Abstract
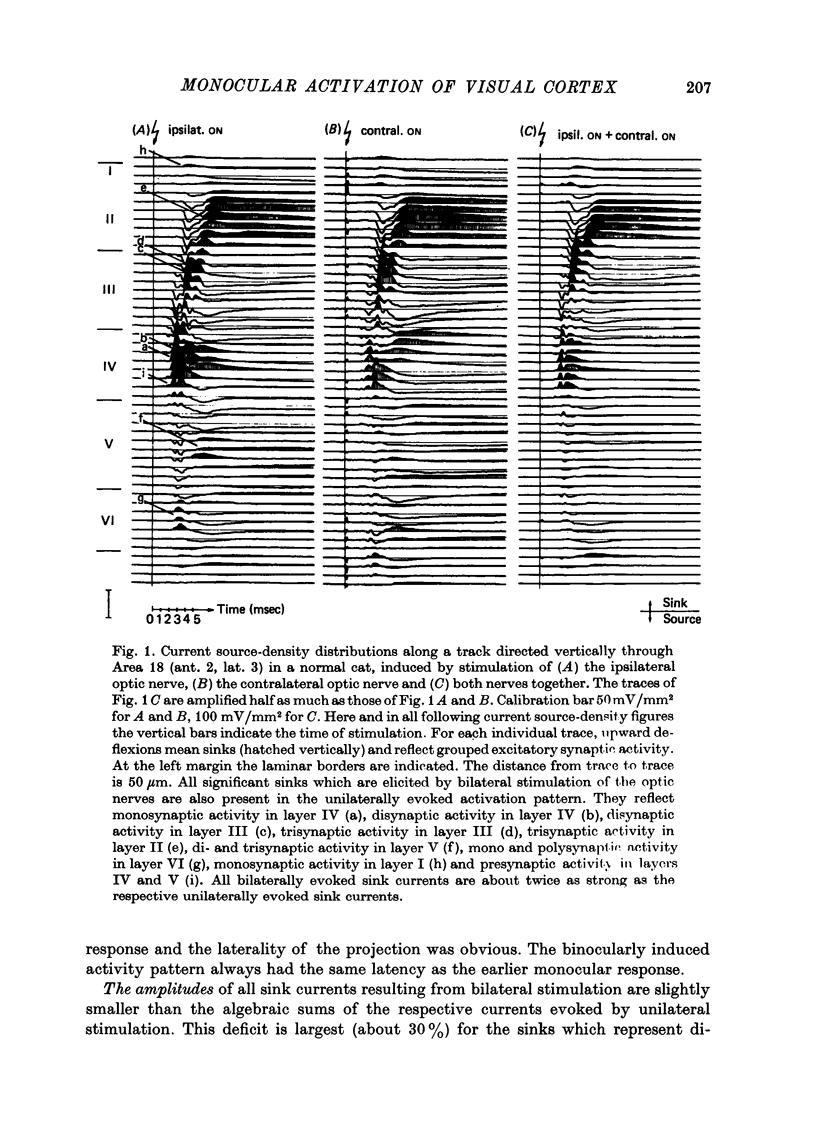
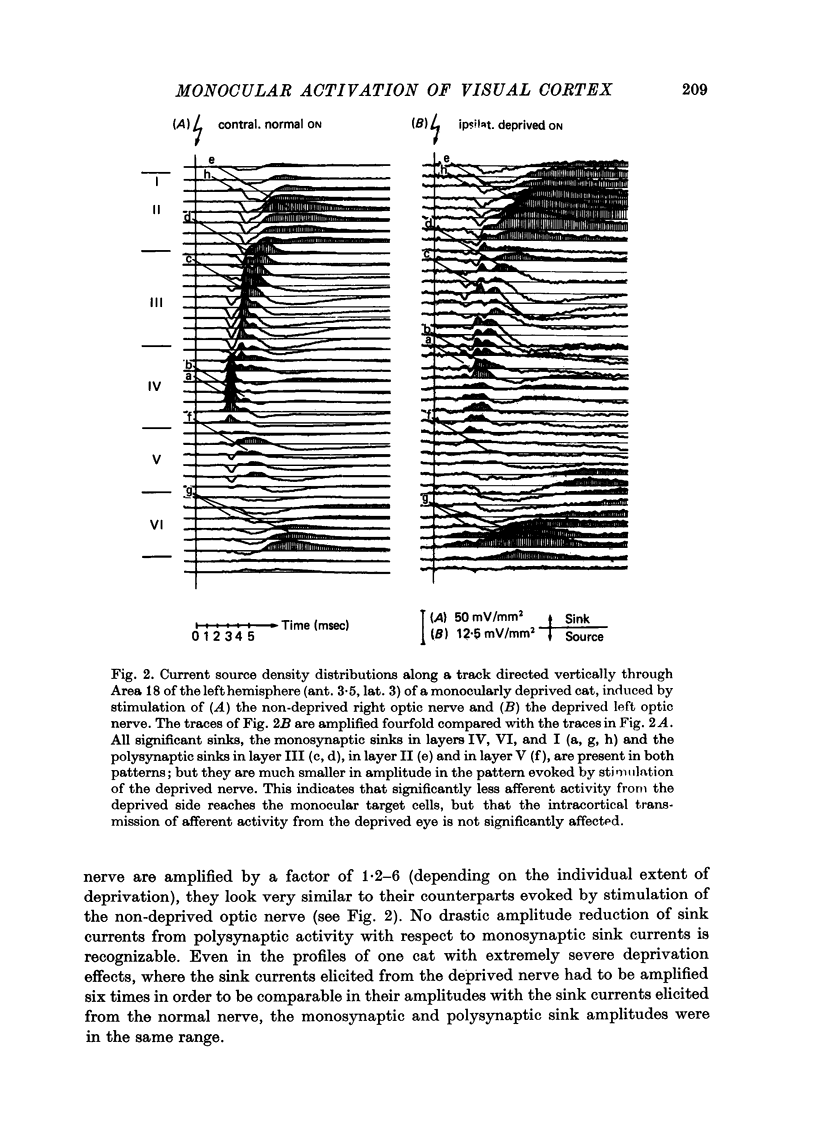
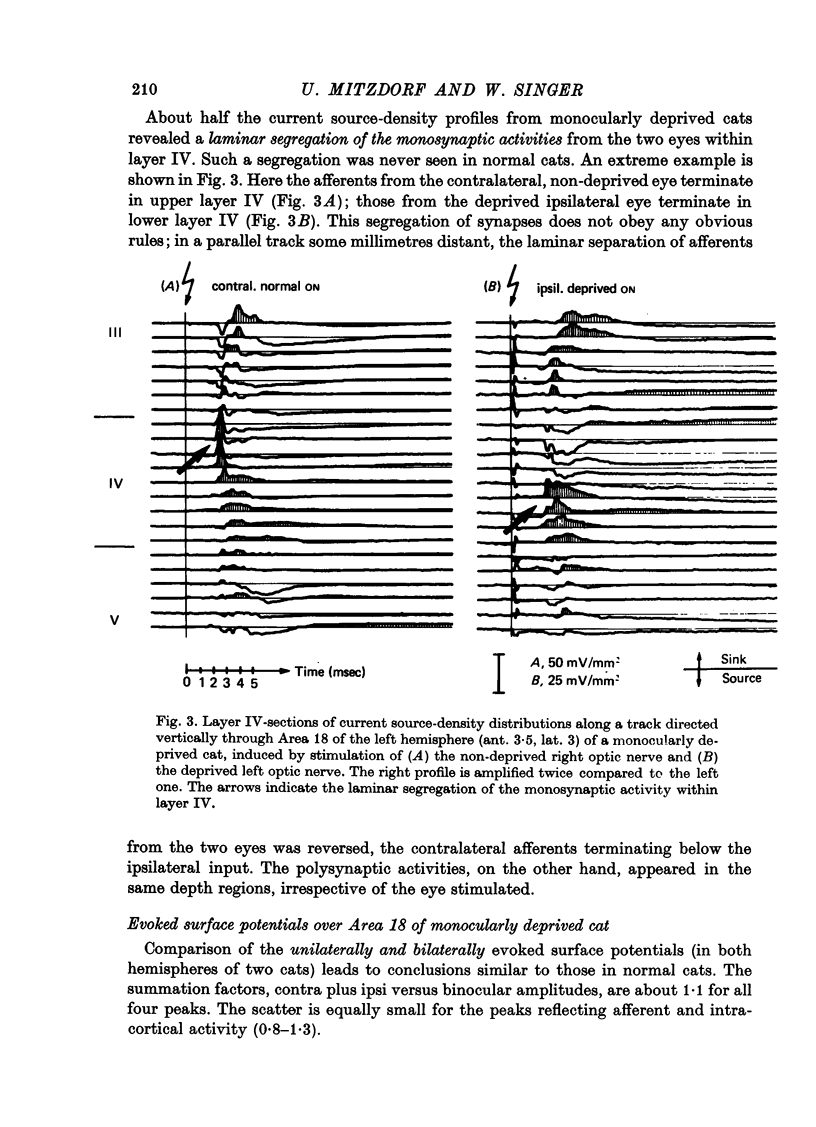
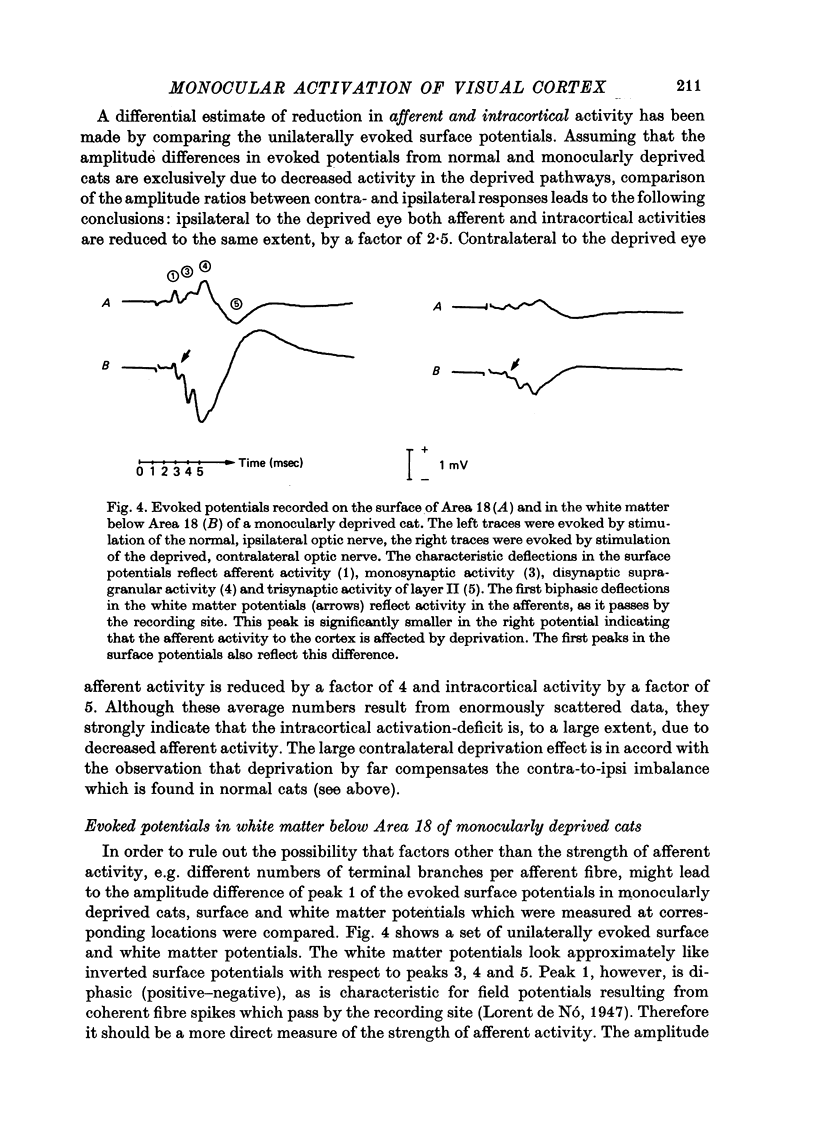
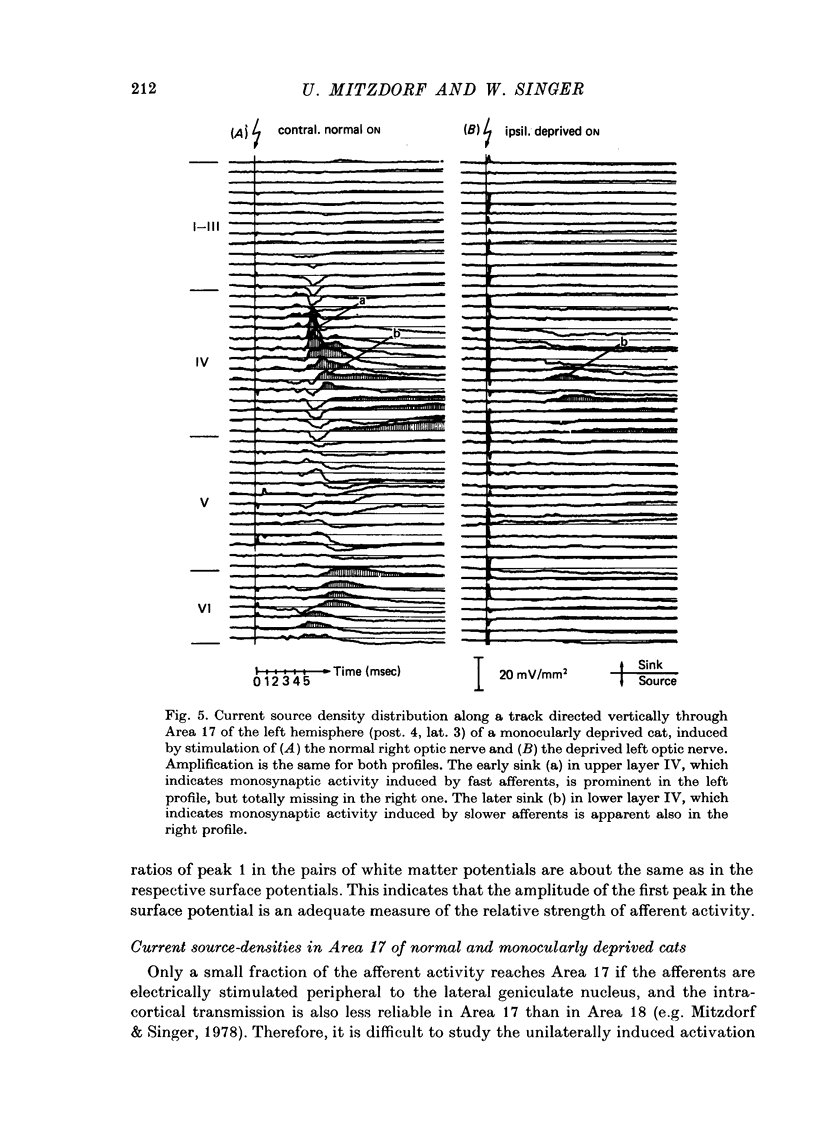
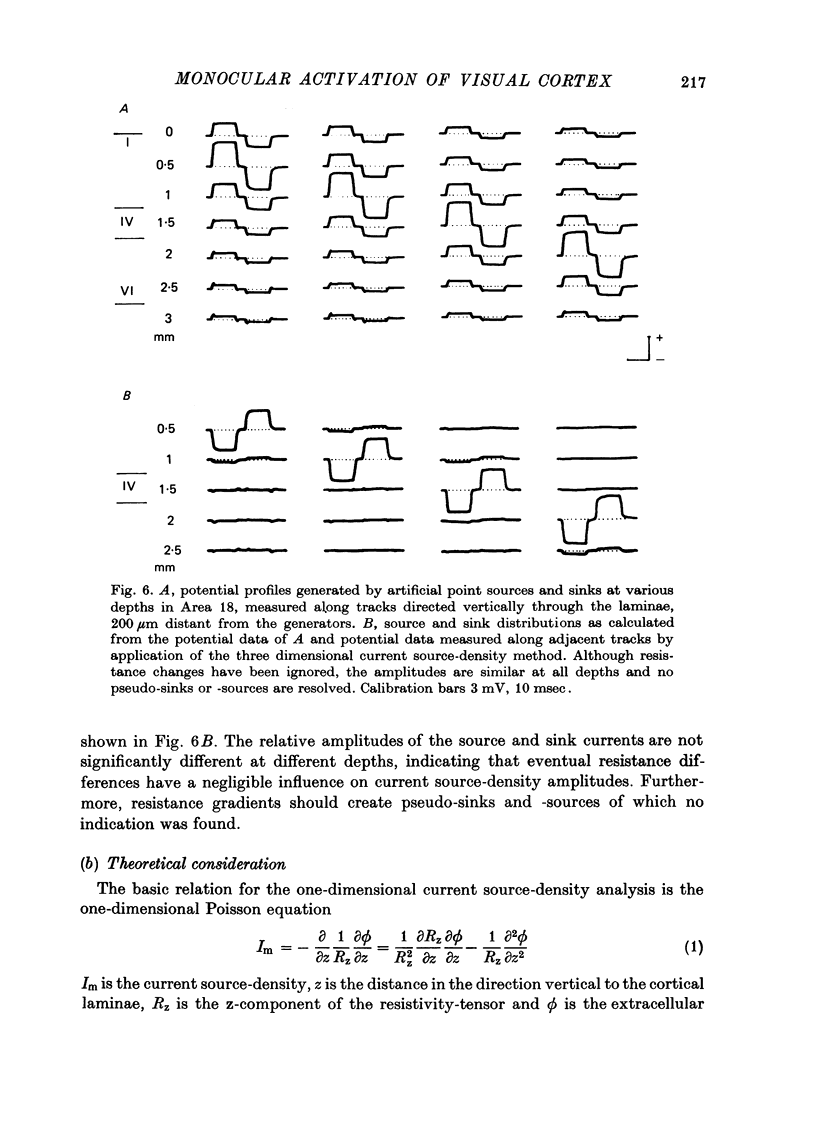
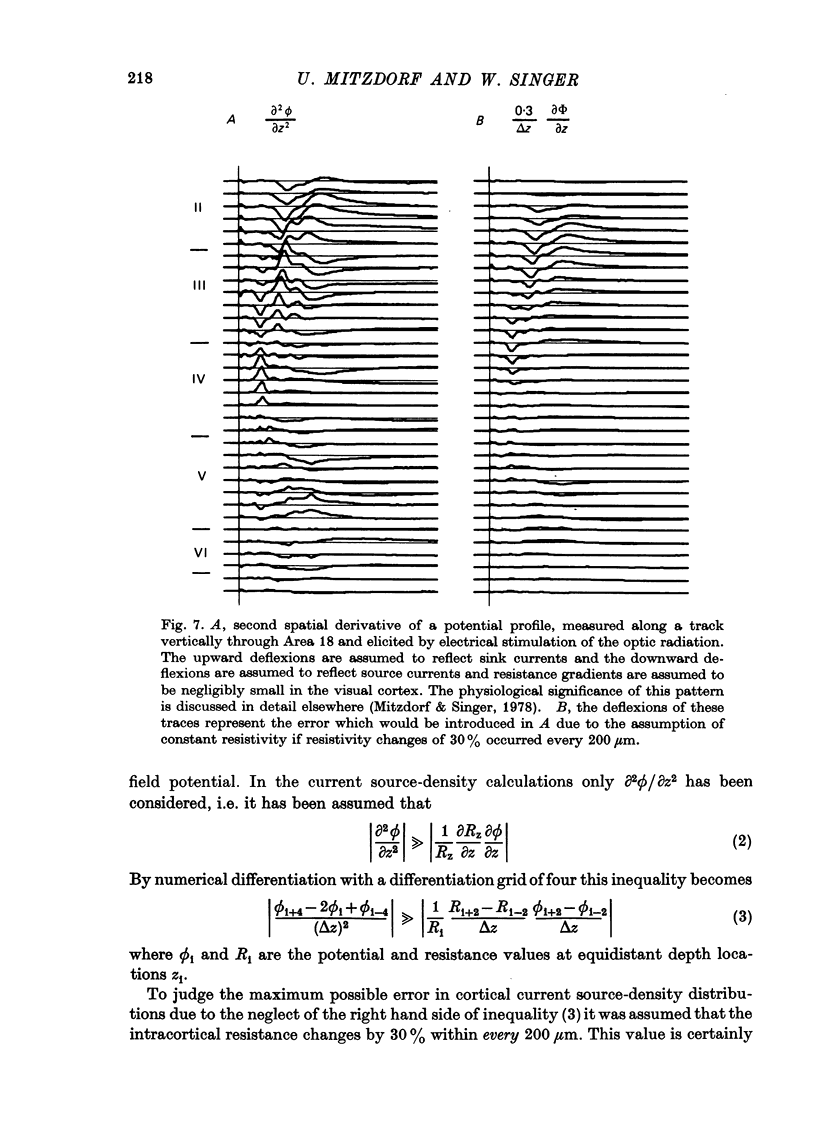
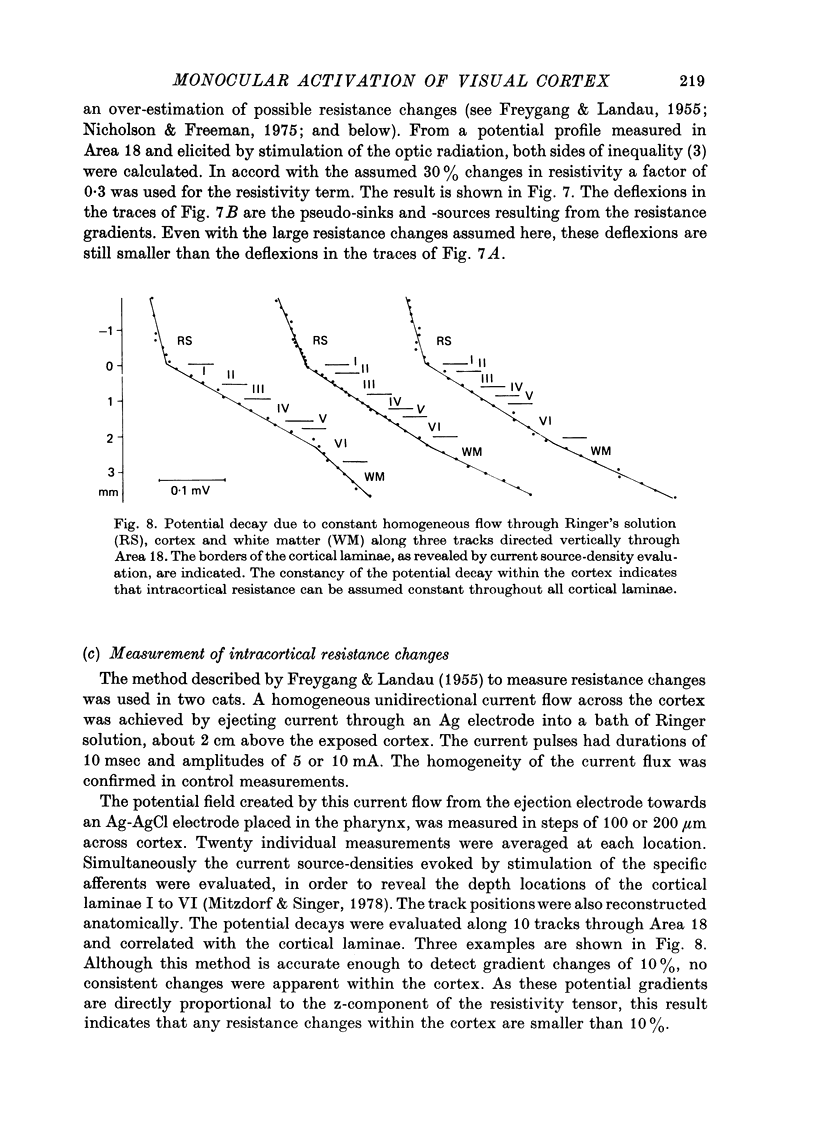
The unilaterally induced patterns of prominent excitatory post-synaptic activity within Areas 17 and 18 were investigated in normal and monocularly deprived cats. They were elicited by electrical stimulation of the optic nerves and evaluated with the one-dimensional current source-density method. 1. In Area 18 of normal cats the unilaterally and bilaterally induced current source-density patterns closely resemble each other. None of the mono-, di- or tri-synaptic activities is potentiated by binocular convergence. 2. In Area 18 of monocularly deprived cats the synaptic currents elicited by stimulating the nerve on the deprived side lead to approximately the same spatial and temporal distribution of sinks and sources as those induced from the normal eye; but the amplitudes are considerably smaller. This reduction is similar for mono-, di- and trisynaptic responses which indicates (a) that the imbalance between activity from the deprived and non-deprived eye is mainly due to reduced input to the cortical target cells from the deprived eye and (b) that the activity from the deprived eye still relayed to these cells is passed on to supra- and infragranular layers without diminution and in the same way as activity from the normal eye. 3. The imbalance of afferent activity from the deprived and non-deprived eye is apparent in the evoked potentials recorded from the white matter. This indicates that activity from the deprived eye is already strongly reduced in the thalamo-cortical fibres. 4. In monocularly deprived, but not in normal cats the monosynaptic activities from the two eyes are often segregated in depth within layer IV. 5. In Area 17 of both normal and deprived cats only a small fraction of the potential monosynaptic activity can be elicited by electrical stimulation of the optic nerves because of transmission failure in the lateral geniculate nucleus. Comparison of the current source-density patterns elicited from the normal and deprived nerve in monocularly deprived cats indicates that activity produced by fast conducting afferents is more affected (reduced) by deprivation that that conveyed by slower afferents.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albus K. Predominance of monocularly driven cells in the projection area of the central visual field in cat's striate cortex. Brain Res. 1975 May 23;89(2):341–347. doi: 10.1016/0006-8993(75)90725-8. [DOI] [PubMed] [Google Scholar]
- Dreyer F., Peper K. Iontophoretic application of acetylcholine: advantages of high resistance micropipettes in connection with an electronic current pump. Pflugers Arch. 1974 Apr 22;348(3):263–272. doi: 10.1007/BF00587417. [DOI] [PubMed] [Google Scholar]
- FREYGANG W. H., Jr, LANDAU W. M. Some relations between resistivity and electrical activity in the cerebral cortex of the cat. J Cell Physiol. 1955 Jun;45(3):377–392. doi: 10.1002/jcp.1030450305. [DOI] [PubMed] [Google Scholar]
- Freeman J. A., Nicholson C. Experimental optimization of current source-density technique for anuran cerebellum. J Neurophysiol. 1975 Mar;38(2):369–382. doi: 10.1152/jn.1975.38.2.369. [DOI] [PubMed] [Google Scholar]
- Ganz L., Fitch M., Satterberg J. A. The selective effect of visual deprivation on receptive field shape determined neurophysiologically. Exp Neurol. 1968 Dec;22(4):614–637. doi: 10.1016/0014-4886(68)90153-2. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Sanides D., Creutzfeldt O. D. A study of binocular convergence in cat visual cortex neurons. Exp Brain Res. 1977 May 23;28(1-2):21–35. doi: 10.1007/BF00237083. [DOI] [PubMed] [Google Scholar]
- LeVay S., Ferster D. Relay cell classes in the lateral geniculate nucleus of the cat and the effects of visual deprivation. J Comp Neurol. 1977 Apr 15;172(4):563–584. doi: 10.1002/cne.901720402. [DOI] [PubMed] [Google Scholar]
- Mitzdorf U., Neumann G. Effects of monocular deprivation in the lateral geniculate nucleus of the cat: an analysis of evoked potentials. J Physiol. 1980 Jul;304:221–230. doi: 10.1113/jphysiol.1980.sp013321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitzdorf U., Singer W. Laminar segregation of afferents to lateral geniculate nucleus of the cat: an analysis of current source density. J Neurophysiol. 1977 Nov;40(6):1227–1244. doi: 10.1152/jn.1977.40.6.1227. [DOI] [PubMed] [Google Scholar]
- Mitzdorf U., Singer W. Prominent excitatory pathways in the cat visual cortex (A 17 and A 18): a current source density analysis of electrically evoked potentials. Exp Brain Res. 1978 Nov 15;33(3-4):371–394. doi: 10.1007/BF00235560. [DOI] [PubMed] [Google Scholar]
- Nicholson C., Freeman J. A. Theory of current source-density analysis and determination of conductivity tensor for anuran cerebellum. J Neurophysiol. 1975 Mar;38(2):356–368. doi: 10.1152/jn.1975.38.2.356. [DOI] [PubMed] [Google Scholar]
- Nicholson C. Theoretical analysis of field potentials in anisotropic ensembles of neuronal elements. IEEE Trans Biomed Eng. 1973 Jul;20(4):278–288. doi: 10.1109/TBME.1973.324192. [DOI] [PubMed] [Google Scholar]
- Shatz C. J., Lindström S., Wiesel T. N. The distribution of afferents representing the right and left eyes in the cat's visual cortex. Brain Res. 1977 Aug 5;131(1):103–116. doi: 10.1016/0006-8993(77)90031-2. [DOI] [PubMed] [Google Scholar]
- Singer W. The effect of monocular deprivation on cat parastriate cortex: asymmetry between crossed and uncrossed pathways. Brain Res. 1978 Nov 24;157(2):351–355. doi: 10.1016/0006-8993(78)90040-9. [DOI] [PubMed] [Google Scholar]
- Singer W., Tretter F., Cynader M. Organization of cat striate cortex: a correlation of receptive-field properties with afferent and efferent connections. J Neurophysiol. 1975 Sep;38(5):1080–1098. doi: 10.1152/jn.1975.38.5.1080. [DOI] [PubMed] [Google Scholar]
- Stone J., Dreher B. Projection of X- and Y-cells of the cat's lateral geniculate nucleus to areas 17 and 18 of visual cortex. J Neurophysiol. 1973 May;36(3):551–567. doi: 10.1152/jn.1973.36.3.551. [DOI] [PubMed] [Google Scholar]
- Tretter F., Cynader M., Singer W. Cat parastriate cortex: a primary or secondary visual area. J Neurophysiol. 1975 Sep;38(5):1099–1113. doi: 10.1152/jn.1975.38.5.1099. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N., HUBEL D. H. SINGLE-CELL RESPONSES IN STRIATE CORTEX OF KITTENS DEPRIVED OF VISION IN ONE EYE. J Neurophysiol. 1963 Nov;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]


