Abstract
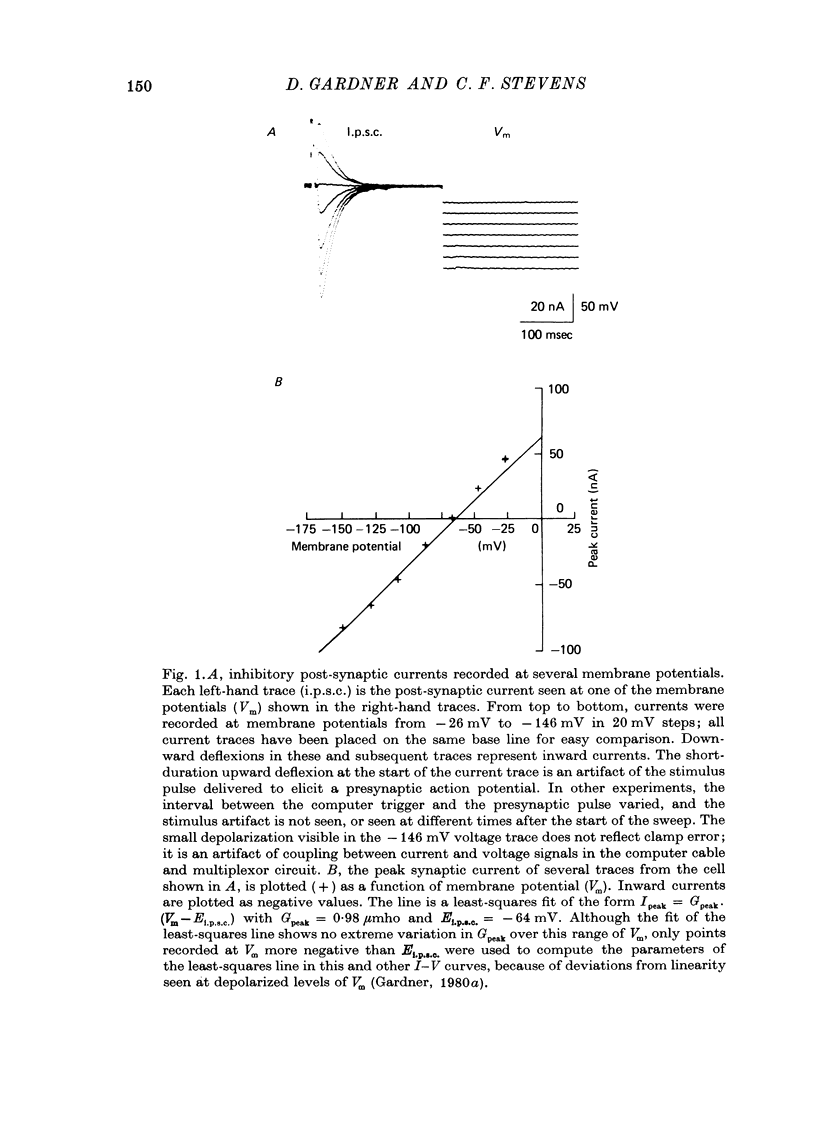
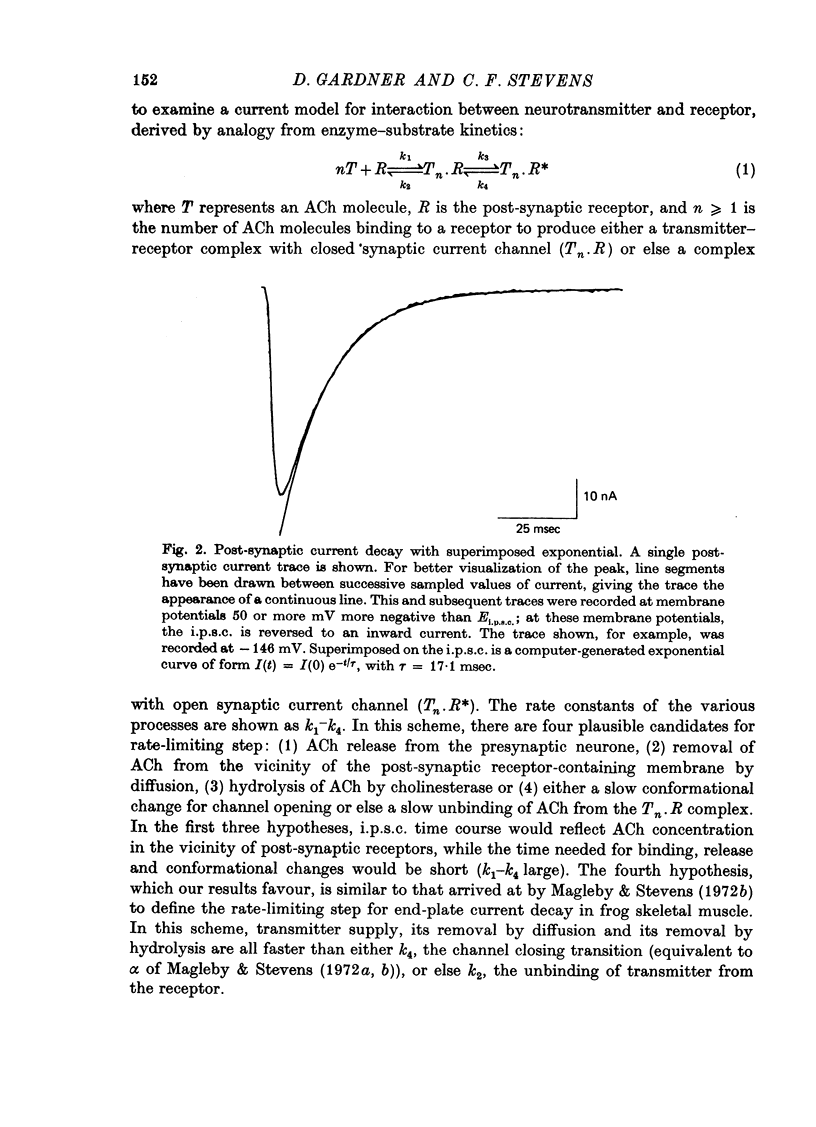
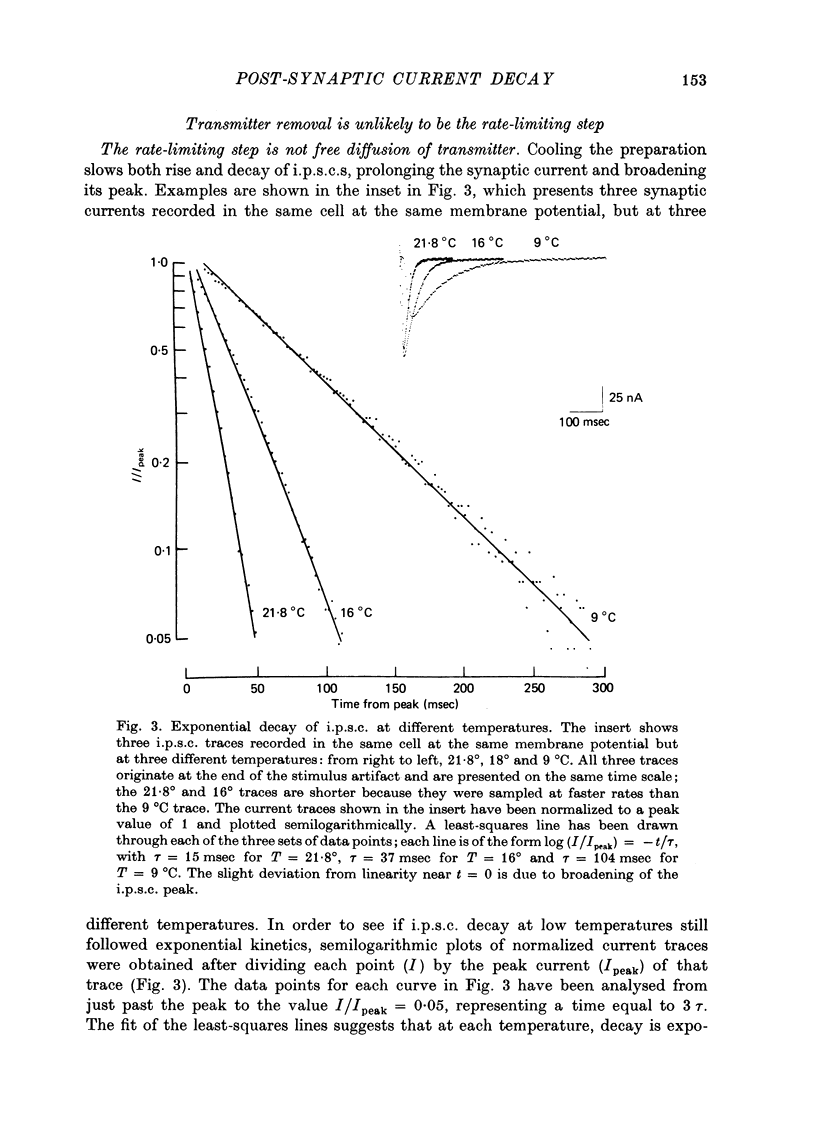
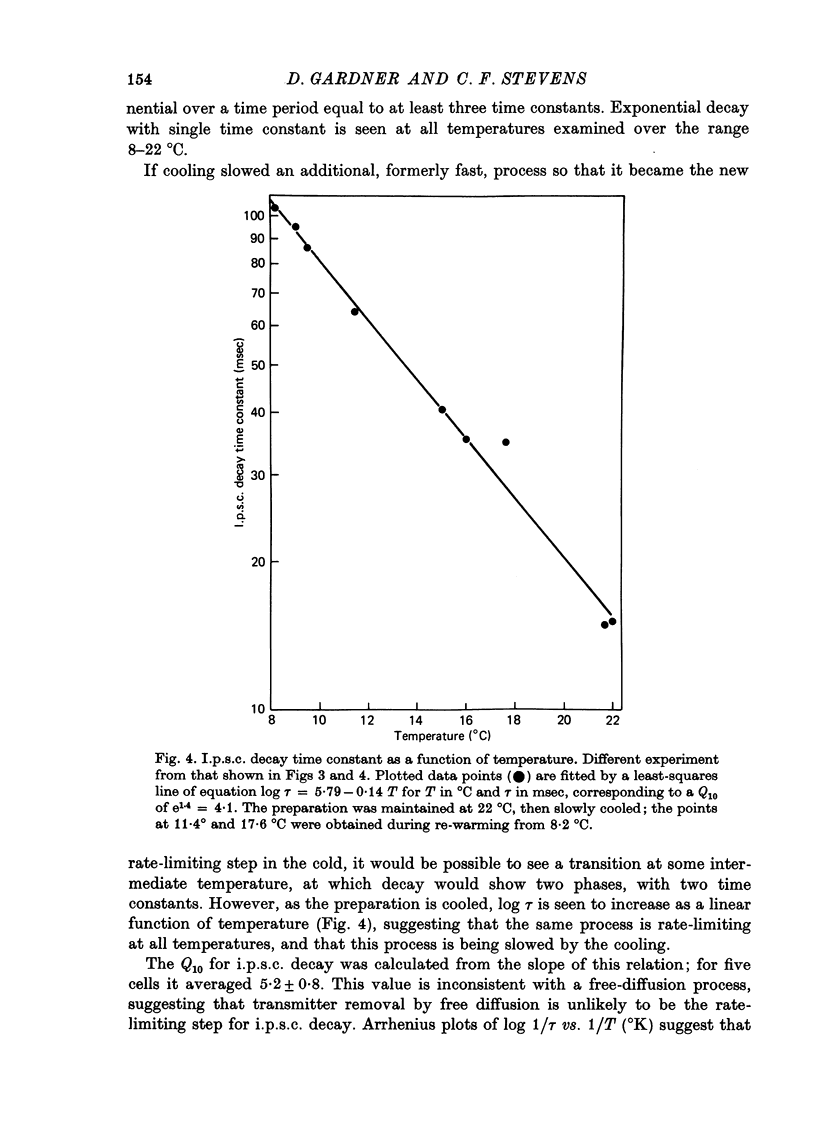
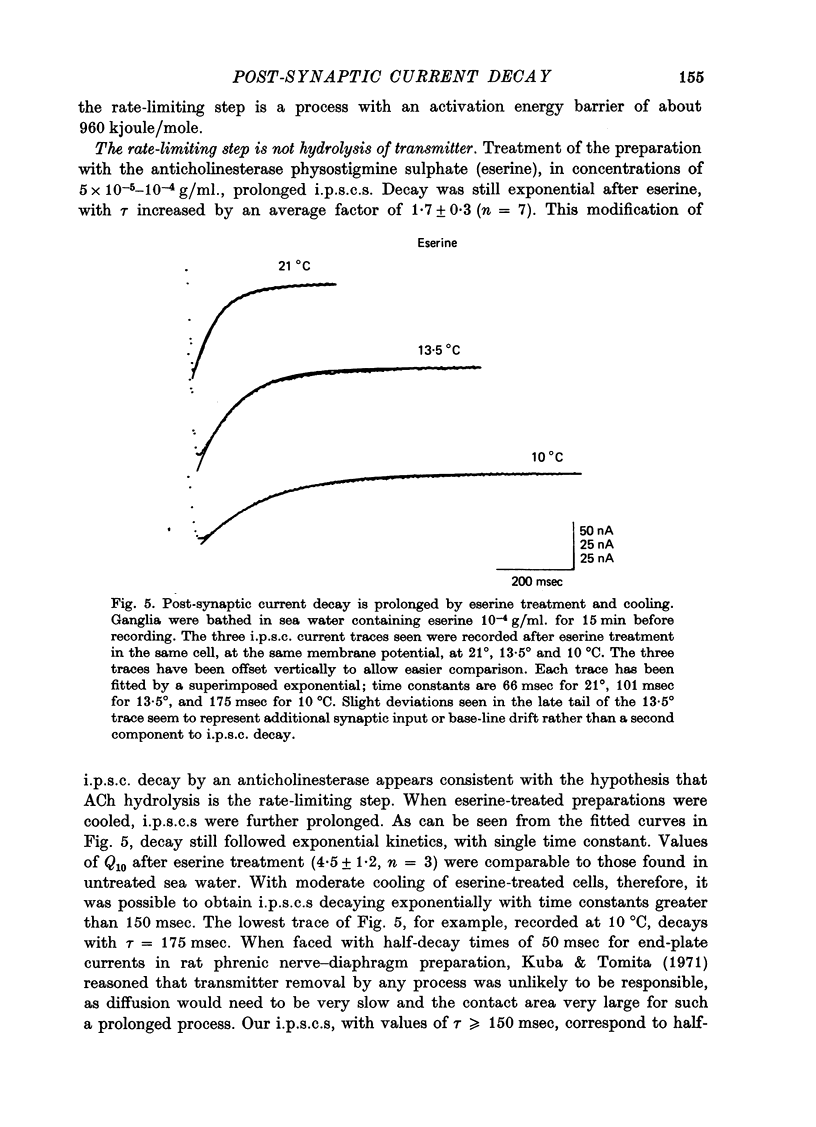
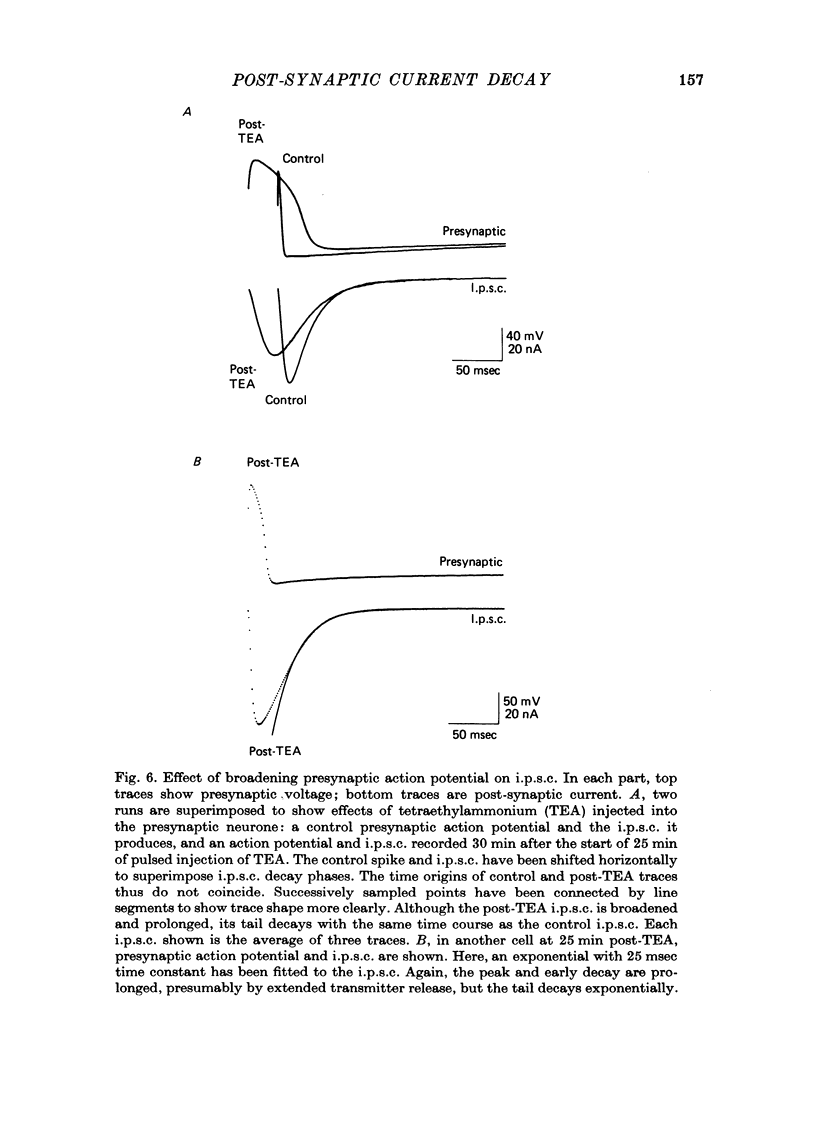
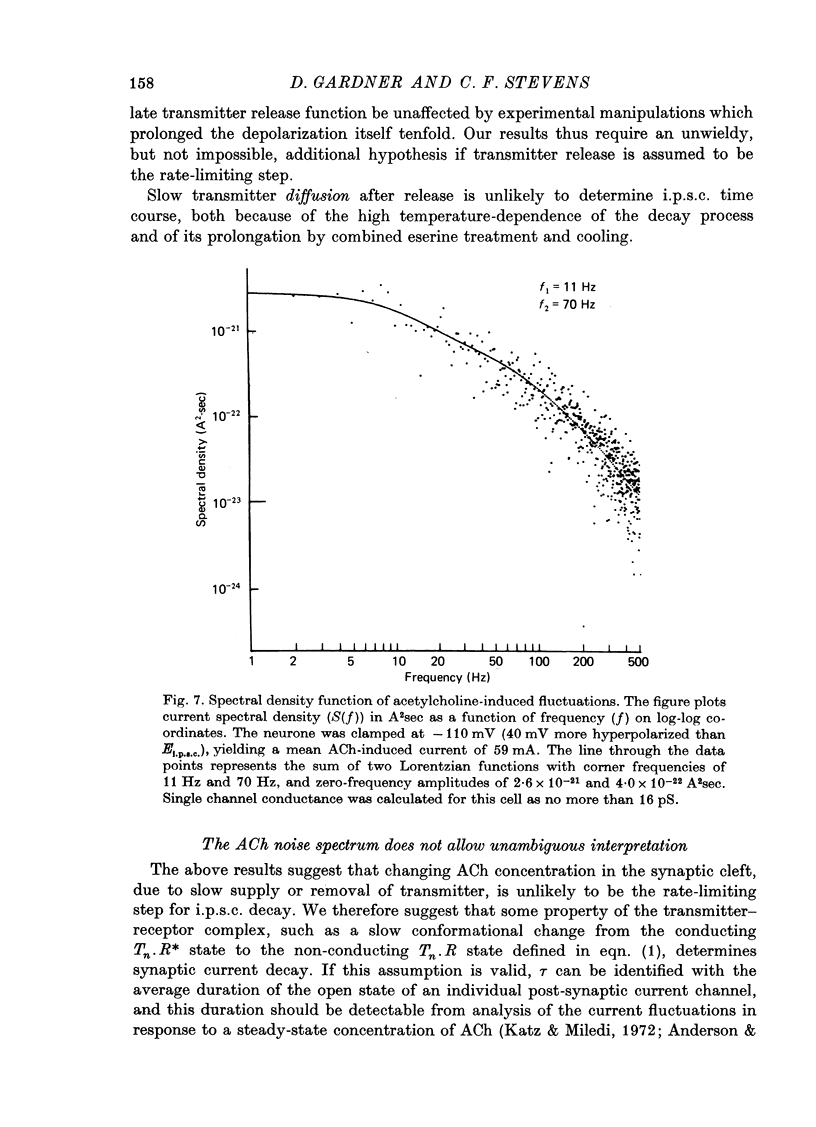
1. In neurones BL and BR 3, 6, 8, 9, 10 and 11 of Aplysia buccal ganglia, cholinergic inhibitory post-synaptic potentials are produced by activity in either of two presynaptic cells. In order to analyse the synaptic conductance change, neurones were voltage-clamped inhibitory post-synaptic currents (i.p.s.c.) recorded. 2. The synaptic conductance change rises to an average peak value of 0.65 micromho and decays exponentially with single time constant tau of 19 msec. 3. We have attempted to identify the rate-limiting step responsible for i.p.s.c. decay from among the following possibilities: (1) acetylcholine (ACh) supply, (2) ACh removal by diffusion, (3) ACh removal by hydrolysis or (4) a slow unbinding or conformational change closing open synaptic current channels. 4. Cooling prolongs tau, with Q10 of 5.2. Cooling and eserine treatment together produce greatly prolonged, exponentially decaying i.p.s.c.s with tau > 150 msec. These results suggest that ACh removal, either by diffusion or hydrolysis, is not the rate-limiting step. 5. Prolonging synaptic action potential time course with intracellular injection of tetraethylammonium broadens the i.p.s.c. peak but does not affect the decay tail, suggesting that the rate-limiting step is not ACh release. 6. The spectrum of ACh-induced current fluctuations is fitted by a double Lorentzian with cut-off frequencies of 7.8 and 47 Hz. The frequency of the slower component corresponds to the macroscopic i.p.s.c. decay tau. 7. We conclude that a slow conformational change closing open synaptic current channels is likely to determine i.p.s.c. decay. We cannot, however, exclude either delayed diffusion or a late tail of slow ACh release as possibilities.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARAKI T., TERZUOLO C. A. Membrane currents in spinal motoneurons associated with the action potential and synaptic activity. J Neurophysiol. 1962 Nov;25:772–789. doi: 10.1152/jn.1962.25.6.772. [DOI] [PubMed] [Google Scholar]
- Adams D. J., Gage P. W., Hamill O. P. Voltage sensitivity of inhibitory postsynaptic current in Aplysia buccal ganglia. Brain Res. 1976 Oct 22;115(3):506–511. doi: 10.1016/0006-8993(76)90368-1. [DOI] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Marty A., Neild T. O. Life time and elementary conductance of the channels mediating the excitatory effects of acetylcholine in Aplysia neurones. J Physiol. 1978 May;278:177–206. doi: 10.1113/jphysiol.1978.sp012299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam K. G. A voltage-clamp study of the effect of two lidocaine derivatives on the time course of end-plate currents. J Physiol. 1976 Jun;258(2):279–300. doi: 10.1113/jphysiol.1976.sp011420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Inward and delayed outward membrane currents in isolated neural somata under voltage clamp. J Physiol. 1971 Feb;213(1):1–19. doi: 10.1113/jphysiol.1971.sp009364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C. Membrane time constants of cat motoneurons and time courses of synptic action. Exp Neurol. 1961 Jul;4:1–22. doi: 10.1016/0014-4886(61)90074-7. [DOI] [PubMed] [Google Scholar]
- Gage P. W., Armstrong C. M. Miniature end-plate currents in voltage-clamped muscle fibre. Nature. 1968 Apr 27;218(5139):363–365. doi: 10.1038/218363b0. [DOI] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N. Effects of membrane potential, temperature and neostigmine on the conductance change caused by a quantum or acetylcholine at the toad neuromuscular junction. J Physiol. 1975 Jan;244(2):385–407. doi: 10.1113/jphysiol.1975.sp010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Moore J. W. Synaptic current at the squid giant synapse. Science. 1969 Oct 24;166(3904):510–512. doi: 10.1126/science.166.3904.510. [DOI] [PubMed] [Google Scholar]
- Gardner D. Bilateral symmetry and interneuronal organization in the buccal ganglia of Aplysia. Science. 1971 Aug 6;173(3996):550–553. doi: 10.1126/science.173.3996.550. [DOI] [PubMed] [Google Scholar]
- Gardner D. Interconnections of identified multiaction interneurons in buccal ganglia of Aplysia. J Neurophysiol. 1977 Mar;40(2):349–361. doi: 10.1152/jn.1977.40.2.349. [DOI] [PubMed] [Google Scholar]
- Gardner D. Interconnections of identified multiaction interneurons in buccal ganglia of Aplysia. J Neurophysiol. 1977 Mar;40(2):349–361. doi: 10.1152/jn.1977.40.2.349. [DOI] [PubMed] [Google Scholar]
- Gardner D., Kandel E. R. Diphasic postsynaptic potential: a chemical synapse capable of mediating conjoint excitation and inhibition. Science. 1972 May 12;176(4035):675–678. doi: 10.1126/science.176.4035.675. [DOI] [PubMed] [Google Scholar]
- Gardner D. Membrane-potential effects on an inhibitory post-synaptic conductance in Aplysia buccal ganglia. J Physiol. 1980 Jul;304:165–180. doi: 10.1113/jphysiol.1980.sp013317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner D. Time integral of synaptic conductance. J Physiol. 1980 Jul;304:181–191. doi: 10.1113/jphysiol.1980.sp013318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner D. Voltage-clamp analysis of a self-inhibitory synaptic potential in the buccal ganglia of Aplysia. J Physiol. 1977 Jan;264(3):893–920. doi: 10.1113/jphysiol.1977.sp011701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., TASAKI I. A study on the mechanism of impulse transmission across the giant synapse of the squid. J Physiol. 1958 Aug 29;143(1):114–137. doi: 10.1113/jphysiol.1958.sp006048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. J., Miller S., Porter R., Redman S. J. The time course of minimal excitory post-synaptic potentials evoked in spinal motoneurones by group Ia afferent fibres. J Physiol. 1971 Jun;215(2):353–380. doi: 10.1113/jphysiol.1971.sp009474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Tomita T. Effect of prostigmine on the time course of the end-plate potential in the rat diaphragm. J Physiol. 1971 Mar;213(3):533–544. doi: 10.1113/jphysiol.1971.sp009398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan H., Tauc L. Acetylcholine receptors: topographic distribution and pharmacological properties of two receptor types on a single molluscan neurone. J Physiol. 1972 May;222(3):537–558. doi: 10.1113/jphysiol.1972.sp009813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Terrar D. A. Factors affecting the time course of decay of end-plate currents: a possible cooperative action of acetylcholine on receptors at the frog neuromuscular junction. J Physiol. 1975 Jan;244(2):467–495. doi: 10.1113/jphysiol.1975.sp010808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellmar T. C., Wilson W. A. Unconventional serotonergic excitation in Aplysia. Nature. 1977 Sep 1;269(5623):76–78. doi: 10.1038/269076a0. [DOI] [PubMed] [Google Scholar]
- RALL W. Membrane potential transients and membrane time constant of motoneurons. Exp Neurol. 1960 Oct;2:503–532. doi: 10.1016/0014-4886(60)90029-7. [DOI] [PubMed] [Google Scholar]
- Ruff R. L. A quantitative analysis of local anaesthetic alteration of miniature end-plate currents and end-plate current fluctuations. J Physiol. 1977 Jan;264(1):89–124. doi: 10.1113/jphysiol.1977.sp011659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C. F. Inferences about membrane properties from electrical noise measurements. Biophys J. 1972 Aug;12(8):1028–1047. doi: 10.1016/S0006-3495(72)86141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Active phase of frog's end-plate potential. J Neurophysiol. 1959 Jul;22(4):395–411. doi: 10.1152/jn.1959.22.4.395. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Electrical changes in pre- and postsynaptic axons of the giant synapse of Loligo. J Gen Physiol. 1962 Jul;45:1181–1193. doi: 10.1085/jgp.45.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]


