Abstract
Although neutrophils have been identified as sources of inflammatory cytokines and chemokines, little is known about their immunologic function during mycobacterial infection in the lungs. In this study, we examined the growth of Mycobacterium bovis BCG in the lungs under experimental conditions that altered neutrophil recruitment to the lungs. Depletion and recruitment of neutrophils was associated with respective increases and decreases in M. bovis BCG growth. Thus, neutrophils may enhance mycobacteriocidal immunity in the lungs.
Protective immunity against Mycobacterium tuberculosis depends on recruitment and activation of CD4- and CD8-positive T lymphocytes to sites of infection (4). In addition, macrophages phagocytose, kill, and sequester mycobacteria and produce T-cell-activating cytokines (18). The interaction between infected macrophages and T cells involves both proinflammatory and downregulatory cytokines, resulting in killing of infecting M. tuberculosis organisms. However, a few bacteria survive and account for latent infection, persistent immune activation, and the risk of reactivation disease (12, 18).
Neutrophils are important for early control of acute bacterial infections and thus are considered pivotal to protective innate immunity (6, 28). However, it is not clear whether neutrophils have immunologic functions during mycobacterial infections which are primarily controlled by T lymphocytes (27, 32, 34). In mice, neutrophils are recruited to sites of mycobacterial infection and may be important since the beige mutation or neutropenia enhances the growth of M. avium (1-3, 32). Although recruitment of neutrophils to bronchoalveolar spaces has been described during active human tuberculosis and associated with local chemokine expression (31, 33), it is not known whether neutrophils have direct bacteriocidal or immunologic functions. In vitro studies suggest that human neutrophils are mycobacteriocidal and activated by soluble mycobacterial antigens (5, 10, 15, 21, 22, 25). Likewise, a role for neutrophil-derived defensins has not been clearly established in humans, although growth of M. tuberculosis in mice and in vitro may be partially impaired by treatment with human neutrophil defensins (23, 36). In addition, relapsing and intractable tuberculosis has been described in patients with a defective gp91phox gene, a gene that is important for reactive oxygen radical production and oxidative killing of intracellular pathogens (19).
Neutrophils produce and respond to cytokines and chemokines and therefore may contribute to acquired T-cell immunity against mycobacteria (16, 17, 28). In mice, mutation of γδ T-cell receptors does not impair the control of M. tuberculosis growth but results in the formation of pyogenic granulomas, suggesting interactions between neutrophils and γδ- T cells (9). Gamma interferon (IFN-γ) gene-disrupted mice develop a pronounced granulocytosis in the blood, liver, and spleen following intravenous M. bovis BCG Pasteur infection, suggesting that IFN-γ may modulate granulocyte recruitment (24). In addition, enhanced growth of M. tuberculosis in lungs of mice rendered partially neutropenic with depleting antibody treatments has been reported (27). Other studies demonstrate that neutrophil depletion enhances the growth of rapid-growing nontuberculous mycobacteria while the growth of M. tuberculosis remains unaffected (35). Expression of surface class I and class II major histocompatibility complex molecules and antigen presentation capabilities suggest that neutrophils may function as “auxillary” antigen-presenting cells for T cells (11, 29, 37). Whether neutrophils contribute to the development of innate and/or T-cell-mediated immunity against mycobacteria remains unclear. In this study, neutrophil recruitment to the lungs was modulated to determine its effect on mycobacterial immunity.
We have previously characterized pulmonary immune responses to intratracheal M. bovis BCG infection in C57BL/6 mice and observed immune cell recruitment and activation in bronchoalveolar spaces and lung parenchyma (12). Pathogen-free C57BL/6 female mice (10 to 12 weeks of age) were infected intratracheally with 3 × 103 to 5 × 103 CFU of M. bovis BCG, and bronchoalveolar cells (BAC) were isolated by lavage 2, 21, 28, 42, and 63 days after infection as previously described (12). Cytospin slides of 2 × 104 cells were prepared using a Cytospin 3 centrifuge (Shandon, Pittsburgh, Pa.) (600 rpm for 6 min) and stained with Diff-Quik (Fisher, Pittsburgh, Pa.). Differential cell counts were determined by examining 200 to 400 cells, and the total number of neutrophils, lymphocytes, and macrophages was calculated. During the first 2 weeks of infection, BAC composition in control and infected mice was similar, with neutrophils and lymphocytes representing fewer than 5% of the cells. After 2 to 3 weeks of infection, a statistically significant increase (compared to age-matched, uninfected control mice) in the numbers of bronchoalveolar neutrophils and lymphocytes was observed although macrophages remained the predominant cell type at all times (Table 1). Peak neutrophil recruitment occurred by day 28 and preceded maximal lymphocyte and macrophage recruitment by 1 to 2 weeks. These data suggest that neutrophils may help mediate the recruitment of lymphocytes and macrophages. In contrast, bronchoalveolar lymphocytes and macrophages persisted through weeks 9 and 10 of infection. After 6 to 7 weeks of infection, the number of neutrophils returned to control levels. Similar cellular recruitment patterns have been described for both intratracheal and intraperitoneal infections with virulent M. tuberculosis (14, 27). Acid-fast staining of BAC cytospin preparations did not demonstrate intracellular mycobacteria, since the CFU of M. bovis BCG had been reduced to less than 1% of the inoculum (data not shown). To determine if neutrophil and lymphocyte activation had occurred within bronchoalveolar spaces during M. bovis BCG infection, we measured macrophage inflammatory protein 2 (MIP-2) and IFN-γ expression in bronchoalveolar lavage fluids by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, Minn.). As shown in Fig. 1, expression of MIP-2 and IFN-γ was significantly increased compared to controls and paralleled the migration and activation of neutrophils and lymphocytes (Table 1) as well as the decrease in M. bovis BCG CFU in bronchoalveolar spaces (data not shown).
TABLE 1.
BAC recruitment during M. bovis BCG infectiona
| Time (days) after infection | 103 No. of BAC (mean ± SEM)b
|
||
|---|---|---|---|
| Neutrophils | Lymphocytes | Macrophages | |
| 2 | 4.51 ± 1.08 | 1.89 ± 0.55 | 130.33 ± 9.24 |
| 21 | 16.06 ± 7.29* | 12.93 ± 6.30* | 171.20 ± 25.45 |
| 28 | 116.05 ± 21.28* | 170.73 ± 46.44* | 550.52 ± 74.07* |
| 42 | 25.85 ± 6.19* | 245.54 ± 39.33* | 703.68 ± 66.87* |
| 63 | 4.04 ± 0.96 | 75.72 ± 24.24* | 509.66 ± 86.85* |
Female C57BL/6 mice 10 to 12 weeks old, were infected intratracheally with 3.0 × 103 to 5.0 × 103 CFU of M. bovis BCG. Total BAC numbers were determined, and cytospin preparations of 15,000 to 20,000 cells were prepared and stained with Diff-Quik. Then 200 to 400 cells were examined, and the percentage of neutrophils and lymphocytes was determined.
Absolute neutrophil, lymphocyte, and macrophage counts were calculated by multiplying the percentage by the total cell number. Five mice were examined at each time point, and the mean number of BAC from a representative experiment is displayed. Statistical comparisons to day 2 values were made using the Wilcoxon rank sum test (*, P < 0.05).
FIG. 1.
M. bovis BCG lung infection results in MIP-2 and IFN-γ expression in bronchoalveolar spaces. Female C57BL/6 mice, 10 to 12 weeks old, were infected intratracheally with 0.5 × 103 to 1.0 × 103 M. bovis BCG organisms. At designated times, mice were exsanguinated and their lungs were lavaged with 1.0 ml of phosphate-buffered saline. Lavage fluids were collected and stored at −70°C. MIP-2 and IFN-γ levels were measured by enzyme-linked immunosorbent assay as specified by the manufacturer. Mean levels of MIP-2 (left) and IFN-γ (right) are shown from a representative experiment. Error bars indicate standard error of the mean. Statistical comparisons were made using the Wilcoxon rank sum test, and significant differences are designated (∗, P < 0.05).
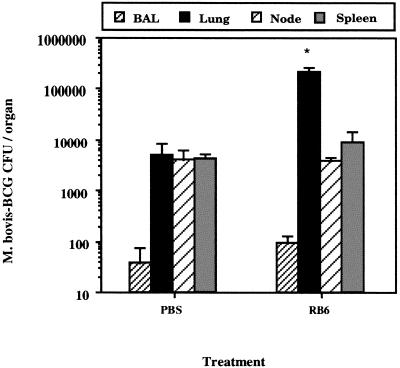
Since neutrophils are phagocytic and potentially mycobacteriocidal (5, 15, 21), we determined whether neutrophil depletion would increase M. bovis BCG growth in the lungs. We first determined that purified rat anti-mouse neutrophil antibody (RB6) depleted peripheral blood neutrophils for at least 72 h after a single 0.5-mg intraperitoneal dose (data not shown). Therefore, mice were infected intratracheally with 0.5 × 104 to 1.0 × 104 CFU of M. bovis BCG and treated intraperitoneally with either 0.5 mg of rat immunoglobulin G (IgG) (Sigma, St. Louis, Mo.) or RB6 on days 14, 16, 18, and 20, when neutrophil recruitment is occurring (12). On day 21, three to five mice per group were sacrificed with tribromoethanol (240 mg/kg), BAC were collected by lavage, lung homogenates were prepared, and M. bovis BCG CFU were determined as previously described (12). In a representative experiment (Fig. 2), a significant 10-fold increase (P ≤ 0.05 [by Wilcoxon rank sum testing]) in M. bovis BCG CFUs was detected in the lungs, suggesting that mycobacterial growth was enhanced as a result of neutrophil depletion. However, M. bovis BCG growth in BAC, mediastinal lymph modes and the spleen was not significantly affected. On day 21, lung cells also were stained with biotinylated RB6 (BD Pharmingen, San Diego, Calif.) and streptavidin-phycoerythrin (Caltag, Burlingame, Calif.) to determine the number of neutrophils in the lungs. In a representative experiment, we detected a 25% decrease in the number of neutrophils (2.98 × 106 ± 1.13 × 106 [RB6 group] versus 4.15 × 106 ± 0.26 × 106 [control group]) which correlated with increased growth of M. bovis BCG. A decrease in the number of bronchoalveolar neutrophils also was detected (75,225.0 ± 43,834.7 [for controls] versus 14,750.0 ± 10,856.1 [for RB6-treated mice]) and was associated with a reduction (13 to 56%) in the number of bronchoalveolar lymphocytes and macrophages (data not shown). However, no change in the numbers of CD4+ or CD8+ T cells was detected in the lungs (data not shown), in contrast with a study demonstrating a concurrent decrease in the number of mesenteric lymph node T cells during gastrointestinal listeriosis (8). However, our results did not achieve statistical significance compared to those obtained with control mice. In a different experiment, delaying treatment with RB6 (on days 17, 20, and 23 days of infection) had no effect on lung CFU measured on day 35 (data not shown). Since depletion of neutrophils in bronchoalveolar spaces or lung parenchyma was not complete (data not shown), we next treated mice during early infection with multiple doses of antibody to generate more profound neutropenia. For this experiment, mice were infected intratracheally with 0.5 × 103 to 1.0 × 103 M. bovis BCG organisms and treated intraperitoneally with RB6 (0.5 mg) on days 3, 6, 9, 12, 15, and 18 after infection. After 21 days of infection, lung homogenates were prepared and a 10% increase in M. bovis BCG lung CFU (P ≥ 0.05) was measured (data not shown). However, we determined that serum from RB6-treated mice contained a high titer (≥1:1,000 dilution) of mouse anti-RB6 immunoglobulin, confirming that the mice had been immunized to the depleting antibody (data not shown). A recent study also has reported the development of mouse anti-RB6 antibodies after only two intraperitoneal doses of RB6 antibody (35). Thus, the immunogenic properties of RB6 antibody precluded its further use in studies to determine the role of neutrophils during prolonged M. bovis BCG infections in the lung. As a result, we used an alternative experimental approach to determine whether neutrophils could modify M. bovis BCG infection in the lungs.
FIG. 2.
Neutrophil depletion enhances M. bovis BCG growth in the lungs. Female C57BL/6 mice, 10 to 12 weeks old, were infected intratracheally with 0.5 × 104 to 1.0 × 104 M. bovis BCG organisms. Mice subsequently received 0.5 mg of rat IgG or neutrophil-depleting antibody RB6 intraperitoneally on days 14, 16, 18, and 20. On day 21, BAC and lung homogenates were prepared, and CFU were determined (n = 4 to 6). Data for BAC, lung, spleen, and lymph node cells are shown; results are representative of three independent experiments. Error bars indicate standard error of the mean. Statistical comparison to IgG-treated controls was made using the Wilcoxon rank sum test, and significant differences are designated (∗, P < 0.05).
Since neutrophil depletion appeared to enhance M. bovis BCG growth in the lungs, we next hypothesized that enhanced early recruitment of neutrophils (Table 1) would decrease mycobacterial growth. First, we demonstrated that intranasal instillation of MIP-2 resulted in neutrophil recruitment to bronchoalveolar spaces. Mice were anesthetized with tribromoethanol (180 mg/kg) as reported above and treated intranasally with 0.5 μg (in 25.0 μl) of recombinant murine MIP-2 (R & D Systems.) resuspended in 0.1% bovine serum albumin (BSA). Control mice were given 25 μl of 0.1% BSA. After 24, 48, and 72 h, the mice were killed and BAC were harvested by lavage. Cytospin preparations were stained with Diff-Quik, and neutrophils and lymphocytes were counted. As shown in Fig. 3, a single intranasal MIP-2 treatment caused an influx of neutrophils which lasted for at least 48 hs and did not affect the numbers of lymphocytes or macrophages. Also, BSA treatment did not alter the cellular composition of the bronchoalveolar lavage fluid. After 72 h, normal numbers of bronchoalveolar neutrophils were observed.
FIG. 3.
Intranasal MIP-2 administration results in recruitment of neutrophils to bronchoalveolar spaces. Female C57BL/6 mice, 10 to 12 weeks old, were given 0.5 μg of recombinant murine MIP-2 intranasally. After 24, 48, and 72 h, the mice were killed and BAC were isolated. Cytospin preparations of 20,000 cells were fixed and stained with Diff-Quik. Numbers of neutrophils and lymphocytes were determined by counting 200 to 400 cells and multiplying the percentage by the total number of cells collected. The mean number of cells from three mice is shown. Error bars indicate standard error of the mean. Control mice were treated with 0.1% BSA in phosphate-buffered saline. Statistical comparisons to controls were made using the Wilcoxon rank sum test, and significant differences are designated (∗, P < 0.05). PMN, polymorphonuclear leukocytes.
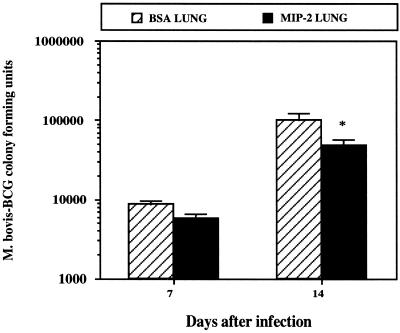
Next, we treated M. bovis BCG-infected mice with 0.5 μg of MIP-2 (1.3- to 3.3-fold higher than the 50% effective dose according to the manufacturer) or BSA intranasally every 48 h (days −1 through +9) and measured M. bovis BCG CFU in the lungs 14 days after infection. Bronchoalveolar neutrophilia and a 20% decrease in M. bovis BCG numbers in the lungs were measured (data not shown). Therefore, we used larger treatment doses of MIP-2 to better ensure neutrophil recruitment before and during early infection. C57BL/6 mice were treated with MIP-2 (2.5 μg/dose [5.0- to 16.6-fold higher than the 50% effective dose) before (days −2 and −1) and after (days +1 and +3) intratracheal infection with 5.0 × 103 to 10.0 × 103 CFU of M. bovis BCG. After 7 and 14 days, bronchoalveolar and lung cells were prepared as described above and CFU were determined. As shown in Fig. 4, MIP-2 treatment resulted in a significant reduction in bacterial growth in the lungs on day 14. However, histopathologic examination of lung tissue did not reveal obvious differences in inflammatory infiltrates (data not shown). In addition, no statistically significant change in the numbers of M. bovis BCG organisms was detected in mediastinal lymph nodes (593.4 ± 193.4 [control mice] versus 593.0 ± 253.0 [MIP-2-treated mice]) or spleen (77.7 ± 77.7 [control mice] versus 280.0 ± 140.0 [MIP-2-treated mice]). This suggested that the MIP-2-mediated reduction in M. bovis BCG CFU in the lungs was not due to enhanced dissemination of M. bovis BCG to other organs. In contrast, bacterial burdens in the lungs were similar in MIP-2-treated and control mice on day 7 of infection, suggesting that a direct bactericidal mechanism was not operative during early infection as was also suggested by others (27). Although we also detected a 15 to 20% increase in the number of CD3+ T cells in lung cell homogenates analyzed by flow cytometry (data not shown), we were unable to detect enhanced IFN-γ expression in lavage fluids or culture supernatants of lung cells cultured in vitro (data not shown). Since we did not quantitate lung macrophages, we could not exclude the possibility that the increase in CD3+ T-cell numbers enhanced the recruitment of macrophages, which reduced M. bovis BCG growth. However, we did not detect a difference in nitrite production, a surrogate marker of nitric oxide expression and macrophage activation (data not shown). Thus, the effect of MIP-2 treatment on M. bovis-BCG growth may be independent of nitric oxide or IFN-γ expression.
FIG. 4.
Intranasal MIP-2 administration decreases M. bovis BCG growth in the lungs. Female C57BL/6 mice, 10 to 12 weeks old, were treated intranasally with 2.5 μg of recombinant murine MIP-2 (in 25 μl) before (days −2 and −1) and after (days +1 and +3) intratracheal infection with 5.0 × 103 to 10.0 × 103 CFU of M. bovis BCG. Control mice were treated similarly with 0.1% BSA. After 7 and 14 days, BAC and lung cell homogenates were prepared from individual mice (n = 3), and CFU were determined. The results show mean CFU and are representative of three independent experiments. Error bars indicate standard error of the mean. The Wilcoxon rank sum test was used to compare numbers of CFU for control and MIP-2-treated mice, and significant differences are designated (∗, P < 0.05).
Although human neutrophils appear to phagocytose and kill mycobacteria, a role for neutrophils during innate and adaptive immune responses to mycobacteria remains poorly defined. Studies using animal models have likewise failed to demonstrate a clear mechanism whereby neutrophils contribute to innate immunity against mycobacteria (3, 27, 32, 34). Whether neutrophils modulate T-cell responses through expression of chemokines (e.g., MIP-1α and monocyte chemotactic protein 1) which recruit lymphocytes and macrophages or enhanced expression of cytokines (e.g., interleukin-12) which promote IFN-γ expression is not clear (2, 16, 17, 27, 28).
We found that depletion of neutrophils between days 14 and 20 was associated with increased M. bovis BCG growth in the lungs after 21 days. In contrast, other studies report that depletion 2 to 3 weeks after infection had no effect on the growth of M. tuberculosis (Erdman) while early neutrophil depletion enhanced the growth of M. tuberculosis (Erdman) in the lungs. Although neutrophil depletion was associated with a transient decrease in the levels of IFN-γ and inducible nitric oxide synthase mRNA in the liver, it is not known whether a similar effect on IFN-γ and inducible nitric oxide synthase mRNA expression occurs simultaneously in the lungs (27). These contrasting observations may reflect differences in mouse strains (BALB/c versus C57BL/6), inoculum size, route of infection (intravenous versus intratracheal) or route of RB6 treatment (intraperitoneal versus intravenous). In addition, other studies of murine listeriosis have shown that RB6 treatment does not affect neutrophil recruitment or microbiocidal activity equally in the spleen and liver, suggesting that different mechanisms might mediate neutrophil activity in different organs (7). Therefore, since others and we have determined that RB6 antibody is significantly immunogenic (35), we were unable to confidently draw conclusions on the basis of a neutrophil depletion approach.
By utilizing a novel intranasal MIP-2 treatment to enhance neutrophil recruitment to the lungs, we determined that neutrophils partially contributed to the control of M. bovis BCG infection, resulting in a 30 to 40% reduction in growth. However, since growth differences were not detected until day 14 of infection, an early mycobacteriocidal effect did not seem operative. Otherwise, an early reduction in mycobacterial counts might have been measured. Furthermore, although we did not detect differences in IFN-γ expression or nitrite production, a 15 to 20% increase in the number of CD3+ cells (both CD4 and CD8) was measured in the lungs. Whether this causes increased recruitment of mycobacteriocidal macrophages is not known. In the bronchoalveolar spaces, peak neutrophil influx preceded peak lymphocyte and macrophage recruitment. Therefore, our data suggest that neutrophils may have a non phagocytic, immunomodulatory function enhancing lymphocyte and macrophage recruitment to the lungs during mycobacterial infection (27). Indeed, neutrophil-mediated recruitment of macrophages has been suggested to be a critical defense mechanism against Listeria, which also is an intracellular pathogen (20). Further studies are under way to determine whether this effect is associated with an increase in the amount of MIP-1α, which is chemotactic for lymphocytes and is expressed in the lungs of mice infected with M. tuberculosis (30) and by human neutrophils exposed to mycobacterial proteins (16).
Innate and acquired T-cell responses are necessary for the control of mycobacterial infection in the lungs (4, 18). Our data suggest that neutrophils have an indirect function which was not associated with enhanced IFN-γ or nitrite expression in the lungs. To be potential immune effector cells, neutrophils must be recruited to infectious foci since other studies have shown that a peripheral blood neutrophilia does not protect mice from M. tuberculosis growth and dissemination (24). Since neutrophils may have the capacity to function as antigen-presenting cells for mycobacterial antigens and to produce chemokines which enhance T-lymphocyte recruitment, further investigation of their interaction with T lymphocytes may elucidate new approaches to immunotherapy or the development of novel vaccines. In addition, analysis of local pulmonary immune responses will define new strategies whereby aerogenic delivery of chemokines or cytokines may prove useful for treatment of or immunization against M. tuberculosis (13, 26).
Acknowledgments
We thank R. Coffmann (DNAX, Palo Alto, Calif.) for providing the hybridoma producing anti-GR1 antibody (RB6-8C5).
This work was supported in part by a developmental grant to S.A.F. from STERIS Corp. (Mentor, Ohio) and NIH grants K8HL-04299 (S.A.F.) and HL-55967 (W.H.B.).
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Appelberg, R. 1992. Mycobacterial infection primes T cells and macrophages for enhanced recruitment of neutrophils. J. Leukoc. Biol. 51:472-477. [DOI] [PubMed] [Google Scholar]
- 2.Appelberg, R. 1992. Macrophage inflammatory proteins MIP-1 and MIP-2 are involved in T cell-mediated neutrophil recruitment. J. Leukoc. Biol. 52:303-306. [DOI] [PubMed] [Google Scholar]
- 3.Appelberg, R., A. G. Castro, S. Gomes, J. Pedrosa, and M. T. Silva. 1995. Susceptibility of beige mice to Mycobacterium avium: role of neutrophils. Infect. Immun. 63:3381-3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boom, W. H. 1996. The role of T-cell subsets in Mycobacterium tuberculosis infection. Infect. Agents Dis. 5:73-81. [PubMed] [Google Scholar]
- 5.Brown, A. E., T. J. Holzer, and B. R. Andersen. 1987. Capacity of human neutrophils to kill Mycobacterium tuberculosis. J. Infect. Dis. 156:985-989. [DOI] [PubMed] [Google Scholar]
- 6.Conlan, J. W. 1997. Critical roles of neutrophils in host defense against experimental systemic infections of mice by Listeria monocytogenes, Salmonella typhimurium, and Yersinia enterocolitica. Infect. Immun. 65:630-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conlan, J. W., and R. J. North. 1994. Neutrophils are essential for early anti-Listeria defense in the liver, but not the spleen or peritoneal cavity, as revealed by a granulocyte- depleting antibody. J. Exp. Med. 179:259-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czuprynski, C. J., C. Theisen, and J. F. Brown. 1996. Treatment with the antigranulocyte monoclonal antibody RB6-8C5 impairs resistance of mice to gastrointestinal infection with Listeria monocytogenes. Infect. Immun. 64:3946-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Souza, C. D., A. M. Cooper, A. A. Frank, R. J. Mazzaccaro, B. R. Bloom, and I. M. Orme. 1997. An anti-inflammatory role for γδ T lymphocytes in acquired immunity to Mycobacterium tuberculosis. J. Immunol. 158:1217-1221. [PubMed] [Google Scholar]
- 10.Faldt, J., C. Dahlgren, A. Karlsson, A. M. S. Ahmed, D. E. Minnikin, and M. Ridell. 1999. Activation of human neutrophils by mycobacterial phenolic glycolipids. Clin. Exp. Immunol. 118:253-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fanger, N. A., C. Liu, P. M. Guyre, K. Wardewell, J. O'Neil, T. L. Guo, T. P. Christian, S. P. Mudzinski, and E. J. Gosselin. 1997. Activation of human T cells by major histocompatability complex class II expressing neutrophils: proliferation in the presence of superantigen, but not tetanus toxoid. Blood 89:4128-4135. [PubMed] [Google Scholar]
- 12.Fulton, S. A., T. D. Martin, R. W. Redline, and W. H. Boom. 2000. Pulmonary immune responses during primary Mycobacterium bovis BCG-Calmette-Guerin bacillus infection in C57Bl/6 mice. Am. J. Respir. Cell Mol. Biol. 22:333-343. [DOI] [PubMed] [Google Scholar]
- 13.Giosue, S., M. Casarini, L. Alemanno, G. Galluccio, P. Mattia, G. Pedicelli, L. Rebek, A. Bisetti, and F. Ameglio. 1998. Effects of aerosolized interferon-α in patients with pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 158:1156-1162. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez-Pando, R. Orozcoe, A. Sampieri, L. Pavon, C. Velasquillo, J. Larriva-Sahd, J. M. Alcocer, and M. V. Madrid. 1996. Correlation between the kinetics of Th1/Th2 cells and pathology in a murine model of experimental pulmonary tuberculosis. Immunology 80:26-31. [PMC free article] [PubMed] [Google Scholar]
- 15.Jones, G. S., H. J. Amirault, and B. R. Andersen. 1990. Killing of Mycobacterium tuberculosis by neutrophils. A nonoxidative process. J. Infect. Dis. 162:700-704. [DOI] [PubMed] [Google Scholar]
- 16.Kasahara, K., I. Sato, K. Ogura, H. Takeuchi, K. Kobayashi, and M. Adachi. 1998. Expression of chemokines and induction of rapid cell death in human blood neutrophils by Mycobacterium tuberculosis. J. Infect. Dis. 178:127-137. [DOI] [PubMed] [Google Scholar]
- 17.Kasama, T., R. M. Strieter, N. W. Lukacs, P. M. Lincoln, M. D. Burdick, and S. L. Kunkel. 1995. Interferon gamma modulates the expression of neutrophil-derived chemokines. J. Investig. Med. 43:58-67. [PubMed] [Google Scholar]
- 18.Kaufmann, S. H. 1993. Immunity to intracellular bacteria. Annu. Rev. Immunol. 11:129-163. [DOI] [PubMed] [Google Scholar]
- 19.Lau, Y. L., G. C. F. Chan, S. Y. Ha, Y. F. Hui, and K. Y. Yuen. 1998. The role of phagocytic respiratory burst in host defense against Mycobacterium tuberculosis. Clin. Infect. Dis. 26:226-227. [DOI] [PubMed] [Google Scholar]
- 20.Lopez, S., A. J. Marco, N. Prats, and C. J. Czuprynski. 2000. Critical role of neutrophils in eliminating Listeria monocytogenes from the central nervous system during experimental murine listeriosis. Infect. Immun. 68:4789-4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Majeed, M., N. Perskvist, J. D. Ernst, K. Orselius, and O. Stendahl. 1998. Roles of calcium and annexins in phagocytosis and elimination of an attenuated strain of Mycobacterium tuberculosis in human neutrophils. Microb. Pathog. 24:309-320. [DOI] [PubMed] [Google Scholar]
- 22.May, M. E., and P. J. Spagnuolo. 1987. Evidence for activation of a respiratory burst in the interaction of human neutrophils with Mycobacterium tuberculosis. Infect. Immun. 55:2304-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyakawa, Y., P. Ratnakar, G. Rao, M. L. Costello, O. Mathieu-Costello, R. I. Lehrer, and A. Catanzaro. 1996. In vitro activity of the antimicrobial peptides human and rabbit defensins and porcine leukocyte protegrin against Mycobacterium tuberculosis. Infect. Immun. 64:926-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray, P. J., R. A. Young, and G. Q. Daley. 1998. Hematopoietic remodeling in interferon-γ-deficient mice infected with mycobacteria. Blood 91:2914-2924. [PubMed] [Google Scholar]
- 25.Neufert, C., R. K. Pai, E. H. Noss, M. Berger, W. H. Boom, and C. V. Harding. 2001. Mycobacterium tuberculosis 19-kDa lipoprotein promotes neutrophil activation. J. Immunol. 167:1542-1549. [DOI] [PubMed] [Google Scholar]
- 26.Palmero, D., K. Eiguchi, P. Rendo, L. Castro Zorrilla, E. Abbate, and L. G. Gonzalez Montaner. 1999. Phase II trial of recombinant interferon-α2b in patients with advanced intractable multidrug-resistant pulmonary tuberculosis: long-term follow up. Int. J. Tuberc. Lung Dis. 3:214-218. [PubMed] [Google Scholar]
- 27.Pedrosa, J., B. M. Saunders, R. Appelberg, I. M. Orme, M. T. Silva, and A. M. Cooper. 2000. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infect. Immun. 68:577-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrofsky, M., and L. E. Bermudez. 1999. Neutrophils from Mycobacterium avium- infected mice produce TNF-α, IL-12 and IL-1β and have a putative role in early host responses. Clin. Immunol. 91:354-358. [DOI] [PubMed] [Google Scholar]
- 29.Potter, N. S., and C. V. Harding. 2001. Neutrophils process exogenous bacteria via an alternate class I MHC processing pathway for presentation of peptides to T lymphocytes. J. Immunol. 167:2538-2546. [DOI] [PubMed] [Google Scholar]
- 30.Rhoades, E. R., A. M. Cooper, and I. M. Orme. 1995. Chemokine response in mice infected with Mycobacterium tuberculosis. Infect. Immun. 63:3871-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadek, M. I., E. Sada, Z. Toossi, S. K. Schwander, and E. A. Rich. 1998. Chemokines induced by infection of mononuclear phagocytes with mycobacteria and present in lung alveoli during active pulmonary tuberculosis. Am. J. Respir. Cell Mol. Biol. 19:513-521. [DOI] [PubMed] [Google Scholar]
- 32.Saunders, B. M., and C. Cheers. 1996. Intranasal infection of beige mice with Mycobacterium avium complex: role of neutrophils and natural killer cells. Infect. Immun. 64:4236-4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwander, S. K., E. Sada, M. Torres, D. Escobedo, J. G. Sierra, S. Alt, and E. A. Rich. 1996. T lymphocytic and immature macrophage alveolitis in active pulmonary tuberculosis. J. Infect. Dis. 173:1267-1272. [DOI] [PubMed] [Google Scholar]
- 34.Segal, B. H., T. M. Doherty, T. A. Wynn, A. W. Cheever, A. Sher, and S. M. Holland. 1999. The p47phox−/− mouse model of chronic granulomatous disease has normal granuloma formation and cytokine responses to Mycobacterium avium and Schistosoma mansoni eggs. Infect. Immun. 67:1659-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seiler, P., P. Aichele, B. Raupach, B. Odermatt, U. Steinhoff, and S. H. E. Kaufmann. 2000. Rapid neutrophil response controls fast-replicating intracellular bacteria but not slow-replicating Mycobacterium tuberculosis. J. Infect. Dis. 181:671-680. [DOI] [PubMed] [Google Scholar]
- 36.Sharma, S., I. Verma, and G. K. Khuller. 2001. Therapeutic potential of human neutrophil peptide 1 against experimental tuberculosis. Antimicrob. Agents Chemother. 45:639-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith, W. B., L. Guida, Q. Sun, E. I. Korpelainen, C. van den Heuvel, D. Gillis, C. M. Hawrylowicz, M. A. Vadas, and A. F. Lopez. 1995. Neutrophils activated by granulocyte-macrophage colony-stimulating factor express receptors for interleukin-3 which mediate class II expression. Blood 63:3938-3944. [PubMed] [Google Scholar]