif.) as described previously (10). Nuclease-protected RNA fragments were resolved on a 5% polyacrylamide sequencing gel. The bands were observed after autoradiography. The band intensities shown in autoradiography were digitized by scanning the images and analyzed with TINA software (Raytest Isotopenmeßgeräte GmbH, Straubenhardt, Germany). The densitometric intensity was normalized with respect to the intensities of the band for the housekeeping gene, the glyceraldehyde-3-phosphate dehydrogenase gene.
Reverse transcription (RT)-PCR.
HUVEC were plated onto six-well dishes and allowed to reach confluence. Then, cells were infected with O. tsutsugamushi for 3 h in the absence or presence of inhibitors of AP-1 activation. The medium was removed from each well, and total RNA was extracted from the cells using an RNeasy kit. cDNA synthesis was conducted using 10 U of avian myeloblastosis virus reverse transcriptase (Promega, Madison, Wis.) and 200 ng of oligo(dT) primer (Promega), together with manufacturer's buffer, 20 nmol of deoxynucleoside triphosphate, and 12.5 nmol of MgSO4 for 1 h at 42°C. PCR amplification consisted of 25 cycles of 30 s of denaturation at 94°C, 30 s of annealing at 58°C (MCP-1) or 55°C (β-actin), and extension at 72°C for 1 min as described previously (11). The sequences of primers used for the amplifications and the size of PCR products were as follows: MCP-1 (135 bp), 5′-AAT GCC CCA GTC ACC TGC TGC TAT-3′ and 5′-TCT CCT TGG CCA CAA TGG TCT TGA-3′; β-actin (349 bp), 5′-TGG AAT CCT GTG GCA TCC ATG AAA C-3′ and 5′-TAA AAC GCA GCT CAG TAA CAG TCC G-3′. The amplified DNA fragments were resolved in 1.2% agarose gels, and the densities of the DNA bands were analyzed as described previously (11). The densitometric intensity was normalized by comparing the ratio of MCP-1 bands with that of β-actin.
Nuclear extraction and EMSA.
Electrophoretic mobility shift assays (EMSA) were performed as described previously (10). Equal amounts of nuclear extracts (10 μg of protein) from each sample were incubated for 30 min at 25°C in 20 μl of binding buffer [10 mM Tris-HCl (pH 7.5), 50 mM NaCl, 0.5 mM dithiothreitol, 0.5 mM EDTA, 4% glycerol, 1 mM MgCl2, 1 μg of poly(dI:dC)] containing 30,000 cpm of an NF-κB-specific or AP-1-specific oligonucleotide probe. Probes were radiolabeled with [γ-32P]ATP (Amersham Ltd., Little Chalfont, England). The sequence of the NF-κB-specific probe was 5′-AGT TGA GGG GAC TTT CCC AGG C-3′, and the sequence of the AP-1-specific probe was 5′-TGC TTG ATG AGT CAG CCG GAA-3′. To ascertain the specific binding of nuclear extracts with NF-κB or AP-1 probe, a competition assay was performed with a 50-fold molar excess of unlabeled oligonucleotides. Nuclear translocation of the NF-κB heterodimer was analyzed by a supershift assay with anti-p65 antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.). The nuclear extracts were mixed with 1 μg of anti-p65 antibody and were hybridized with the NF-κB-specific oligonucleotide probe. The supershifted bands were analyzed after separation on 5% nondenaturing polyacrylamide gels and autoradiography.
Construction of plasmid.
The plasmid pGL3-basic (Promega) was used to construct MCP-1-luciferase hybrids. To obtain pMCP560, pMCP121, pMCP90, pMCP73, pMCP65, and pMCP62, DNA fragments covering position −560 to +43, position −121 to +43, position −90 to +43, position −73 to +43, position −65 to +43, and position −62 to +43 were amplified by using the standard PCR technique with chemically synthesized primers that corresponded to nucleotides −560 to −537 of the sense strand, 5′GGG ACG CGT CCT ACA GTT CTG CTA GGC TTC TA3′; −121 to −98 of the sense strand, 5′GGG ACG CGT CAG CAG ATT TAA CAG CCC ACT TA3′; −90 to −69 of the sense strand, 5′GGG ACG CGT GGA AGA TCC CTC CTC CTG CTT3′; −73 to −53 of the sense strand, 5′GGG ACG CGT GCT TGA CTC CGC CCT CTC TC3′; −65 to −46 of the sense strand, 5′GGG ACG CGT CCG CCC TCT CTC CCT CTG C3′; −62 to −43 of the sense strand, 5′GGG ACG CGT GCC CTC TCT CCC TCT GCC CG3′; and +20 to +40 of the antisense strand, 5′GAA GAT CTG AGC TTC AGT TTG AGA ATT GGA TG3′. MluI or BglII linkers were incorporated in the 5′ end of these oligonucleotides. Human genomic DNA was isolated from HUVEC and was used as the PCR template. PCR products were digested with MluI and BglII and then cloned into the MluI-BglII site of the pGL3-basic plasmid. To obtain pMCP560-En, the DNA sequence from position −2805 to −2787 of the sense strand, 5′GGG GTA CCT TGT GTG TCC CCA AGC G3′ and position −2101 to −2081 of the antisense strand, 5′GGG AGC TCG TGC ATC CTT TAC CAT GAA CT3′. KpnI or SacI linkers were incorporated in the 5′ end of these oligonucleotides. The amplified DNA was then digested with KpnI and SacI and cloned into the KpnI-SacI site of the pMCP560. The orientation of the positive clone was determined by DNA sequencing. Site-directed mutagenesis was performed by using a method previously reported (29). Two-step PCRs were conducted. The primers used are as follows: MA1 primer pair (position −2650 to −2627), 5′ACG GGA TCT aGa gAC TTC CAA AGC3′; MA2 primer pair (position −2616 to −2591), 5′GAG TGG GAA TTc ggA CTC ACT TCT CT3′; and MA3 primer pair (position −2282 to −2259), 5′AAC AAG TGA Gga gTG CCA CAG GAT3′. Mutated bases are designated by lowercase letters. The resulting mutated DNA fragments were cloned into the KpnI-SacI site of the pMCP560 to yield pMCP560-EnMA1, pMCP560-EnMA2, pMCP560-EnMA3, pMCP560-EnMA4, and pMCP560-EnMA5.
Cell transfection and luciferase assay.
HUVEC were transfected using Lipofectin (Life Technologies) as specified by manufacturer. Briefly, cells of 50 to 60% confluent monolayers were washed with OPTI-MEM I reduced serum medium (Life Technologies) and incubated with combined solution containing 10 μl of Lipofectin and 3 μg of reporter plasmid for 3 h in six-well culture. After incubation, the DNA-containing medium was replaced with culture medium and cells were further incubated for 18 h before infection with O. tsutsugamushi. Following stimulation, the cells were washed once with phosphate-buffered saline and lysed with 100 μl of reporter lysis buffer (Promega). The luciferase activity of lysed cells was determined using a luciferase assay system (Promega) and immediately analyzed for light production in a TD-20/20 luminometer (Turner designs, Sunnyvale, Calif.). Transfection efficiencies were monitored and normalized by cotransfection of a pCMV-βgal vector (1 μg) for β-galactosidase (19).
RESULTS
MCP-1 mRNA expression was increased by O. tsutsugamushi.
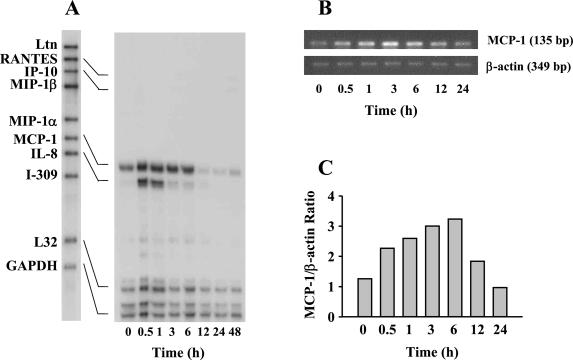
Before and after exposure of HUVEC to O. tsutsugamushi, the chemokine transcript levels were assayed at various time points with RNase protection assays (Fig. 1A). Figure 1 represents the result of three separate experiments. Basal levels of MCP-1 and interleukin-8 (IL-8) messages were detected in the uninfected cells. The levels of the transcripts for MCP-1 and IL-8 increased as early as 30 min after infection, with peak responses at 3 and 1 h, respectively. While the transcripts for MCP-1 were sustained for 6 h after infection, the RNA messages for IL-8 persisted for 1 h after infection. The transcripts for RANTES and IP-10 were also detectable at 6 h after infection in HUVEC. The levels of transcripts for RANTES and IP-10 were significantly lower than those of MCP-1 and IL-8 and were gradually increased by 48 h after infection. To confirm the induction of the MCP-1 gene, semiquantitative RT-PCR was performed at various time points after infection with O. tsutsugamushi (Fig. 1B and C). A similar result was obtained from the RT-PCR analysis. The normalized levels of MCP-1 gene at 3 and 6 h after infection were increased by approximately three times when compared with those of the cells uninfected.
FIG. 1.
Time course of O. tsutsugamushi-stimulated chemokine induction in HUVEC. (A) The levels of chemokine mRNAs at each time point were assayed by RNase protection. (B) The mRNA levels of MCP-1 gene were analyzed by semiquantitative RT-PCR in 1.2% agarose gel. The levels of β-actin transcripts were also determined to normalize the transcripts of MCP-1. (C) The band densities were determined with TINA software, and the mRNA expression level for MCP-1 was normalized with respect to the intensities of the bands of β-actin. Abbreviations: MIP-1α, macrophage inflammatory protein 1α; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Activation of transcription factors NF-κB and AP-1 by O. tsutsugamushi.
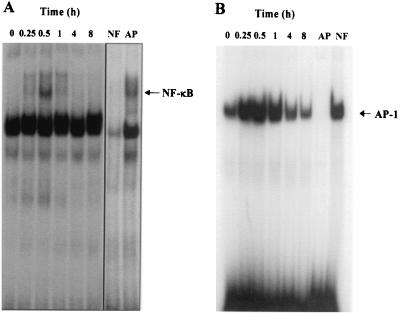
Infection of HUVEC with O. tsutsugamushi may induce alterations in signal transduction pathways that modulate cellular transcription factors. Furthermore, the activation of transcription factors and chemokine genes such as MCP-1 may be coordinately inducible in O. tsutsugamushi-stimulated HUVEC. To elucidate the mechanism(s) by which O. tsutsugamushi induces the transcriptional activity of the MCP-1 gene, we analyzed the binding activities of NF-κB and AP-1 in O. tsutsugamushi-exposed HUVEC by EMSA. At different time points after challenge, nuclear extracts were assayed for NF-κB or AP-1 DNA binding activity using radiolabeled specific oligonucleotide probe. As shown in Fig. 2, nuclear extracts from uninfected HUVEC contained basal levels of AP-1 binding activity. Inducible binding activities of both transcription factors, NF-κB and AP-1, were detected after infection with O. tsutsugamushi. In accordance with the kinetics of O. tsutsugamushi-induced MCP-1 mRNA induction, inducible NF-κB and AP-1 binding activities were detected within 15 min and up-regulated up to 4 h following infection with O. tsutsugamushi. At 8 h after infection, the levels of NF-κB or AP-1 binding activity decreased to levels similar to cells incubated with medium alone. Cold oligonucleotide competition demonstrated that the binding of NF-κB or AP-1 was specific, as the unlabeled NF-κB or AP-1 oligonucleotide prevented formation of radiolabeled protein-DNA complexes. This indicates that O. tsutsugamushi activates the DNA-binding activity of both transcription factors, NF-κB and AP-1, in the human endothelial cell.
FIG. 2.
EMSA showing activation of transcription factors, NF-κB (A) and AP-1 (B), in O. tsutsugamushi-infected HUVEC at each time point. A competitive inhibition assay was performed with nuclear extracts that were preincubated with 50-fold molar excesses of either unlabeled NF-κB (NF) or AP-1 (AP) consensus oligonucleotide.
Effect of the inhibitors of NF-κB activation on the expression of MCP-1 mRNA.
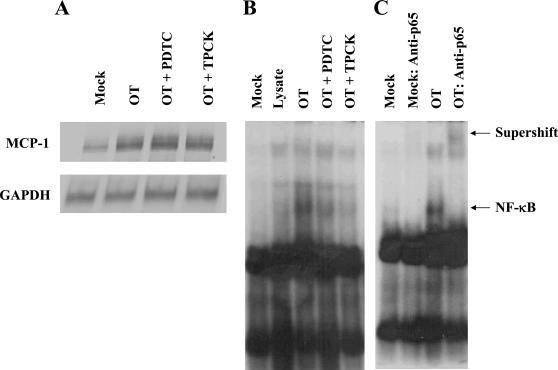
In order to examine whether NF-κB activation is involved in MCP-1 expression of O. tsutsugamushi-exposed HUVEC, we used two inhibitors of NF-κB activation, the antioxidant PDTC (3, 42) and the proteasome inhibitor TPCK (3, 13, 36). Interestingly, when the endothelial cells were incubated with O. tsutsugamushi in the presence of 50 μM PDTC or 100 μM TPCK, induction of MCP-1 and IL-8 was not significantly affected in HUVEC (Fig. 3A). Rather, the level of MCP-1 was increased by approximately 15% in cells infected with O. tsutsugamushi in the presence of PDTC compared to that of cells infected with O. tsutsugamushi alone. The results were reproduced in three separate trials. We, therefore, have investigated whether the activation of NF-κB is affected by the inhibitors in HUVEC infected with O. tsutsugamushi (Fig. 3B). Although the basal levels of NF-κB complexes were detected in the cells treated with medium or L-cell lysate, the activation and nuclear translocation of NF-κB was increased when stimulated with O. tsutsugamushi. In the presence of the inhibitors of NF-κB activation, the levels of NF-κB complex were remarkably decreased and comparable to those of control groups. The proteasome inhibitor, TPCK, was more effective in inhibiting NF-κB activation than PDTC. These results showed that the inhibitors in the concentrations used were effective in inhibiting the activation and nuclear translocation of NF-κB. The specificity of the activated NF-κB and DNA complexes were demonstrated by supershift assay using anti-p65 antibody (Fig. 3B).
FIG. 3.
Effect of PDTC (50 μM) or TPCK (100 μM) on O. tsutsugamushi-induced MCP-1 mRNA level and NF-κB in HUVEC. (A) MCP-1 mRNA level was analyzed using an RNase protection assay with total RNA samples that were prepared from uninfected cells (Mock), cells infected with O. tsutsugamushi for 3 h (OT), and infected cells in the presence of PDTC (OT + PDTC) or TPCK (OT + TPCK). (B) EMSA was performed to analyze the activation of NF-κB using nuclear extracts prepared from HUVEC treated for 2 h with medium (Mock), L-929 cell lysate (Lysate), or O. tsutsugamushi. Nuclear extracts from cells pretreated with PDTC (OT + PDTC) or TPCK (OT + TPCK) for 1 h before infection with O. tsutsugamushi were also analyzed. (C) In the supershift assay, nuclear extracts from HUVEC were preincubated with antibodies against the p65 subunit of NF-κB.
Effect of the inhibitors of AP-1 activation on the expression of MCP-1 mRNA.
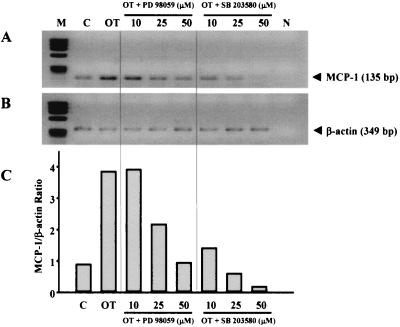
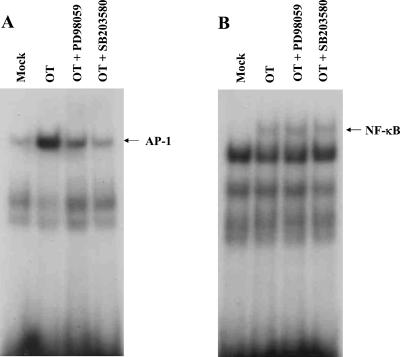
Since infection of O. tsutsugamushi also caused an increase in AP-1 DNA-binding activity, the roles of the MAP kinase pathways able to activate this transcription factor complex were examined using specific inhibitors of MAP kinase pathways. The compound PD 098059 selectively blocks activation of the extracellular signal-related kinase (ERK) kinase MEK1, which in turn prevents activation of ERK1/2 (16), whereas SB 203580 specifically inhibits p38/CSBP kinase activities (33). HUVEC were pretreated with PD 098059 or SB 203580 and thereafter infected with O. tsutsugamushi. As shown in Fig. 4, infection of HUVEC with O. tsutsugamushi in the absence of inhibitors led to a significant increase in MCP-1 mRNA. Addition of PD 098059 or SB 203580 reduced the levels of MCP-1 transcripts following infection with O. tsutsugamushi by 75% (50 μM PD 098059) or by 95% (50 μM SB 203580), respectively (normalized for β-actin). In particular, the levels of MCP-1 transcripts in cells infected in the presence of SB 203580 (25 or 50 μM) were lower than those in cells treated with medium only. These results demonstrate a transcriptional control of MCP-1 by MEK or p38 MAP kinase in O. tsutsugamushi-infected HUVEC. These MAP kinase pathway-related kinases modulate regulation of AP-1 activity through phosphorylation (64). In addition, recent studies have suggested that the p38 MAP kinase pathway may be involved in the activation of NF-κB (5, 63). We therefore investigated the effect of the inhibitors on O. tsutsugamushi-induced AP-1 and NF-κB activation in HUVEC (Fig. 5). HUVEC were treated with PD 098059 (25 μM) or SB 203580 (25 μM) and subsequently infected with O. tsutsugamushi for 1 h. As shown in Fig. 5A, both PD098059 and SB203580 significantly inhibited the O. tsutsugamushi-induced activation of AP-1, as determined by EMSA. In an identical nuclear extract used in AP-1 studies, it was found that PD 098059 and SB 203580 were not able to prevent O. tsutsugamushi-induced NF-κB-DNA binding activity (Fig. 5B), suggesting that inhibition of the MAP kinase pathways did not interfere with release of NF-κB from IκB.
FIG. 4.
Effect of PD 098059 and SB 203580 on MCP-1 induction. (A) HUVEC were pretreated for 1 h with the indicated concentrations of PD 098059 or SB 203580 and then infected with O. tsutsugamushi for 3 h. The mRNA levels of MCP-1 gene were analyzed by semiquantitative RT-PCR in 1.2% agarose gel. (B) The levels of β-actin transcripts were also determined to normalize the transcripts of MCP-1. (C) The band densities were determined with TINA software, and the mRNA expression level of MCP-1 was normalized with respect to the intensities of the bands of the β-actin. Abbreviations: M, ΦX174 DNA digested with HaeIII; C, mock-treated HUVEC; N, PCR-negative control (reactions performed without any cDNA).
FIG. 5.
Effects of PD 098059 and SB 203580 on the activation of NF-κB or AP-1. (A) HUVEC were pretreated for 1 h with PD 098059 (25 μM), SB 203580 (25 μM), or medium and subsequently exposed to medium (mock) or O. tsutsugamushi for 2 h. Nuclear extracts were analyzed by EMSA using a specific 32P-labeled NF-κB consensus oligonucleotide. (B) The nuclear extracts used in panel A were also analyzed for AP-1 activity by EMSA.
Functional analysis of MCP-1 gene regulation.
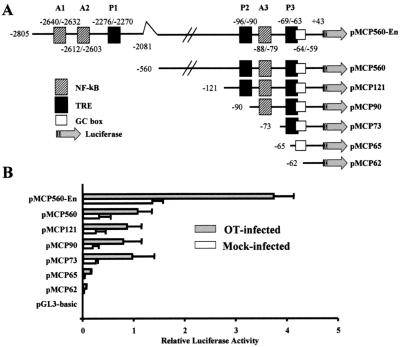
To elucidate the mechanism of MCP-1 transcriptional regulation in HUVEC infected with O. tsutsugamushi, the enhancer region (base −2805 to −2081) and proximal promoter region (base −560 to +43) were cloned into a luciferase reporter plasmid (54). The human MCP-1 gene contains two NF-κB binding sites in an enhancer region (A1 and A2) and one site (A3) in the proximal promoter region. The AP-1 binding site (TRE) is also located in the enhancer region (P1) and in the proximal promoter region (P2 and P3). To investigate which regions are involved in the transcriptional activity of MCP-1 in HUVEC by O. tsutsugamushi, a series of progressive 5′-deletion constructs were generated (Fig. 6A). These constructs were used to transfect HUVEC transiently and then assayed for luciferase activity in the presence or absence of O. tsutsugamushi. As seen in Fig. 6B, HUVEC transfected with pMCP560-En demonstrated a significant increase in luciferase activity at 24 h after infection with O. tsutsugamushi. The induced levels of luciferase activity were twofold to threefold greater than that of uninfected cells. Deletion of the enhancer region resulted in a significant reduction in response to O. tsutsugamushi as well as a basal level of luciferase activity, although the luciferase activities inducible by O. tsutsugamushi were showed in the absence of enhancer region. The deletion of the upstream region of the proximal promoter (position −121 to −560), P2, and A3 did not affect the basal activity and inducible activity of the luciferase gene in response to O. tsutsugamushi infection (Fig. 6B). However, deletion of P3 decreased both basal levels and inducibility of the luciferase gene. Further deletion of the GC box significantly reduced basal and inducible levels.
FIG. 6.
Deletion analysis of the MCP-1 5′-flanking region. (A) A series of clones was used to characterized the MCP-1 promoter region. pMCP560-En contains a proximal promoter region extending from bp +43 to −560 and an enhancer region extending from bp −2081 to −2805 relative to the transcription start site. Numbers indicate nucleotides relative to transcription start site and the first nucleotide inserted into each plasmid DNA. All of the insert DNA extends to nucleotide +43 of the MCP-1 gene. (B) Luciferase activity of HUVEC transfected transiently with clones in panel A. HUVEC were transiently transfected as described and allowed to recover for 18 h. The cells were then stimulated with O. tsutsugamushi (filled bars) or left untreated (open bars) and allowed to incubate for 24 h. Luciferase activity was normalized according to β-galactosidase activity obtained from the pCMV-βgal plasmid which was cotransfected. Results are given as means + standard deviations (error bars) of three independent transfection experiments.
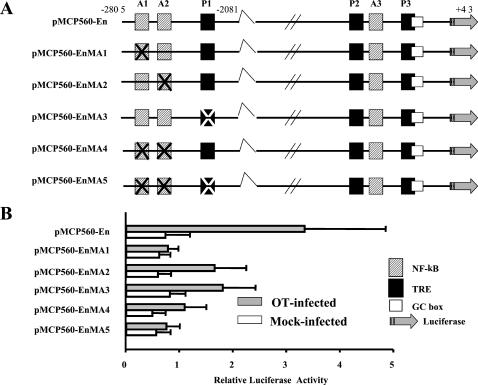
Next, we further investigated whether the two κB-binding sites and a TRE are required for the maximal O. tsutsugamushi induction of the MCP-1 promoter, HUVEC were transfected with plasmids containing mutations in the specific binding sites. A series of mutant constructs were generated (Fig. 7A) and analyzed by transient transfection in HUVEC (Fig. 7B). Site-directed mutagenesis of the κB-binding site located at −2640 to −2632 bp (pMCP560-EnMA1) resulted in an almost complete loss of inducible activity of luciferase in HUVEC infected with O. tsutsugamushi. pMCP560-EnMA2, which has a mutation at another κB-binding site (position −2612 to −2603 bp), and pMCP560-EnMA3, which has a mutation at TRE (position −2276 to −2270), also showed reduced induction of luciferase activity, with a reduction of 50% compared with pMCP560-En. Mutations in all the binding sites also resulted in a decrease in the amount of O. tsutsugamushi-induced luciferase activity. These mutations were not significantly affected the transcriptional activity of uninfected cell.
FIG. 7.
Mutational analysis of transcriptional control elements in the MCP-1 enhancer region. (A) A series of clones was used to characterize the MCP-1 enhancer region. pMCP560-En contains insert DNA as described in the legend to Fig. 6. Nucleotides of the NF-κB consensus site (hatched box) and/or AP-1 consensus site (filled box) in the enhancer region were substituted as described in Material and Methods and are indicated (X). (B) Luciferase activity of HUVEC transfected transiently with clones in panel A. The extracts from cells stimulated with O. tsutsugamushi (closed bars) or left untreated (open bars) were assayed for luciferase activity. Luciferase activity was normalized as described in the legend to Fig. 6. Results are given as means + standard deviations (error bars) of three independent transfection experiments.
DISCUSSION
In human endothelial cells, the regulation of MCP-1 expression has been studied intensively (27, 37, 45, 61). The expression of the MCP-1 gene is controlled in a stimulus-specific manner and involves differential activation of the redox-responsive transcription factors, AP-1 and NF-κB (6, 10, 37, 39, 40). Inflammatory cytokines, including IL-1 and tumor necrosis factor alpha (TNF-α), activate NF-κB and AP-1 and induce the expression of the MCP-1 gene in endothelial cells (37). Induction of the chemokine by infectious pathogens such as Chlamydia pneumoniae or Borrelia burgdorferi also requires the activation NF-κB (17, 40). Membrane microparticles produced by platelets or leukocytes, however, do not activated NF-κB, and the production of MCP-1 may be mediated by the activation of AP-1 in HUVEC (39). Previously, we have also reported that the expression of MCP-1 by O. tsutsugamushi might be independent of the activation of NF-κB and was probably mediated by activation of AP-1 in human microvascular endothelial cell line (10). In this report, we have showed that the induction of MCP-1 by O. tsutsugamushi was not blocked by the inhibitors of NF-κB activation, although the transcription factor was activated by the stimulation of O. tsutsugamushi and the activation of NF-κB was blocked by the inhibitors used in HUVEC (Fig. 3). The levels and kinetics of chemokine transcription were slightly different in the two types of endothelial cells, HUVEC and HMEC (10). The levels of mRNAs for MCP-1 and IL-8 were more highly induced in HUVEC than those in HMEC-1. And the induction of IP-10 mRNAs was barely detectable in HMEC-1. These differences in the induction of chemokine genes might be due to phenotypic heterogeneity between the transformed microvascular endothelial cells and the primary macrovascular endothelial cells. It has been reported that the microvascular endothelial cells are different from macrovascular endothelial cells in many ways (4, 10, 12, 17, 18). The different levels and kinetics of the transcriptional factor activation (NF-κB and AP-1) could also partly explain the different chemokine expression in two types of endothelial cells (10). Although the antioxidant PDTC has been known to increase the DNA-binding activity of AP-1, it inhibits the induction of IL-8, IL-6, and granulocyte-macrophage colony-stimulating factor in a dose-dependent manner by preventing the induction of NF-κB DNA-binding activity by TNF-α (42). The proteasome inhibitors such as TPCK also block NF-κB translocation to the nucleus and the induction of MCP-1 in a range of 5 to 100 μM by IL-1 (45). Our results showed that the inhibitors used were effective in blocking the activation and nuclear translocation of NF-κB in endothelial cells in response to O. tsutsugamushi infection and suggested that the activated NF-κB probably did not contribute the transcriptional induction of MCP-1.
Another transcription factor, AP-1, was also activated as early as 15 min after stimulation in endothelial cells infected with O. tsutsugamushi, and the kinetics of AP-1 activation was well correlated with the induction of MCP-1 gene (Fig. 2B). We, therefore, have tested the effect of the two inhibitors blocking the MAP kinase pathways on the expression on the transcription of MCP-1 gene. There are at least three main groups of MAP kinases: ERK, c-Jun N-terminal kinase (JNK), and p38 (46, 64). In general, the ERKs are strongly activated on stimulation of cells with mitogens such as epidermal growth factor, platelet-derived growth factor, serum, and phorbol-myristate acetate through sequential activation of the upstream kinases Raf and MEK. In contrast to the ERKs, two other subgroups of the MAP kinase family, namely, the p38 and the JNK subgroups, are only weakly activated by mitogens but are highly stimulated on exposure to inflammatory cytokines such as TNF-α and IL-1 and a wide variety of environmental stress inducers such as lipopolysaccharides. The upstream activator of p38 is the dual specificity kinase MKK6, whereas JNK is activated by MKK4/SEK. These members of the MAP kinase family have been reported to regulate the activation of AP-1 (64). The involvement of the MAP kinase pathways and the transcription factor AP-1 in the regulation of MCP-1 gene in endothelial cells has been tested using the specific inhibitors (15, 38, 53). The transcription of MCP-1 is inhibited by the p38 MAP kinase inhibitor, SB 203580, in endothelial cells stimulated by homocysteine (53) or by the ERK inhibitor, PD 098059, in cells activated by vascular endothelial cell growth factor (38). In HUVEC interacted with activated platelets, the secretion of MCP-1 and monocyte-endothelium adhesion was reduced by SB 203580, but not by PD 098059 (15). These selective activations of the MAP kinase pathway according to the stimulating agents could partly explain the differential regulation of the chemokine gene in endothelial cells. When the MAP kinase inhibitors PD 098059 and SB 203580 were treated in HUVEC, the levels of O. tsutsugamushi-induced MCP-1 transcripts were reduced in a dose-dependent manner (Fig. 4). In addition, the activation of AP-1 induced by O. tsutsugamushi infection was significantly suppressed by the treatment of the inhibitors (Fig. 5A). These results demonstrated that the activation of ERK and p38 MAP kinases is necessary for both the activation of AP-1 and MCP-1 induction by O. tsutsugamushi, but the p38 MAP kinase pathway has a more potent effect on the transcriptional regulation of the gene. In agreement with previous reports indicating that this signaling pathway is required for the activation of AP-1 (64), we have shown that O. tsutsugamushi induction of AP-1 requires activation of the ERK and p38 MAP kinases pathways. Therefore, the effect of PD 098059 and SB 203580 on MCP-1 mRNA (Fig. 4) is probably due in part a decrease in the abundance of the AP-1 components c-Fos and c-Jun. Because there is no available specific inhibitor for JNK pathway, we could not investigate the involvement of the JNK pathway in the activation of AP-1 and induction of MCP-1 in response to O. tsutsugamushi infection. We also studied the MAP kinase pathways, as recent studies have suggested their involvement in the regulation of NF-κB (63). In contrast to the case of AP-1, inhibition of MAP kinase pathways had no effect on NF-κB-DNA binding activity as detected by EMSA in HUVEC infected with O. tsutsugamushi (Fig. 5B). Recently, several investigators have shown that p38 MAP kinase inhibitors (SB 203580 or SB 202190) or ERK kinase inhibitor (PD 098059) are capable of blocking the activation of AP-1 and subsequent induction of cytokines or adhesion molecules (24, 30, 32, 57). In particular, Lawson et al. have suggested that the expression of vascular cell adhesion molecule 1 in endothelial cells by ligation of intercellular adhesion molecule 1 is probably mediated by the activation of AP-1 and NF-κB-independent mechanism (32).
In the functional analysis of the MCP-1 promoter region, however, κB-binding sites in the enhancer region were required for the induction of reporter luciferase activity by O. tsutsugamushi infection (Fig. 7). This functional analysis was originated from the assay performed by Ueda et al. (54). They have suggested that the κB-binding site located between bp −2612 and −2603 was important for cytokines or mitogen-induced enhancer activity in diverse types of cells. Recently, Goebeler et al. have also reported that the mutations in the κB-binding sites in the enhancer region resulted in complete loss of promoter inducibility in HUVEC stimulated with NiCl2 (27). They have suggested that MCP-1 induction in endothelial cells requires activation of the IKKβ/IκBα/NF-κB signaling pathway, resulting in nuclear accumulation of p65 and subsequent recruitment of cofactors, and proper assembly and activity of this transcriptional complex is further modulated by the p38 MAP kinase cascade and a phospholipase C-dependent pathway (27). It seems likely that multiple signaling pathways are activated in endothelial cells stimulated with environmental stresses and the activation of endothelial cells is modulated by the different sets of transcription factors activated by the signaling pathways. Here, we found that the κB-binding site in the enhancer region of MCP-1 gene was important for the inducible induction of MCP-1 by O. tsutsugamushi infection, although AP-1 binding sites in proximal promoter region (position −69 to −63) and enhancer region (position −2276 to −2270) were also required for the inducible expression of MCP-1 in HUVEC. The requirement of the κB-binding site for the inducible expression of MCP-1 might be contrary to the effects of the inhibitors of NF-κB activation. We have tried to investigate the effect of the inhibitors on the reporter gene expression using transient-transfection assay, but the viability of HUVEC was markedly reduced by the chemicals used after transfection, and it was very difficult to assay the effect of the inhibitors. However, these conflicting results could be explained partly by the fact that the transcriptional regulation in promoter regions of many genes integrated in chromosome is different from those of the genes used in transient transfection assays (14, 22, 49, 56, 60). Alteration of chromatin structures by histone modification and subsequent formation of appropriate transcriptional complex are required in the transcriptional activation (8). Recently, some reports have also suggested that histone acetyltransferase activity of cyclic AMP-responsive element binding protein-binding protein or the activation of TFIID are required for the formation of active transcription complex in NF-κB-dependent transcription (7, 50, 58). In the case of the transcriptional regulation of MCP-1 gene, the chromatin structure is also one of the important regulatory mechanisms for the expression of the gene (20). Furthermore, transcriptional coactivators such as p300 or p90 may be involved in the formation of active enhanceosome formation in the NF-κB-mediated transcriptional regulation of MCP-1 gene (23, 27). Hence, appropriate nucleosomal structure is probably the reason why transient transfections of reporter gene construct harboring the promoter region resulted in a different effect of the transcription factor, NF-κB, although the possible involvement of activated NF-κB in the expression of MCP-1 gene could not be excluded thoroughly in endothelial cells infected with O. tsutsugamushi. Interestingly, the role of AP-1 in the alteration of the nucleosome is suggested to be critical in the transcriptional activation (44). Ng et al. have reported that the binding of Fos/Jun to a single high-affinity site incorporated into an acetylated nucleosome resulted in the complete disruption of nucleosomal structure without histone displacement, and this disruption was sufficient to facilitate the subsequent binding of a second transcription factor (44). Furthermore, the DNase I-hypersensitive region, containing an AP-1 binding site, located downstream of the polyadenylation site of the MCP-1 gene and the heterodimerization pattern of AP-1 seem to play an important role for the regulation of MCP-1 gene expression in human fibroblast (20). Wang et al. also reported that adenovirus-mediated overexpression of AP-1 induced MCP-1 in human endothelial cells without affecting the level of NF-κB (61). Taken together, AP-1 activation could circumvent the NF-κB pathway by differential signal transduction pathways, leading to the induction of the MCP-1 gene, although further studies are required to investigate the precise signaling pathways mediated through MAP kinases and the transcriptional complex involving the activated AP-1.
In conclusion, for the first time, we have reported that the expression of the MCP-1 gene in human endothelial cells infected with the intracellular bacterium O. tsutsugamushi is primarily affected by MAP kinase pathways, leading to the activation of AP-1, although it remains to be determined how the activation of NF-κB affects the transcription of MCP-1. Previously, we have also reported that the induction of MCP-1 in HMEC-1 might be an NF-κB-independent mechanism, probably mediated by the activation of AP-1 (10). Our present results suggest that the activation of AP-1 by O. tsutsugamushi infection might play a pivotal role in the activation of endothelial cells and the transcriptional induction of inflammatory genes such as MCP-1 and suggest that the activation of endothelial cells by O. tsutsugamushi is mediated by signaling pathways different from those that mediate other environmental stimuli. These differences resell in the differential activation of signal transduction pathways and transcription factors and thus the differential inflammatory responses.
Acknowledgments
This study was supported by a grant of the Korea Health 21 R&D Project, Ministry of Health & Welfare (grant 01-PJ10-PG6-01GM01-0004).
Editor: R. N. Moore
REFERENCES
- 1.Allen, A. C., and S. Spitz. 1945. A comparative study of the pathology of scrub typhus (tsutsugamushi disease) and other rickettsial disease. Am. J. Pathol. 21:603-681. [PMC free article] [PubMed] [Google Scholar]
- 2.Baggiolini, M., B. Dewald, and B. Moser. 1994. Interleukin-8 and related chemotactic cytokines-CXC and CC chemokines. Adv. Immunol. 55:97-179. [PubMed] [Google Scholar]
- 3.Baldwin, A. S., Jr. 1996. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu. Rev. Immunol. 14:649-683. [DOI] [PubMed] [Google Scholar]
- 4.Beilke, M. A. 1989. Vascular endothelium in immunology and infectious disease. Rev. Infect. Dis. 11:273-283. [DOI] [PubMed] [Google Scholar]
- 5.Beyaert, R., A. Cuenda, W. Vanden Berghe, S. Plaisance, J. C. Lee, G. Haegeman, P. Cohen, and W. Fiers. 1996. The p38/RK mitogen-activated protein kinase pathway regulates interleukin-6 synthesis response to tumor necrosis factor. EMBO J. 15:1914-1923. [PMC free article] [PubMed] [Google Scholar]
- 6.Bouloumie, A., T. Marumo, M. Lafontan, and R. Busse. 1999. Leptin induces oxidative stress in human endothelial cells. FASEB J. 13:1231-1238. [PubMed] [Google Scholar]
- 7.Carter, A. B., K. L. Knudtson, M. M. Monick, and G. W. Hunninghake. 1999. The p38 mitogen-activated protein kinase is required for NF-κB-dependent gene expression. The role of TATA-binding protein (TBP). J. Biol. Chem. 274:30858-30863. [DOI] [PubMed] [Google Scholar]
- 8.Cheung, P., C. D. Allis, and P. Sassone-Corsi. 2001. Signaling to chromatin through histone modifications. Cell 103:263-271. [DOI] [PubMed] [Google Scholar]
- 9.Chi, W. C., J. J. Huang, J. M. Sung, R. R. Lan, W. C. Ko, and F. F. Chen. 1997. Scrub typhus associated with multiorgan failure: a case report. Scand. J. Infect. Dis. 29:634-635. [DOI] [PubMed] [Google Scholar]
- 10.Cho, N. H., S. Y. Seong, M. S. Choi, and I. S. Kim. 2001. Expression of chemokine genes in Human dermal microvascular endothelial cell lines infected with Orientia tsutsugamushi. Infect. Immun. 69:1265-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho, N. H., S. Y. Seong, M. S. Huh, T. H. Han, Y. S. Koh, M. S. Choi, and I. S. Kim. 2000. Expression of chemokine genes in murine macrophages infected with Orientia tsutsugamushi. Infect. Immun. 68:594-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cines, D. B., E. S. Pollak, C. A. Buck, J. Loscalzo, G. A. Zimmerman, R. P. McEver, J. S. Pober, T. M. Wick, B. A. Konkle, B. S. Schwartz, E. S. Barnathan, K. R. McCrae, B. A. Hug, A. M. Schmidt, and D. M. Stern. 1998. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 91:3527-3561. [PubMed] [Google Scholar]
- 13.Cobb, R. R., K. A. Felts, G. C. Parry, and N. Mackman. 1996. Proteasome inhibitors block VCAM-1 and ICAM-1 gene expression in endothelial cells without affecting nuclear translocation of nuclear factor-kappa B. Eur. J. Immunol. 26:839-845. [DOI] [PubMed] [Google Scholar]
- 14.Cook, J. L., Z. Zhang, J. Alam, and R. N. Re. 1999. Effects of chromosomal integration site upon p53 interactions with DNA consensus sequence homologies. Oncogene 18:2373-2379. [DOI] [PubMed] [Google Scholar]
- 15.Dickfeld, T., E. Lengyel, A. E. May, S. Massberg, K. Brand, S. Page, C. Thielen, K. Langenbrink, and M. Gawaz. 2001. Transient interaction of activated platelets with endothelial cells induces expression of monocyte-chemoattractant protein-1 via a p38 mitogen-activated protein kinase mediated pathway. Implications for atherogenesis. Cardiovasc. Res. 49:189-199. [DOI] [PubMed] [Google Scholar]
- 16.Dudley, D. T., L. Pang, S. J. Decker, A. J. Bridges, and A. R. Saltiel. 1995. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc. Natl. Acad. Sci. USA 92:7686-7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebnet, K., K. D. Brown, U. K. Siebenlist, M. M. Simon, and S. Shaw. 1997. Borrelia burgdorferi activates nuclear factor-kappa B and is a potent inducer of chemokine and adhesion molecule gene expression in endothelial cells and fibroblasts. J. Immunol. 158:3285-3292. [PubMed] [Google Scholar]
- 18.Ebnet, K., M. M. Simon, and S. Shaw. 1996. Regulation of chemokine gene expression in human endothelial cells by proinflammatory cytokines and Borrelia burgdorferi. Ann. N. Y. Acad. Sci. 797:107-117. [DOI] [PubMed] [Google Scholar]
- 19.Felgner, J. H., R. Kumar, C. N. Sridhar, C. J. Wheeler, Y. J. Tsai, R. Border, P. Ramsey, M. Martin, and P. L. Felgner. 1994. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. J. Biol. Chem. 269:2550-2561. [PubMed] [Google Scholar]
- 20.Finzer, P., U. Soto, H. Delius, A. Patzelt, J. F. Coy, A. Poustka, H. zur Hausen, and F. Rosl. 2001. Differential transcriptional regulation of the monocyte-chemoattractant protein-1 (MCP-1) gene in tumorigenic and non-tumorigenic HPV 18 positive cells: the role of the chromatin structure and AP-1 composition. Oncogene 19:3235-3244. [DOI] [PubMed] [Google Scholar]
- 21.Foletta, V. C., D. H. Segal, and D. R. Cohen. 1998. Transcriptional regulation in the immune system: all roads lead to AP-1. J. Leukoc. Biol. 63:139-152. [DOI] [PubMed] [Google Scholar]
- 22.Frenkel, B., M. Montecino, J. Green, F. Aslam, R. Desai, C. Banerjee, J. L. Stein, J. B. Lian, and G. S. Stein. 1996. Basal and vitamin D-responsive activity of the rat osteocalcin promoter in stably transfected osteosarcoma cells: requirement of upstream sequences for control by the proximal regulatory domain. Endocrinology 137:1080-1088. [DOI] [PubMed] [Google Scholar]
- 23.Freter, R. R., J. A. Alberta, G. Y. Hwang, A. L. Wrentmore, and C. D. Stiles. 1996. Platelet-derived growth factor induction of the immediate-early gene MCP-1 is mediated by NF-kappaB and a 90-kDa phosphoprotein coactivator. J. Biol. Chem. 271:17417-17424. [DOI] [PubMed] [Google Scholar]
- 24.Garcia, J., B. Lemercier, S. Roman-Roman, and G. Rawadi. 1998. A Mycoplasma fermentans-derived synthetic lipopeptide induces AP-1 and NF-kappaB activity and cytokine secretion in macrophages via the activation of mitogen-activated protein kinase pathways. J. Biol. Chem. 273:34391-34398. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16:225-260. [DOI] [PubMed] [Google Scholar]
- 26.Gimbrone, M. A., Jr., R. S. Cotran, and J. Folkman. 1974. Human vascular endothelial cells in culture. Growth and DNA synthesis. J. Cell Biol. 60:673-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goebeler, M., R. Gillitzer, K. Kilian, K. Utzel, E. B. Brocker, U. R. Rapp, and S. Ludwig. 2001. Multiple signaling pathways regulate NF-kappaB-dependent transcription of the monocyte chemoattractant protein-1 gene in primary endothelial cells. Blood 97:46-55. [DOI] [PubMed] [Google Scholar]
- 28.Goebeler, M., K. Kilian, R. Gillitzer, M. Kunz, T. Yoshimura, E. B. Brocker, U. R. Rapp, and S. Ludwig. 1999. The MKK6/p38 stress kinase cascade is critical for tumor necrosis factor-alpha-induced expression of monocyte-chemoattractant protein-1 in endothelial cells. Blood 93:857-865. [PubMed] [Google Scholar]
- 29.Ho, S. N., H. D. Hunt, R. M. Horton, J. K. Pullen, and L. R. Pease. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51-59. [DOI] [PubMed] [Google Scholar]
- 30.Hobbie, S., L. M. Chen, R. J. Davis, and J. E. Galan. 1997. Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J. Immunol. 159:5550-5559. [PubMed] [Google Scholar]
- 31.Jalali, S., Y. S. Li, M. Sotoudeh, S. Yuan, S. Li, S. Chien, and J. Y. Shyy. 1998. Shear stress activates p60src-Ras-MAPK signaling pathways in vascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 18:227-234. [DOI] [PubMed] [Google Scholar]
- 32.Lawson, C., M. Ainsworth, M. Yacoub, and M. Rose. 1999. Ligation of ICAM-1 on endothelial cells leads to expression of VCAM-1 via a nuclear factor-κB-independent mechanism. J. Immunol. 162:2990-2996. [PubMed] [Google Scholar]
- 33.Lee, J. C., and P. R. Young. 1996. Role of CSB/p38/RK stress response kinase in LPS and cytokine signaling mechanisms. J. Leukoc. Biol. 59:152-157. [DOI] [PubMed] [Google Scholar]
- 34.Leonard, E. J., and T. Yoshimura. 1990. Human monocyte chemoattractant protein-1 (MCP-1). Immunol. Today 11:97-101. [DOI] [PubMed] [Google Scholar]
- 35.Locati, M., and P. M. Murphy. 1999. Chemokines and chemokine receptors: biology and clinical relevance in inflammation and AIDS. Annu. Rev. Med. 50:425-440. [DOI] [PubMed] [Google Scholar]
- 36.Mackman, N. 1994. Protease inhibitors block lipopolysaccharide induction of tissue factor gene expression in human monocytic cells by preventing activation of c-Rel/p65 heterodimers. J. Biol. Chem. 269:26363-26367. [PubMed] [Google Scholar]
- 37.Martin, T., P. M. Cardarelli, G. C. Parry, K. A. Felts, and R. R. Cobb. 1997. Cytokine induction of monocyte chemoattractant protein-1 gene expression in human endothelial cells depends on the cooperative action of NF-kappa B and AP-1. Eur. J. Immunol. 27:1091-1097. [DOI] [PubMed] [Google Scholar]
- 38.Marumo, T., V. B. Schini-Kerth, and R. Busse. 1999. Vascular endothelial growth factor activates nuclear factor-kappaB and induces monocyte chemoattractant protein-1 in bovine retinal endothelial cells. Diabetes 48:1131-1137. [DOI] [PubMed] [Google Scholar]
- 39.Mesri, M., and D. C. Altieri. 1999. Leukocyte microparticles stimulate endothelial cell cytokine release and tissue factor induction in a JNK1 signaling pathway. J. Biol. Chem. 274:23111-23118. [DOI] [PubMed] [Google Scholar]
- 40.Molestina, R. E., R. D. Miller, A. B. Lentsch, J. A. Ramirez, and J. T. Summersgill. 2001. Requirement for NF-κB in transcriptional activation of monocyte chemotactic protein 1 by Chlamydia pneumoniae in human endothelial cells. Infect. Immun. 68:4282-4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Molestina, R. E., R. D. Miller, J. A. Ramirez, and J. T. Summersgill. 1999. Infection of human endothelial cells with Chlamydia pneumoniae stimulates transendothelial migration of neutrophils and monocytes. Infect. Immun. 67:1323-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Munoz, C., D. Pascual-Salcedo, M. C. Castellanos, A. Alfranca, J. Aragones, A. Vara, M. J. Redondo, and M. O. de Landazuri. 1996. Pyrrolidine dithiocarbamate inhibits the production of interleukin-6, interleukin-8, and granulocyte-macrophage colony-stimulating factor by human endothelial cells in response to inflammatory mediators: modulation of NF-kappa B and AP-1 transcription factors activity. Blood 88:3482-3490. [PubMed] [Google Scholar]
- 43.Murao, K., T. Ohyama, H. Imachi, T. Ishida, W. M. Cao, H. Namihira, M. Sato, N. C. Wong, and J. Takahara. 2000. TNF-alpha stimulation of MCP-1 expression is mediated by the Akt/PKB signal transduction pathway in vascular endothelial cells. Biochem. Biophys. Res. Commun. 276:791-796. [DOI] [PubMed] [Google Scholar]
- 44.Ng, K. W., P. Ridgway, D. R. Cohen, and D. J. Tremethick. 1997. The binding of a Fos/Jun heterodimer can completely disrupt the structure of a nucleosome. EMBO J. 16:2072-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parry, G. C., T. Martin, K. A. Felts, and R. R. Cobb. 1998. IL-1beta-induced monocyte chemoattractant protein-1 gene expression in endothelial cells is blocked by proteasome inhibitors. Arterioscler. Thromb. Vasc. Biol. 18:934-940. [DOI] [PubMed] [Google Scholar]
- 46.Robinson, M. J., and M. H. Cobb. 1997. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 9:180-186. [DOI] [PubMed] [Google Scholar]
- 47.Roebuck, K. A., L. R. Carpenter, V. Lakshminarayanan, S. M. Page, J. N. Moy, and L. L. Thomas. 1999. Stimulus-specific regulation of chemokine expression involves differential activation of the redox-responsive transcription factors AP-1 and NF-κB. J. Leukoc. Biol. 65:291-298. [DOI] [PubMed] [Google Scholar]
- 48.Rovin, B. H., T. Yoshiumura, and L. Tan. 1992. Cytokine-induced production of monocyte chemoattractant protein-1 by cultured human mesangial cells. J. Immunol. 148:2148-2153. [PubMed] [Google Scholar]
- 49.Schreiber, K. L., A. Calderone, and H. Rindt. 2001. Distant upstream regulatory domains direct high levels of beta-myosin heavy chain gene expression in differentiated embryonic stem cells. J. Mol. Cell Cardiol. 32:585-598. [DOI] [PubMed] [Google Scholar]
- 50.Sheppard, K. A., D. W. Rose, Z. K. Haque, R. Kurokawa, E. McInerney, S. Westin, D. Thanos, M. G. Rosenfeld, C. K. Glass, and T. Collins. 1999. Transcriptional activation by NF-κB requires multiple coactivators. Mol. Cell. Biol. 19:6367-6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shyy, J. Y., M. C. Lin, J. Han, Y. Lu, M. Petrime, and S. Chien. 1995. The cis-acting phorbol ester “12-O-tetradecanoylphorbol 13-acetate”-responsive element is involved in shear stress-induced monocyte chemotactic protein 1 gene expression. Proc. Natl. Acad. Sci. USA 92:8069-8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shyy, Y. J., Y. S. Li, and P. E. Kolattukudy. 1993. Activation of MCP-1 gene expression is mediated through multiple signaling pathways. Biochem. Biophys. Res. Commun. 192:693-699. [DOI] [PubMed] [Google Scholar]
- 53.Sung, F. L., Y. L. Slow, G. Wang, E. G. Lynn, and O. Karmin. 2001. Homocysteine stimulates the expression of monocyte chemoattractant protein-1 in endothelial cells leading to enhanced monocyte chemotaxis. Mol. Cell Biochem. 216:121-128. [DOI] [PubMed] [Google Scholar]
- 54.Ueda, A., K. Okuda, S. Ohno, A. Shirai, T. Igarashi, K. Matsunaga, J. Fukushima, S. Kawamoto, Y. Ishigatsubo, and T. Okubo. 1994. NF-kappa B and Sp1 regulate transcription of the human monocyte chemoattractant protein-1 gene. J. Immunol. 153:2052-2063. [PubMed] [Google Scholar]
- 55.Vaddi, K., M. Keller, and R. C. Newton. 1997. The chemokine FactsBook. Academic Press, Inc., New York, N.Y.
- 56.Valerie, K., A. Singhal, J. C. Kirkham, W. S. Laster, and M. Rosenberg. 1995. Activation of human immunodeficiency virus gene expression by ultraviolet light in stably transfected human cells does not require the enhancer element. Biochemistry 34:15760-15767. [DOI] [PubMed] [Google Scholar]
- 57.Vallejo, J. G., P. Knuefermann, D. L. Mann, and N. Sivasubramanian. 2001. Group B Streptococcus induces TNF-alpha gene expression and activation of the transcription factors NF-kappa B and activator protein-1 in human cord blood monocytes. J. Immunol. 165:419-425. [DOI] [PubMed] [Google Scholar]
- 58.Vanden Berghe, W., K. De Bosscher, E. Boone, S. Plaisance, and G. Haegeman. 1999. The nuclear factor-κB engages CBP/p300 and histone acetyltransferase activity for transcriptional activation of the interleukin-6 gene promoter. J. Biol. Chem. 274:32091-32098. [DOI] [PubMed] [Google Scholar]
- 59.Villiger, P. M., R. Terkeltaub, and M. Lotz. 1992. Production of monocyte chemoattractant protein-1 by inflamed synovial tissue and cultured synoviocytes. J. Immunol. 149:722-727. [PubMed] [Google Scholar]
- 60.Walters, M. C., S. Fiering, J. Eidemiller, W. Magis, M. Groudine, and D. I. Martin. 1995. Enhancers increase the probability but not the level of gene expression. Proc. Natl. Acad. Sci. USA 92:7125-7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang, N., L. Verna, S. Hardy, J. Forsayeth, Y. Zhu, and M. B. Stemerman. 1999. Adenovirus-mediated overexpression of c-Jun and c-Fos induces intercellular adhesion molecule-1 and monocyte chemoattractant protein-1 in human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 19:2078-2084. [DOI] [PubMed] [Google Scholar]
- 62.Watt, G., and D. Strickman. 1994. Life-threatening scrub typhus in a traveler returning from Thailand. Clin. Infect. Dis. 18:624-626. [DOI] [PubMed] [Google Scholar]
- 63.Wesselborg, S., M. K. A. Bauer, M. Vogt, M. L. Schmitz, and K. Schulze-Osthoff. 1997. Activation of transcription factor NF-κB and p38 mitogen-activated protein kinase is mediated by distinct and separate stress effector pathways. J. Biol. Chem. 272:12422-12429. [DOI] [PubMed] [Google Scholar]
- 64.Whitmarsh, A. J., and R. J. Davis. 1996. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. 74:589-607. [DOI] [PubMed] [Google Scholar]
- 65.Yla-Herttuala, S., B. A. Lipton, M. E. Rosenfeld, T. Sarkioja, T. Yoshimura, E. J. Leonard, J. L. Witztum, and D. Steinberg. 1991. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc. Natl. Acad. Sci. USA 88:5252-5256. [DOI] [PMC free article] [PubMed] [Google Scholar]