Abstract
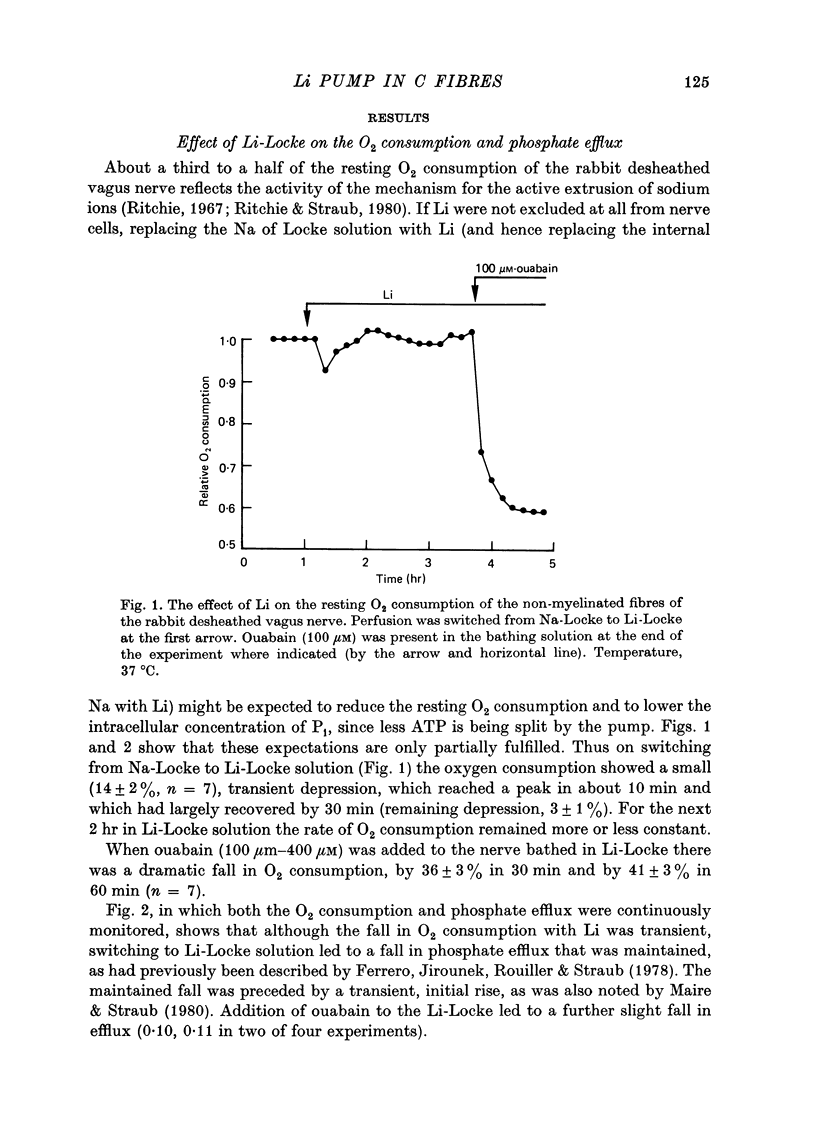
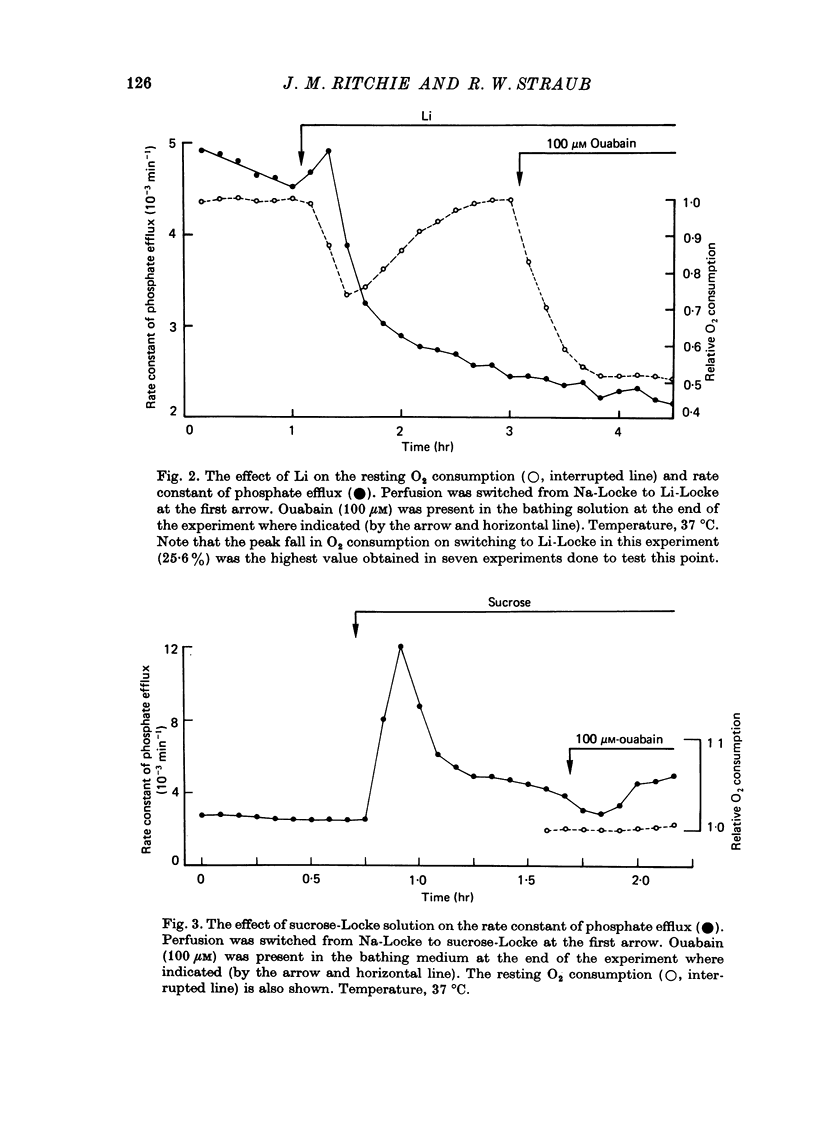
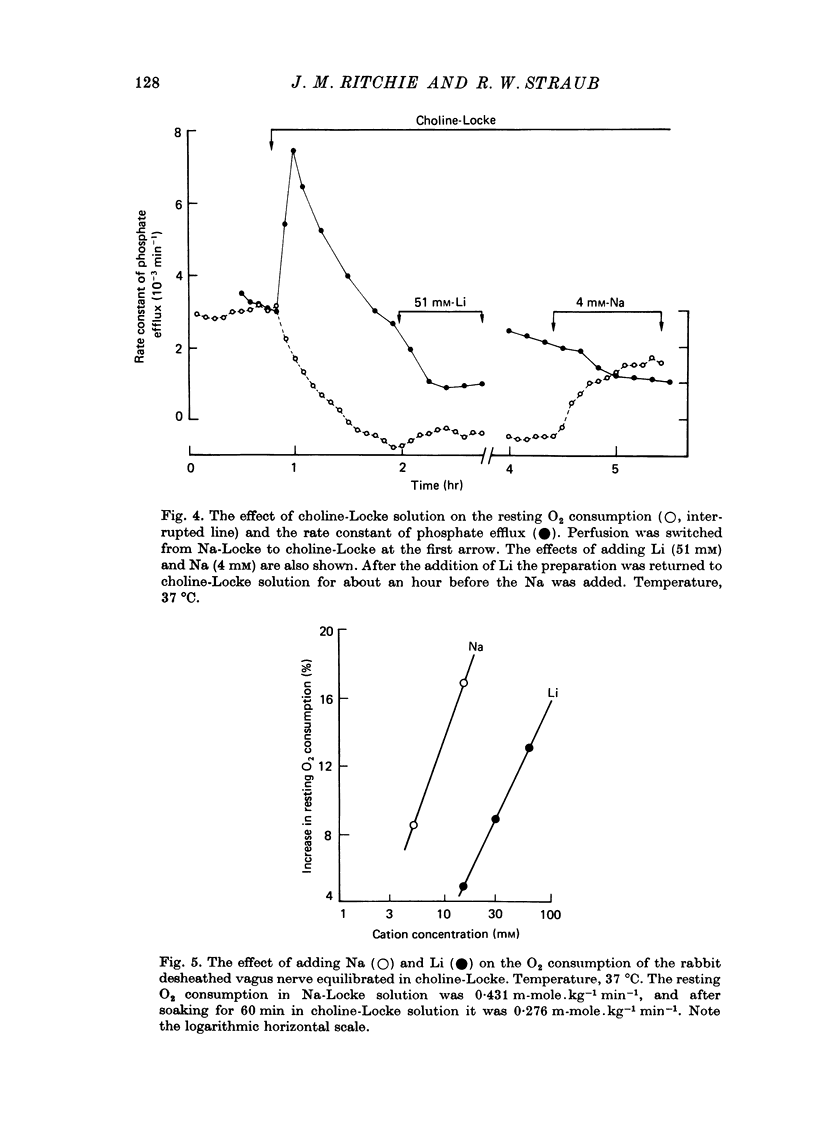
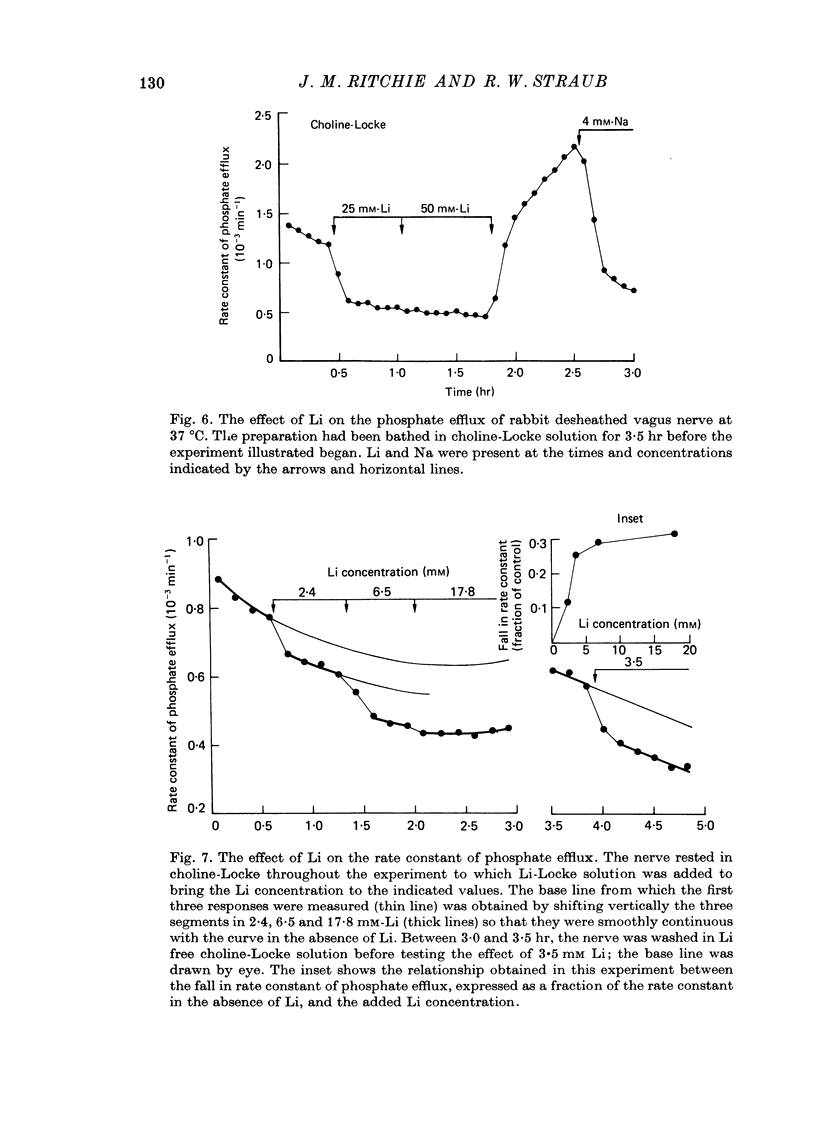
1. A study has been made of the O2 consumption, and the corresponding efflux of labelled phosphate, from the non-myelinated fibres of the desheathed rabbit vagus nerve at 37 degrees C in Locke solutions in which various ions were substituted for Na, and also in the presence of ouabain. 2. Switching from Na-Locke solution to Li-Locke solution produced a small transient decrease in the resting O2 consumption (of 14%), which rapidly recovered to its original value. This was accompanied by an initial brief rise followed by a maintained fall in the resting phosphate efflux. 3. In Li-Locke solution, ouabain (100 microM) produced a fall in the resting O2 consumption of 40%, i.e. similar to that produced in Na-Locke solution. Any depression of the resting phosphate efflux was absent or small. 4. In choline-Locke solution, in Tris-Locke solution, in K-Locke solution or in sucrose-Locke solution the resting O2 consumption, which fell by 30-40%, was insensitive to the addition of ouabain (100 microM). 5. Addition of either Na ions or of Li ions partially restored respiration in choline-Locke solution, Li being an order of magnitude less effective than Na. 6. In choline-Locke solution the internal K content was not affected by ouabain. However, if Li (77 mM) was present in the bathing solution ouabain (100 microM) produced a 30% fall in the internal K content. 7. It is concluded that these effects of Li, and their alteration by ouabain, reflect the activity of a mechanism for the active extrusion of Li ions. It is suggested that the mechanism for the active extrusion of Li is the same as that for Na. 8. There also seems to be a site for Li that controls the phosphate efflux and which is half-maximally activated with external Li concentrations of about 2-4 mM.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armett C. J., Ritchie J. M. On the permeability of mammalian non-myelinated fibres to sodium and to lithium ions. J Physiol. 1963 Jan;165(1):130–140. doi: 10.1113/jphysiol.1963.sp007047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaugé L. Activation by lithium ions of the inside sodium sites in (Na+ + K+)-ATPase. Biochim Biophys Acta. 1978 Dec 8;527(2):472–484. doi: 10.1016/0005-2744(78)90361-3. [DOI] [PubMed] [Google Scholar]
- Duhm J., Becker B. F. Studies on the lithium transport across the red cell membrane. II. Characterization of ouabain-sensitive and ouabain-insensitive Li+ transport. Effects of bicarbonate and dipyridamole. Pflugers Arch. 1977 Jan 17;367(3):211–219. doi: 10.1007/BF00581357. [DOI] [PubMed] [Google Scholar]
- Dunham P. B., Senyk O. Lithium efflux through the Na/K pump in human erythrocytes. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3099–3103. doi: 10.1073/pnas.74.7.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero J., Jirounek P., Rouiller M., Straub R. W. Efflux of inorganic phosphate from mammalian non-myelinated nerve fibres. J Physiol. 1978 Sep;282:507–519. doi: 10.1113/jphysiol.1978.sp012478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth J. V., Keynes R. D., Ritchie J. M. The origin of the initial heat associated with a single impulse in mammalian non-myelinated nerve fibres. J Physiol. 1968 Feb;194(3):745–793. doi: 10.1113/jphysiol.1968.sp008434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., SWAN R. C. The permeability of frog muscle fibres to lithium ions. J Physiol. 1959 Oct;147:626–638. doi: 10.1113/jphysiol.1959.sp006265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Ritchie J. M. The movements of labelled ions in mammalian non-myelinated nerve fibres. J Physiol. 1965 Jul;179(2):333–367. doi: 10.1113/jphysiol.1965.sp007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maire J. C., Straub R. W. Release of inorganic phosphate during activity in mammalian non-myelinated nerve fibres. J Physiol. 1980 Jul;304:135–143. doi: 10.1113/jphysiol.1980.sp013315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RITCHIE J. M., STRAUB R. W. The hyperpolarization which follows activity in mammalian non-medullated fibres. J Physiol. 1957 Apr 3;136(1):80–97. doi: 10.1113/jphysiol.1957.sp005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. On the electrogenic sodium pump in mammalian non-myelinated nerve fibres and its activation by various external cations. J Physiol. 1968 May;196(1):183–221. doi: 10.1113/jphysiol.1968.sp008502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. The dependence on external cations of the oxygen consumption of mammalian non-myelinated fibres at rest and during activity. J Physiol. 1968 May;196(1):163–181. doi: 10.1113/jphysiol.1968.sp008501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie J. M., Straub R. W. Oxygen consumption and phosphate efflux in mammalian non-myelinated nerve fibres. J Physiol. 1980 Jul;304:109–121. doi: 10.1113/jphysiol.1980.sp013313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie J. M., Straub R. W. Phosphate efflux and oxygen consumption in small non-myelinated nerve fibres at rest and during activity. J Physiol. 1979 Feb;287:315–327. doi: 10.1113/jphysiol.1979.sp012661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie J. M. The oxygen consumption of mammalian non-myelinated nerve fibres at rest and during activity. J Physiol. 1967 Feb;188(3):309–329. doi: 10.1113/jphysiol.1967.sp008141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOU M. Biology and pharmacology of the lithium ion. Pharmacol Rev. 1957 Mar;9(1):17–58. [PubMed] [Google Scholar]
- Thomas R. C., Simon W., Oehme M. Lithium accumulation by snail neurones measured by a new Li+-sensitive microelectrode. Nature. 1975 Dec 25;258(5537):754–756. doi: 10.1038/258754a0. [DOI] [PubMed] [Google Scholar]
- Wespi H. H. Active transport and passive fluxes of K, Na, and Li in mammalian non-myelinated nerve fibres. Pflugers Arch. 1969;306(3):262–280. doi: 10.1007/BF00592437. [DOI] [PubMed] [Google Scholar]
- Whittam R., Ager M. E. The connexion between active cation transport and metabolism in erythrocytes. Biochem J. 1965 Oct;97(1):214–227. doi: 10.1042/bj0970214. [DOI] [PMC free article] [PubMed] [Google Scholar]


