Abstract
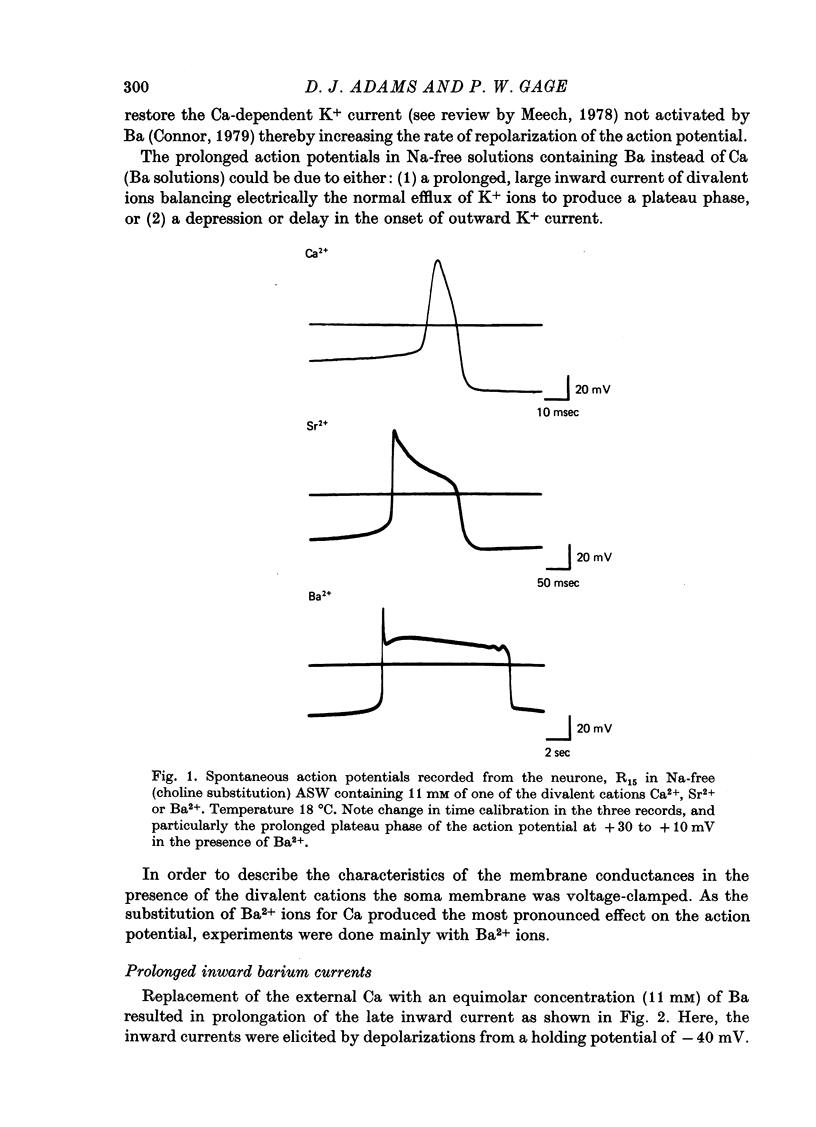
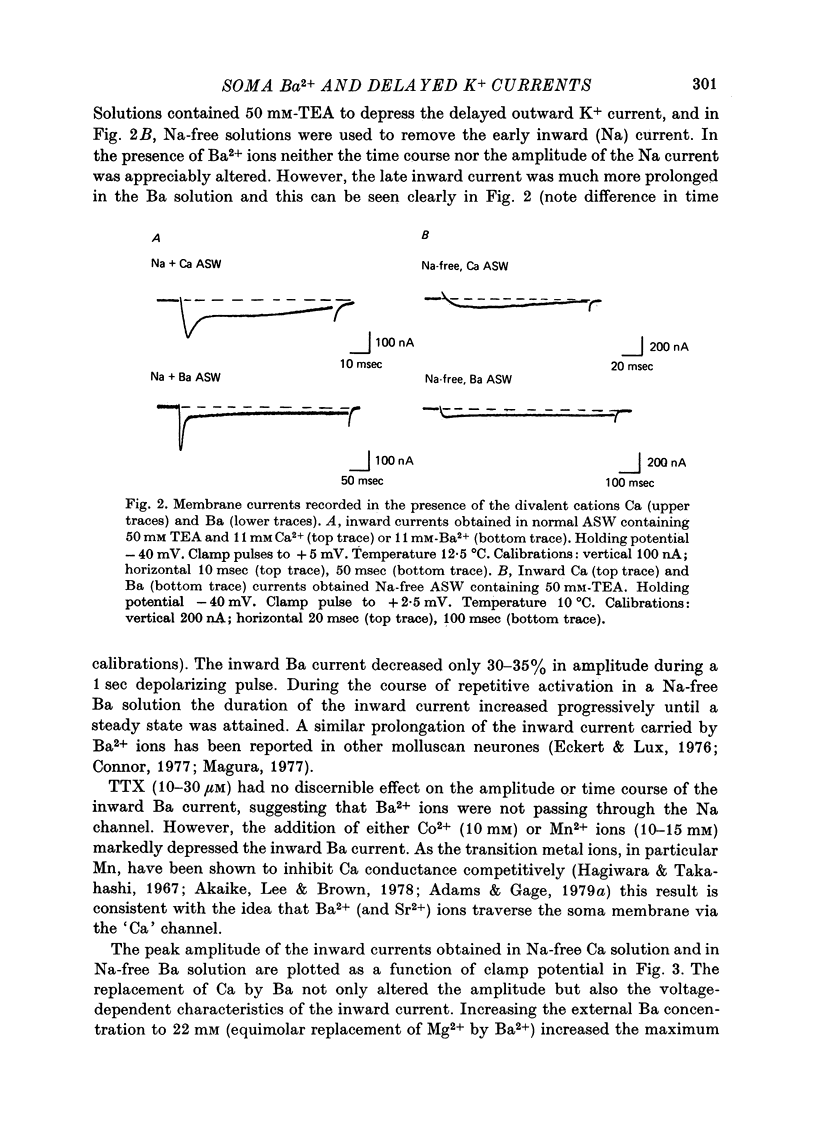
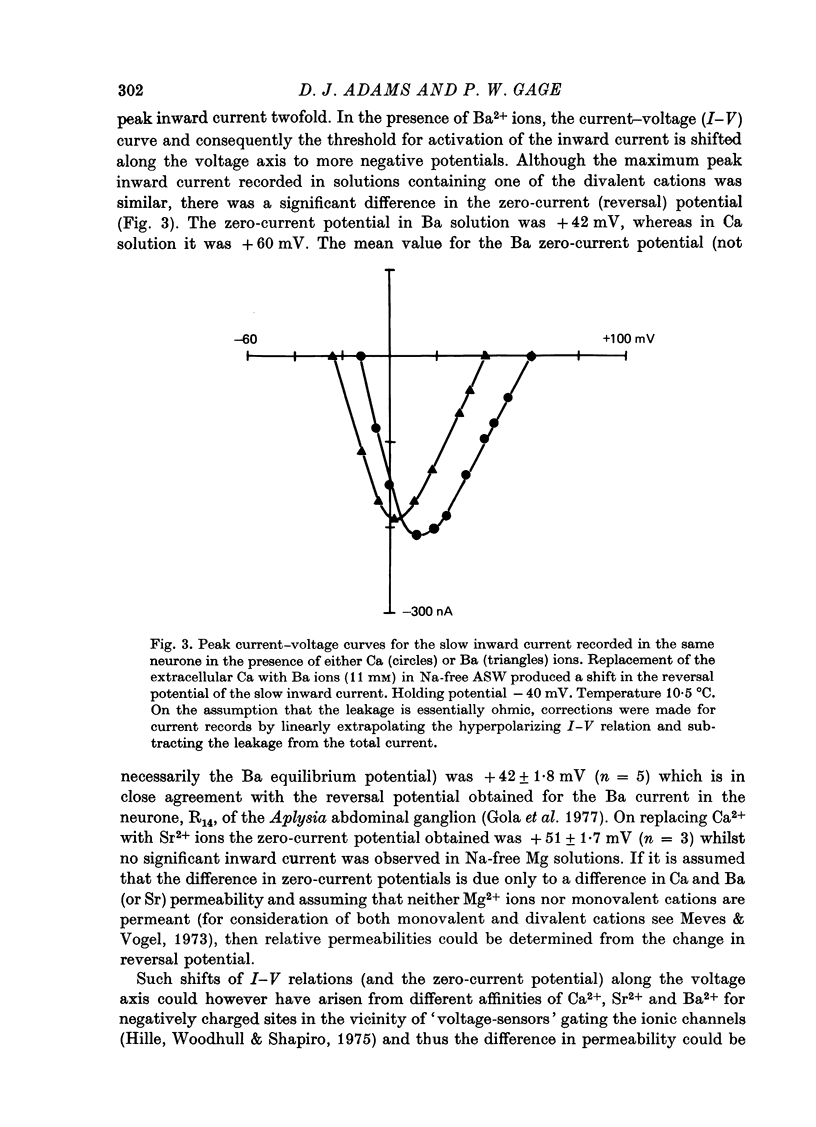
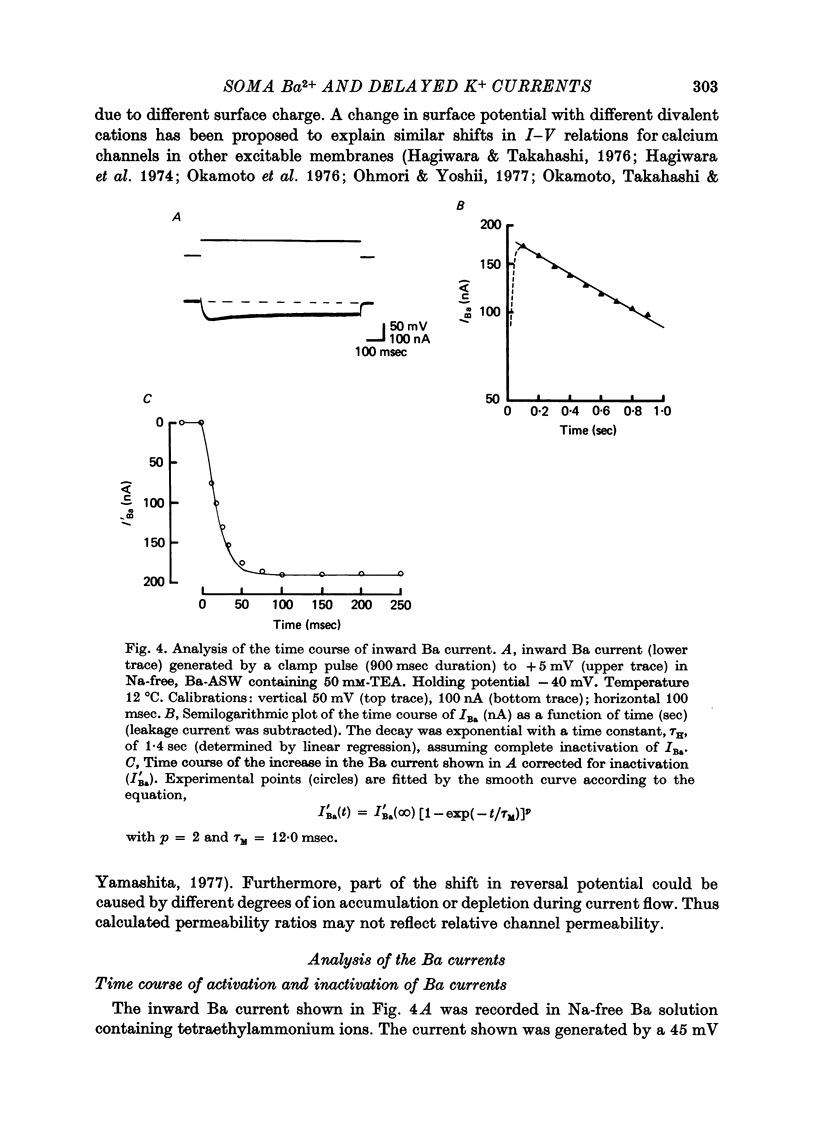
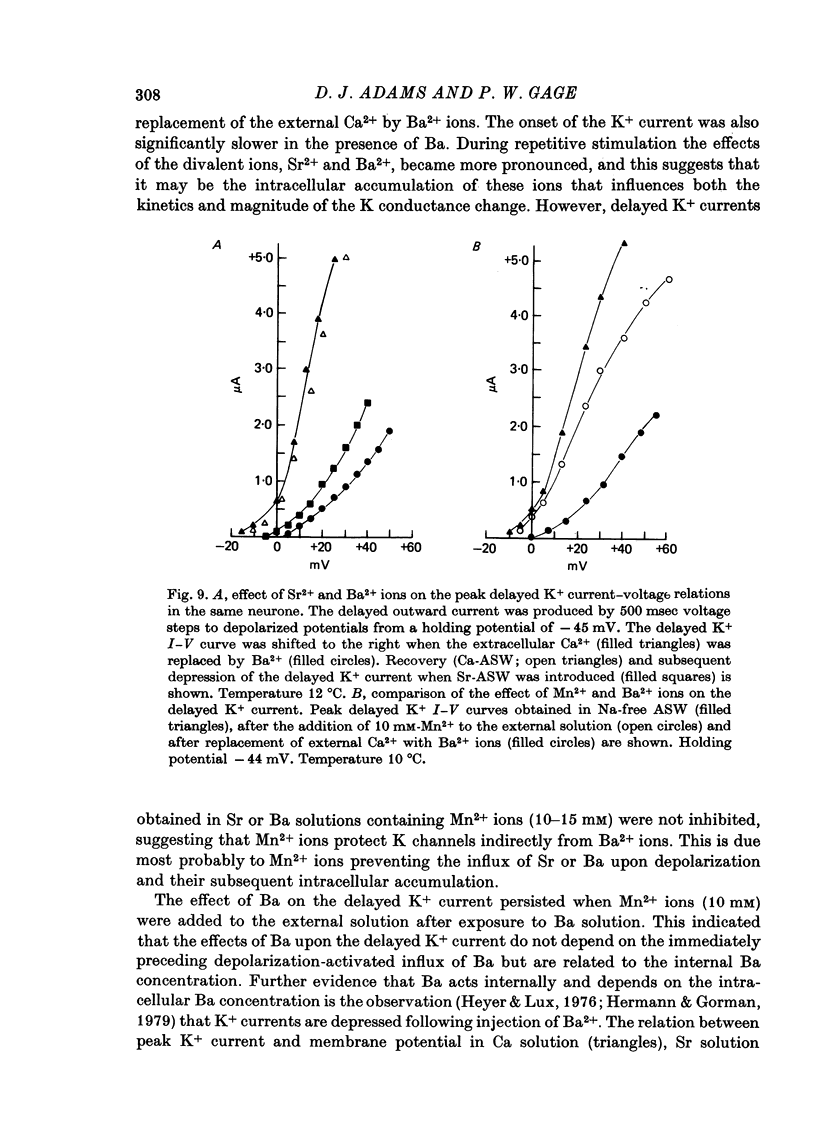
1. In Na- and Ca-free external solutions, Sr or Ba (but not Mg) could act as carriers of inward current during action potentials in the neurone, R15 of the Aplysia abdominal ganglion. These action potentials exhibited a prolonged plateau phase, the duration of which was dependent on the concentration and species of divalent cation and activity of the neurone. 2. Depolarization of the soma membrane in Na-free Ba solution generated a prolonged, 'late' inward current the amplitude of which was dependent on the external Ba concentration. The Ba current was insensitive to tetrodotoxin but could be blocked by Mn2+ and Co2+ ions. 3. The peak current-voltage relation and threshold for activation of the late inward current was shifted to more negative potentials on replacement of Ca with Ba. The zero-current (reversal) potentials for both Sr and Ba were more negative than for Ca, indicating that the 'Ca' channel is less permeable to Sr2+ or Ba2+ ions than to Ca2+ ions. 4. Inactivation of the 'Ca' channel is slower in Ba than in Ca solution. The time course of Ba currents during a maintained depolarization of 2 sec could be reasonably described by the expression, I'Ba(t) = I'Ba (infinity) [1-exp(-t/tau M)]2exp(-t/tau H). 5. Time constants for activation (tau M) and inactivation (tau H) were voltage-dependent. In the range -10 to +30 mV, tau M varied from 15 to 5 msec and tau H from 2.0 to 0.5 sec (12 degrees C). Steady-state Ba conductance (corrected for inactivation) was voltage-dependent, increasing sigmoidally with depolarization to a maximum of approximately 12 microS at potentials beyond +15 mV. 6. Steady-state inactivation of Ba conductance (hBa(infinity)) varied with holding potential (VH). Conditioning holding potentials more negative than the resting potential (-40 to -50 mV) produced depression of Ba currents. Complete inactivation of Ba currents occurred at holding potentials more positive than 0 mV or with repetitive activation at frequencies greater than 1 Hz. 7. The divalent ions, Ba2+ and Sr2+, reversible depressed the total delayed K+ current at a rate dependent on the frequency of activation. Ba and Sr shifted the delayed K+ current-voltage curve to more positive voltages and depressed the delayed outward current at all membrane potentials. 8. Comparison of the effect of Ba on delayed K+ currents with those obtained in the presence of Mn2+ ions indicated that Ba2+ ions depress both the voltage-dependent and Ca-dependent components of the delayed K+ current. However, the mechanism by which Ba acts to inhibit the two components of the delayed K+ current appears to be different.
Full text
PDF








Selected References
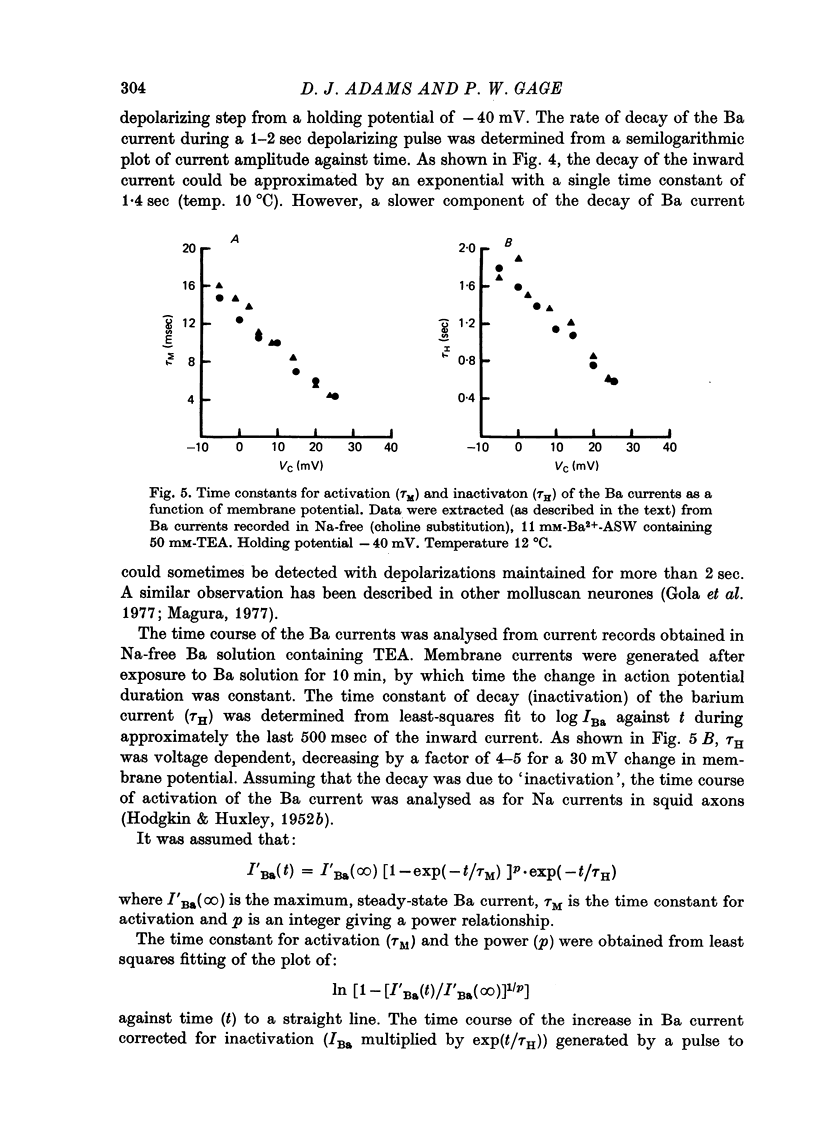
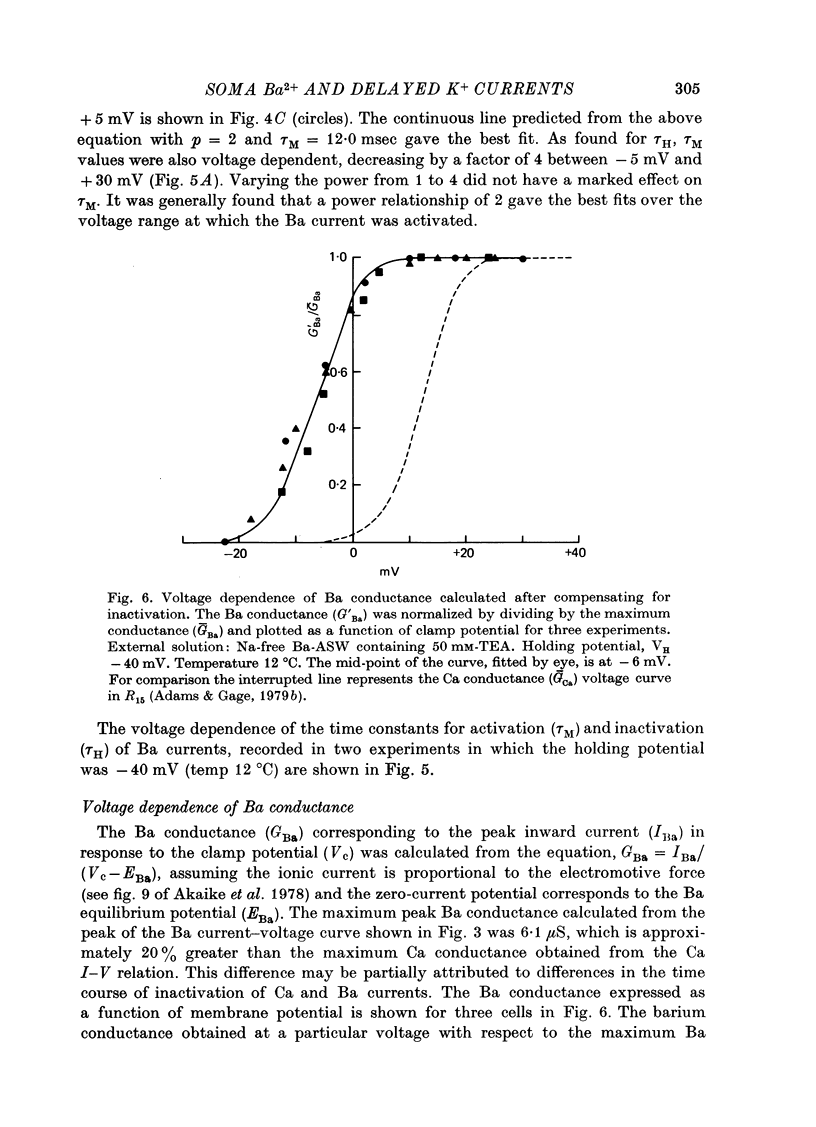
These references are in PubMed. This may not be the complete list of references from this article.
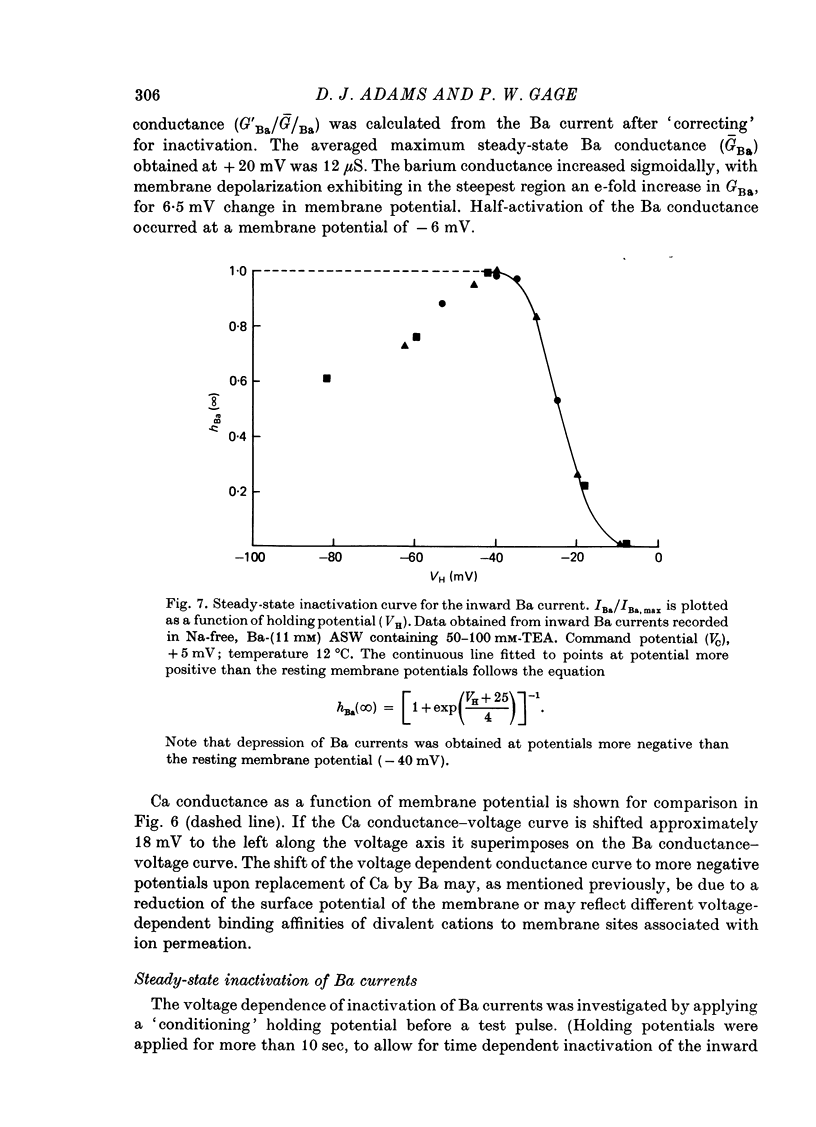
- Adams D. J., Gage P. W. Characteristics of sodium and calcium conductance changes produced by membrane depolarization in an Aplysia neurone. J Physiol. 1979 Apr;289:143–161. doi: 10.1113/jphysiol.1979.sp012729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams D. J., Gage P. W. Ionic currents in response to membrane depolarization in an Aplysia neurone. J Physiol. 1979 Apr;289:115–141. doi: 10.1113/jphysiol.1979.sp012728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Lee K. S., Brown A. M. The calcium current of Helix neuron. J Gen Physiol. 1978 May;71(5):509–531. doi: 10.1085/jgp.71.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich R. W., Jr, Getting P. A., Thompson S. H. Inactivation of delayed outward current in molluscan neurone somata. J Physiol. 1979 Jun;291:507–530. doi: 10.1113/jphysiol.1979.sp012828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm P., Eckert R. Calcium entry leads to inactivation of calcium channel in Paramecium. Science. 1978 Dec 15;202(4373):1203–1206. doi: 10.1126/science.103199. [DOI] [PubMed] [Google Scholar]
- Butler J. N. The thermodynamic activity of calcium ion in sodium chloride-calcium chloride electrolytes. Biophys J. 1968 Dec;8(12):1426–1433. doi: 10.1016/S0006-3495(68)86564-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A. Calcium current in molluscan neurones: measurement under conditions which maximize its visibility. J Physiol. 1979 Jan;286:41–60. doi: 10.1113/jphysiol.1979.sp012606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A. Time course separation of two inward currents in molluscan neurons. Brain Res. 1977 Jan 7;119(2):487–492. doi: 10.1016/0006-8993(77)90330-4. [DOI] [PubMed] [Google Scholar]
- Eckert R., Lux H. D. A voltage-sensitive persistent calcium conductance in neuronal somata of Helix. J Physiol. 1976 Jan;254(1):129–151. doi: 10.1113/jphysiol.1976.sp011225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENGARD P., STRAUB R. W. Restoration by barium of action potentials in sodium-deprived mammalian B and C fibres. J Physiol. 1959 Mar 12;145(3):562–569. doi: 10.1113/jphysiol.1959.sp006162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geduldig D., Junge D. Sodium and calcium components of action potentials in the Aplysia giant neurone. J Physiol. 1968 Dec;199(2):347–365. doi: 10.1113/jphysiol.1968.sp008657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gola M., Ducreux C., Chagneux H. Ionic mechanism of slow potential wave production in barium-treated Aplysia neurons. J Physiol (Paris) 1977;73(4):407–440. [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Fukuda J., Eaton D. C. Membrane currents carried by Ca, Sr, and Ba in barnacle muscle fiber during voltage clamp. J Gen Physiol. 1974 May;63(5):564–578. doi: 10.1085/jgp.63.5.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Nakajima S. Effects of the intracellular Ca ion concentration upon the excitability of the muscle fiber membrane of a barnacle. J Gen Physiol. 1966 Mar;49(4):807–818. doi: 10.1085/jgp.49.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann A., Gorman A. L. Blockade of voltage-dependent and Ca2+-dependent K+ current components by internal Ba2+ in molluscan pacemaker neurons. Experientia. 1979 Feb 15;35(2):229–231. doi: 10.1007/BF01920633. [DOI] [PubMed] [Google Scholar]
- Heyer C. B., Lux H. D. Control of the delayed outward potassium currents in bursting pace-maker neurones of the snail, Helix pomatia. J Physiol. 1976 Nov;262(2):349–382. doi: 10.1113/jphysiol.1976.sp011599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Woodhull A. M., Shapiro B. I. Negative surface charge near sodium channels of nerve: divalent ions, monovalent ions, and pH. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):301–318. doi: 10.1098/rstb.1975.0011. [DOI] [PubMed] [Google Scholar]
- Koketsu K., Nishi S. Calcium and action potentials of bullfrog sympathetic ganglion cells. J Gen Physiol. 1969 May;53(5):608–623. doi: 10.1085/jgp.53.5.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magura I. S. Long-lasting inward current in snail neurons in barium solutions in voltage-clamp conditions. J Membr Biol. 1977 Jul 14;35(3):239–256. doi: 10.1007/BF01869952. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. G., Szabo G., Eisenman G. Divalent ions and the surface potential of charged phospholipid membranes. J Gen Physiol. 1971 Dec;58(6):667–687. doi: 10.1085/jgp.58.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H. The ionic requirements for the production of action potentials in helix pomatia neurones. Pflugers Arch. 1968;304(3):215–241. doi: 10.1007/BF00592126. [DOI] [PubMed] [Google Scholar]
- Meves H., Vogel W. Calcium inward currents in internally perfused giant axons. J Physiol. 1973 Nov;235(1):225–265. doi: 10.1113/jphysiol.1973.sp010386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHI S., SOEDA H., KOKETSU K. EFFECT OF ALKALI-EARTH CATIONS ON FROG SPINAL GANGLION CELL. J Neurophysiol. 1965 May;28:457–472. doi: 10.1152/jn.1965.28.3.457. [DOI] [PubMed] [Google Scholar]
- Neher E., Lux H. D. Rapid changes of potassium concentration at the outer surface of exposed single neurons during membrane current flow. J Gen Physiol. 1973 Mar;61(3):385–399. doi: 10.1085/jgp.61.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H., Yoshii M. Surface potential reflected in both gating and permeation mechanisms of sodium and calcium channels of the tunicate egg cell membrane. J Physiol. 1977 May;267(2):429–463. doi: 10.1113/jphysiol.1977.sp011821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Takahashi K., Yamashita N. Ionic currents through the membrane of the mammalian oocyte and their comparison with those in the tunicate and sea urchin. J Physiol. 1977 May;267(2):465–495. doi: 10.1113/jphysiol.1977.sp011822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Takahashi K., Yoshii M. Two components of the calcium current in the egg cell membrane of the tunicate. J Physiol. 1976 Feb;255(2):527–561. doi: 10.1113/jphysiol.1976.sp011294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillotson D., Horn R. Inactivation without facilitation of calcium conductance in caesium-loaded neurones of Aplysia. Nature. 1978 May 25;273(5660):312–314. doi: 10.1038/273312a0. [DOI] [PubMed] [Google Scholar]
- Tillotson D. Inactivation of Ca conductance dependent on entry of Ca ions in molluscan neurons. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1497–1500. doi: 10.1073/pnas.76.3.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereecke J., Carmeliet E. Sr action potentials in cardiac Purkyne fibres. II. Dependence of the Sr conductance on the external Sr concentration and Sr-Ca antagonism. Pflugers Arch. 1971;322(1):73–82. doi: 10.1007/BF00586666. [DOI] [PubMed] [Google Scholar]
- WERMAN R., GRUNDFEST H. Graded and all-or-none electrogenesis in arthropod muscle. II. The effects of alkali-earth and onium ions on lobster muscle fibers. J Gen Physiol. 1961 May;44:997–1027. doi: 10.1085/jgp.44.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]


