Abstract
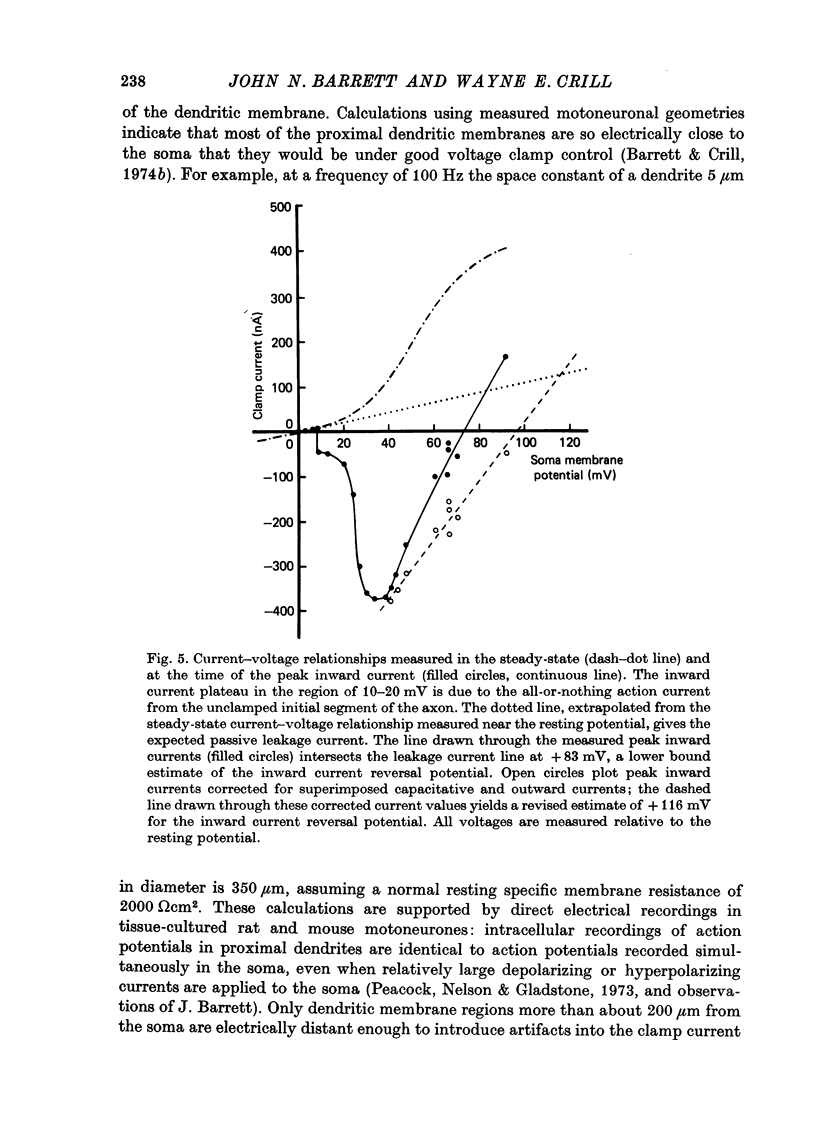
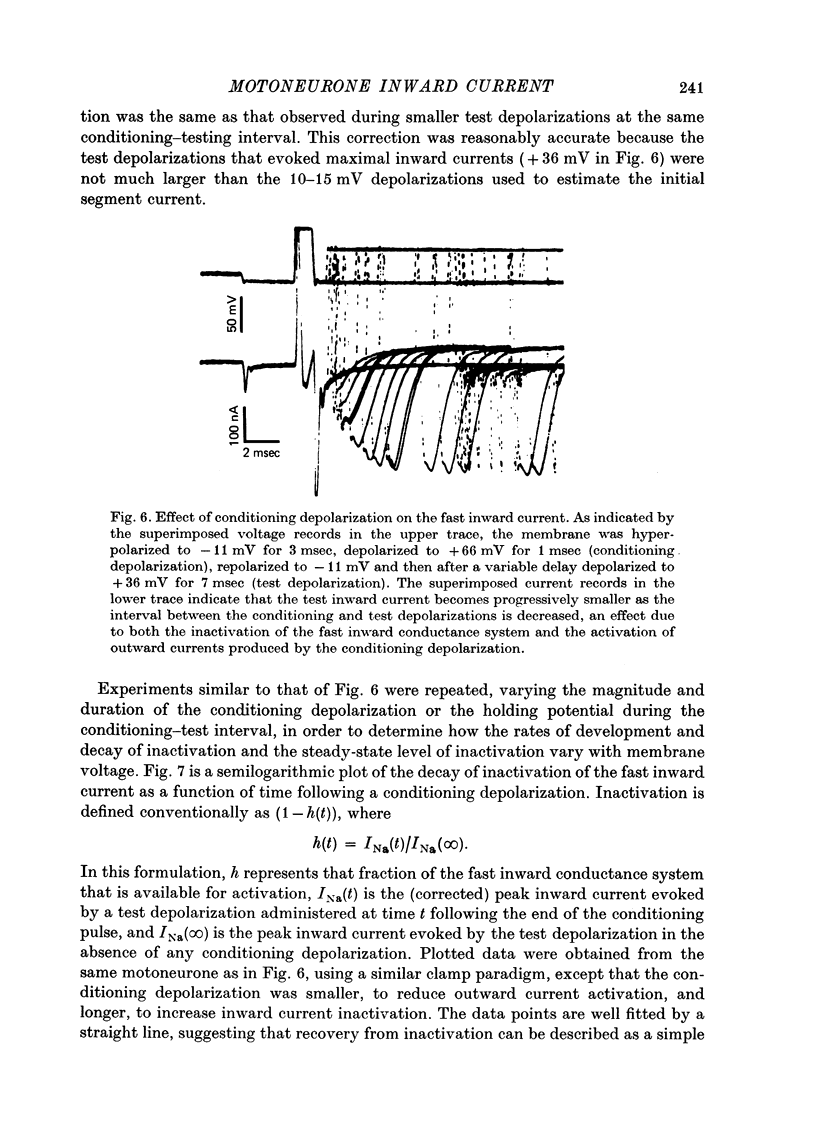
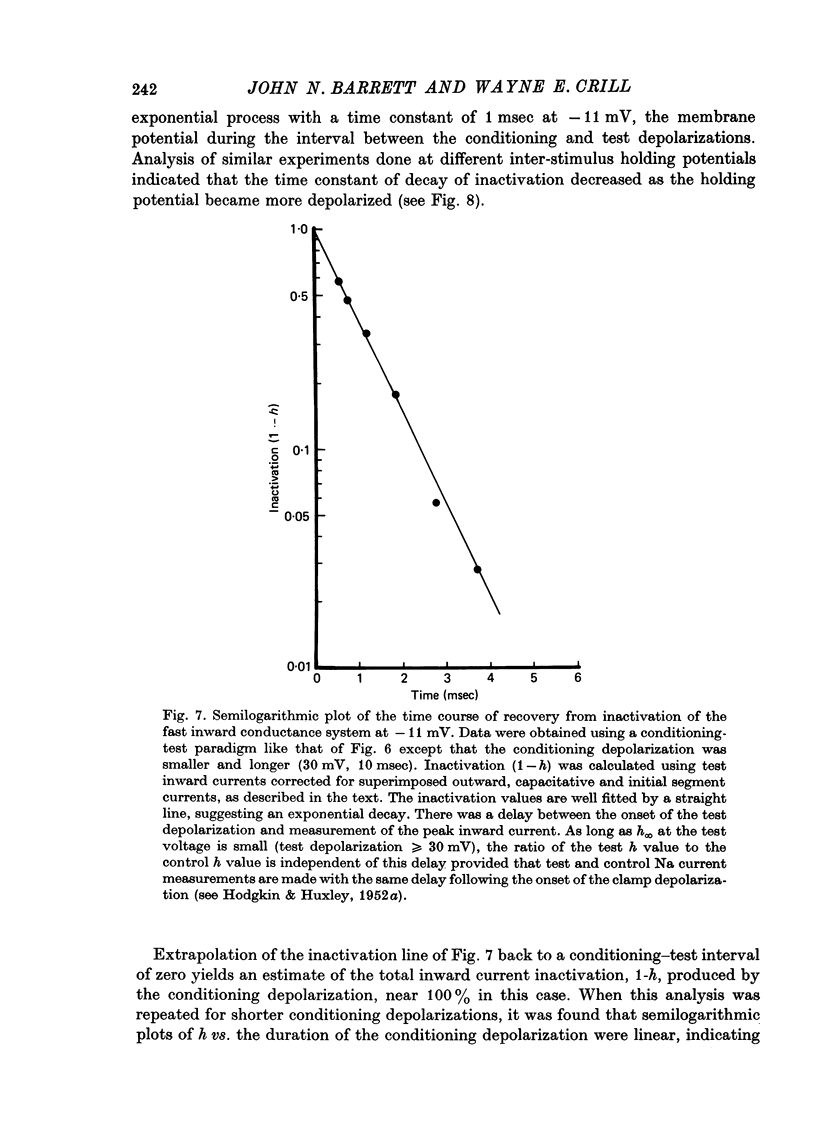
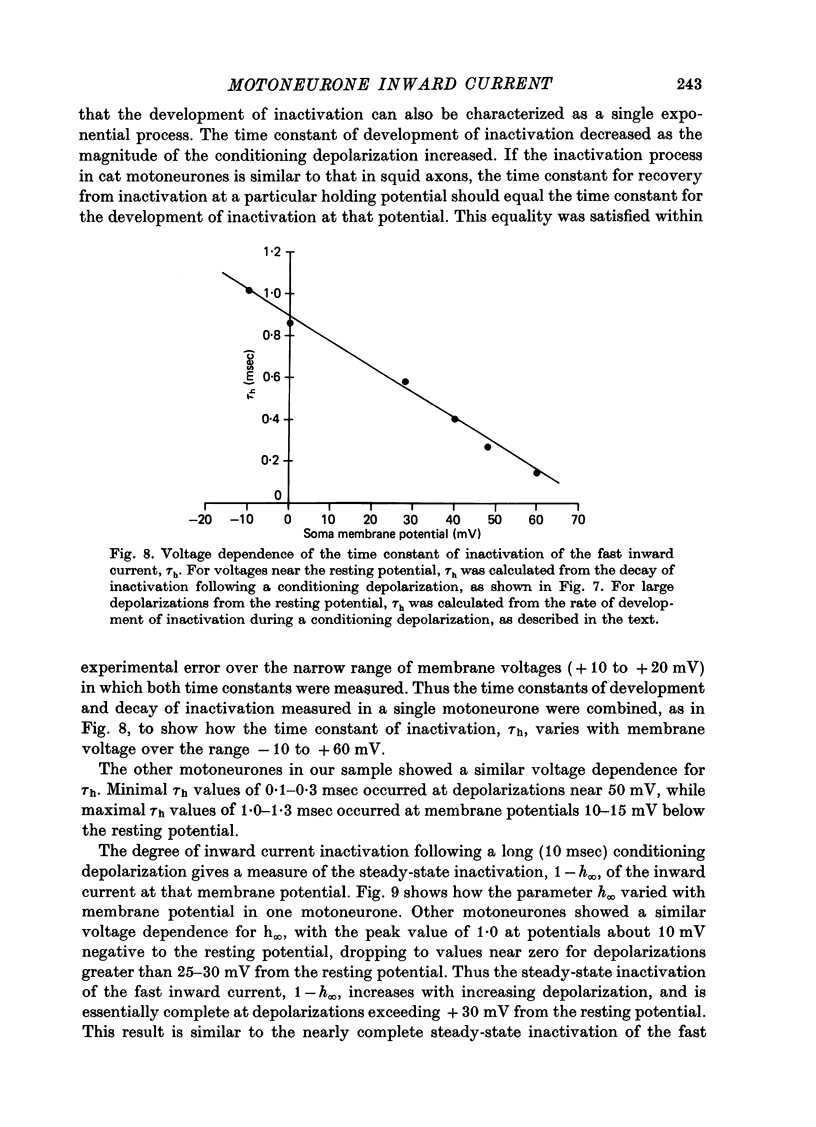
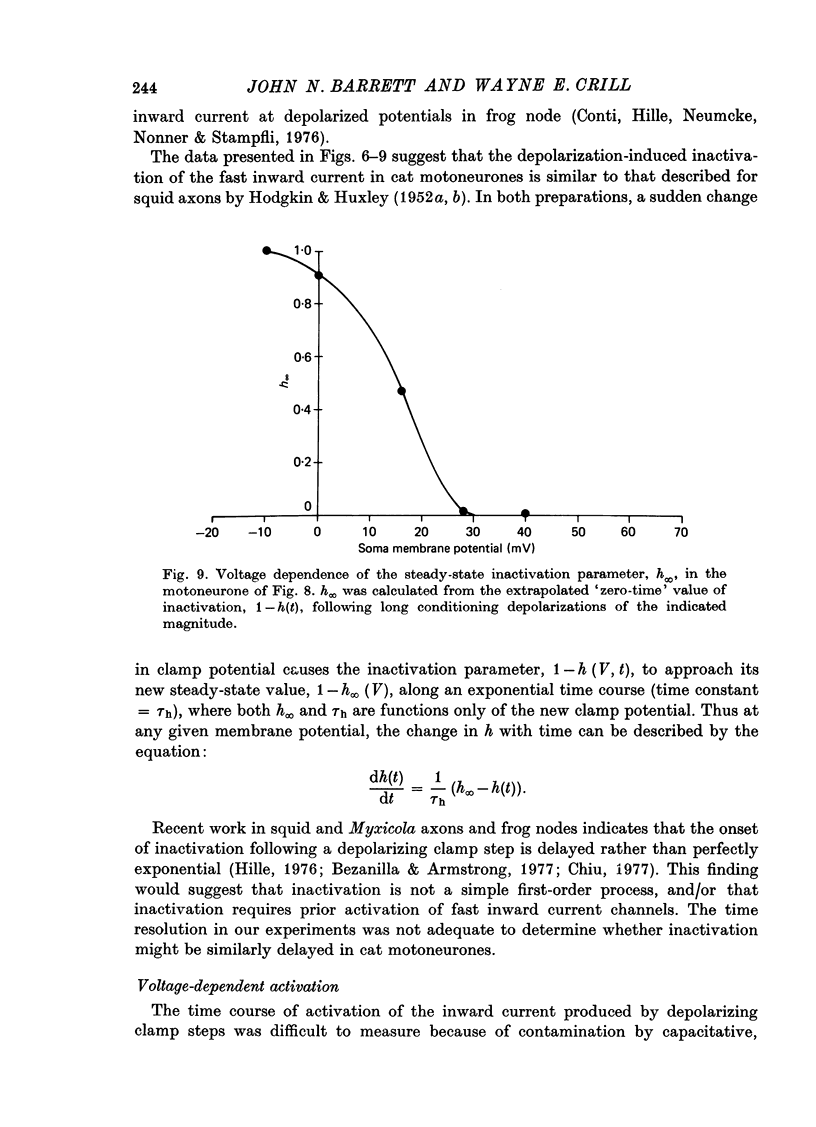
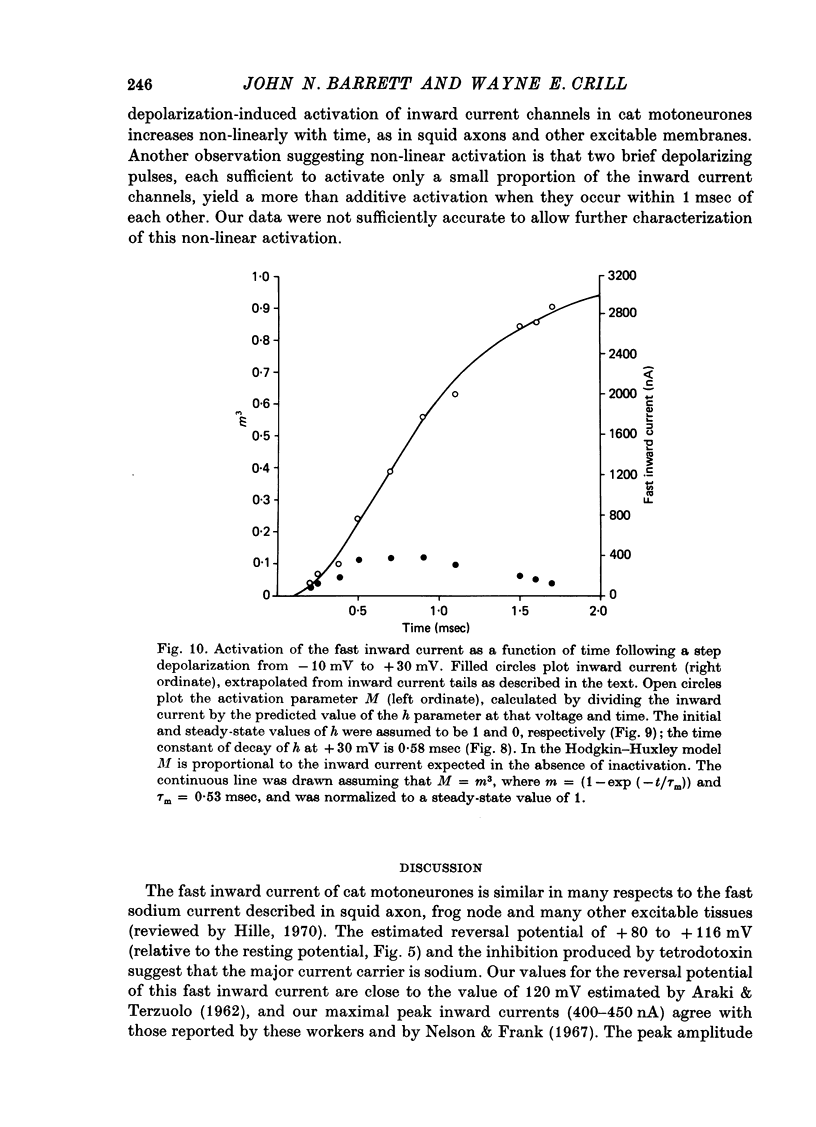
1. The soma membrane of cat spinal motoneurones was voltage clamped using separate intracellular voltage and current electrodes directed into the same motoneurone with a new guide system. 2. Antidromic stimulation of the motoneurone's axon or small depolarizing voltage clamp steps (10-20 mV from the resting potential) evoked a small (30-80 nA) all-or-none action potential current, which was shown by occlusion experiments to originate from the initial segment of the axon. Except for this axonal current spike, there was no indication of active (voltage-dependent) conductance changes in membrane regions not under good voltage clamp control. Calculations based on motoneuronal geometry, and electrophysiological recordings from spinal cord neurones in tissue culture, indicate that the proximal portions of dendritic membranes were also under good voltage clamp control. 3. Clamp depolarizations greater than 20 mV activated a fast, transient inward current, which increased in a smoothly graded manner with depolarization between 20 and 40 mV from the resting potential, reaching a peak magnitude of up to 450 nA, and then decreased smoothly for larger depolarizations. Extrapolation of the current-voltage relationship for this current indicated a reversal potential about 80-116 mV positive to the resting potential. 4. This transient inward current is blocked by tetrodotoxin. After a depolarizing voltage clamp step the conductance system controlling this current first activates with fast, non-linear kinetics, and then inactivates with first-order kinetics. These properties are similar to those of the Na conductance system in squid and frog axons. 5. Conditioning-testing experiments showed that the time constant of inactivation ranges from 1.0-1.3 msec at potentials slightly negative to the resting potential to 0.1-0.3 msec for depolarizations 60 mV from the resting potential. The degree of steady-state inactivation also varied with membrane potential, ranging from total inactivation at depolarizations greater than 30 mV from the resting potential, to minimal inactivation at potentials more than 10 mV negative to the resting potential.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARAKI T., TERZUOLO C. A. Membrane currents in spinal motoneurons associated with the action potential and synaptic activity. J Neurophysiol. 1962 Nov;25:772–789. doi: 10.1152/jn.1962.25.6.772. [DOI] [PubMed] [Google Scholar]
- Barrett E. F., Barret J. N. Separation of two voltage-sensitive potassium currents, and demonstration of a tetrodotoxin-resistant calcium current in frog motoneurones. J Physiol. 1976 Mar;255(3):737–774. doi: 10.1113/jphysiol.1976.sp011306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Barrett J. N., Crill W. E. Voltage-sensitive outward currents in cat motoneurones. J Physiol. 1980 Jul;304:251–276. doi: 10.1113/jphysiol.1980.sp013323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. N., Crill W. E. Influence of dendritic location and membrane properties on the effectiveness of synapses on cat motoneurones. J Physiol. 1974 Jun;239(2):325–345. doi: 10.1113/jphysiol.1974.sp010571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. N., Crill W. E. Specific membrane properties of cat motoneurones. J Physiol. 1974 Jun;239(2):301–324. doi: 10.1113/jphysiol.1974.sp010570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Inactivation of the sodium channel. I. Sodium current experiments. J Gen Physiol. 1977 Nov;70(5):549–566. doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Rojas E., Taylor R. E. Sodium and potassium conductance changes during a membrane action potential. J Physiol. 1970 Dec;211(3):729–751. doi: 10.1113/jphysiol.1970.sp009301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship J. E. Action of tetrodotoxin on spinal motoneurons of the cat. J Neurophysiol. 1968 Mar;31(2):186–194. doi: 10.1152/jn.1968.31.2.186. [DOI] [PubMed] [Google Scholar]
- Brown K. T., Flaming D. G. Instrumentation and technique for beveling fine micropipette electrodes. Brain Res. 1975 Mar 14;86(1):172–180. doi: 10.1016/0006-8993(75)90652-6. [DOI] [PubMed] [Google Scholar]
- Chiu S. Y. Inactivation of sodium channels: second order kinetics in myelinated nerve. J Physiol. 1977 Dec;273(3):573–596. doi: 10.1113/jphysiol.1977.sp012111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F., Hille B., Neumcke B., Nonner W., Stämpfli R. Measurement of the conductance of the sodium channel from current fluctuations at the node of Ranvier. J Physiol. 1976 Nov;262(3):699–727. doi: 10.1113/jphysiol.1976.sp011616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., LIBET B., YOUNG R. R. The behaviour of chromatolysed motoneurones studied by intracellular recording. J Physiol. 1958 Aug 29;143(1):11–40. doi: 10.1113/jphysiol.1958.sp006041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Gating in sodium channels of nerve. Annu Rev Physiol. 1976;38:139–152. doi: 10.1146/annurev.ph.38.030176.001035. [DOI] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J. An electrical description of the motoneurone, and its application to the analysis of synaptic potentials. J Physiol. 1971 Jun;215(2):321–352. doi: 10.1113/jphysiol.1971.sp009473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke B. R., Ogden T. E. A technique for beveling fine micropipettes. Electroencephalogr Clin Neurophysiol. 1974 Mar;36(3):323–326. doi: 10.1016/0013-4694(74)90177-1. [DOI] [PubMed] [Google Scholar]
- Kuno M., Llinás R. Enhancement of synaptic transmission by dendritic potentials in chromatolysed motoneurones of the cat. J Physiol. 1970 Nov;210(4):807–821. doi: 10.1113/jphysiol.1970.sp009243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NELSON P. G., FRANK K. LA PRODUCTION DU POTENTIEL D'ACTION 'ETUDI'EE PAR LA TECHNIQUE DU VOLTAGE IMPOS'EE SUR LE MOTONEURONE DU CHAT. Actual Neurophysiol (Paris) 1964;5:15–35. [PubMed] [Google Scholar]
- Nelson P. G., Lux H. D. Some electrical measurements of motoneuron parameters. Biophys J. 1970 Jan;10(1):55–73. doi: 10.1016/S0006-3495(70)86285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden T. E., Citron M. C., Pierantoni R. The jet stream microbeveler: an inexpensive way to bevel ultrafine glass micropipettes. Science. 1978 Aug 4;201(4354):469–470. doi: 10.1126/science.663670. [DOI] [PubMed] [Google Scholar]
- Peacock J. H., Nelson P. G., Goldstone M. W. Electrophysiologic study of cultured neurons dissociated from spinal cords and dorsal root ganglia of fetal mice. Dev Biol. 1973 Jan;30(1):137–152. doi: 10.1016/0012-1606(73)90053-5. [DOI] [PubMed] [Google Scholar]
- Rall W. Time constants and electrotonic length of membrane cylinders and neurons. Biophys J. 1969 Dec;9(12):1483–1508. doi: 10.1016/S0006-3495(69)86467-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindt P., Crill W. E. A persistent negative resistance in cat lumbar motoneurons. Brain Res. 1977 Jan 14;120(1):173–178. doi: 10.1016/0006-8993(77)90510-8. [DOI] [PubMed] [Google Scholar]


