Abstract
1. ACh-induced secretion from the main excretory duct, as well as ACh-induced hyperpolarizations and resting membrane potentials of superficial, probably acinar, cells were recorded from the rabbit lacrimal gland perfused in vivo with either control or test solutions.
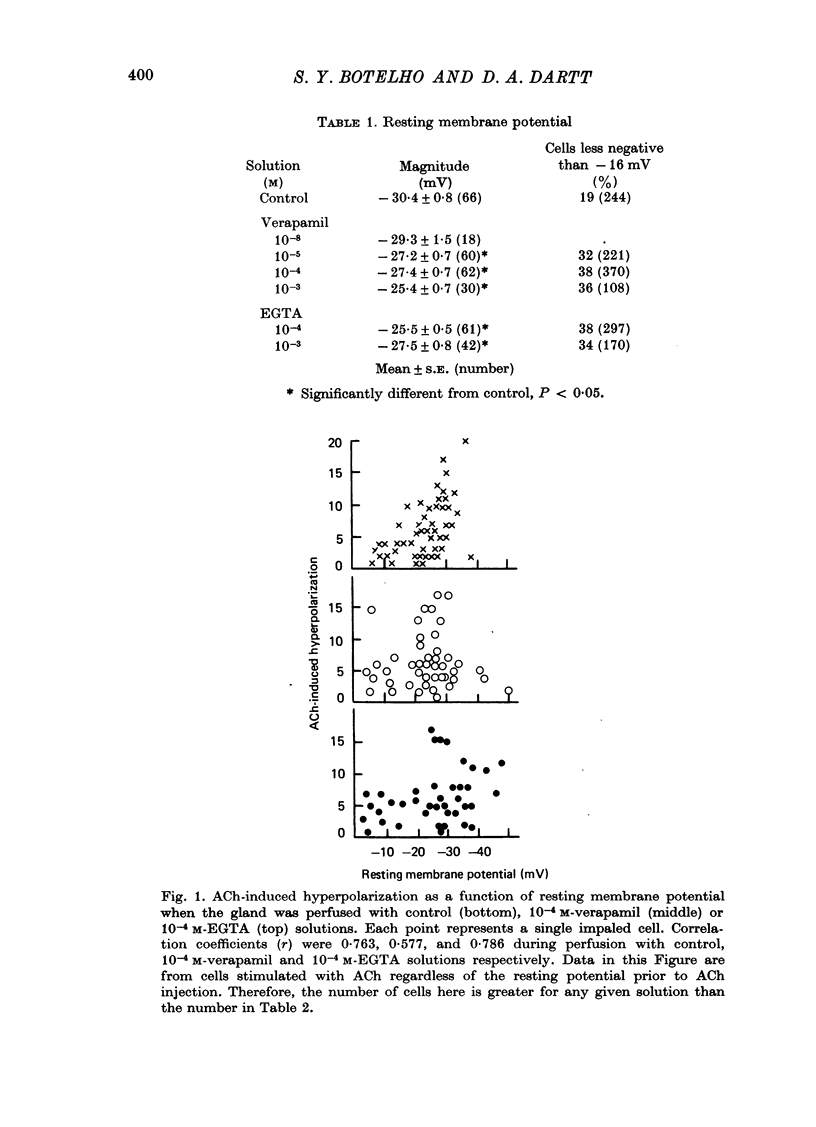
2. During perfusion with test solution containing a Ca antagonist, verapamil (10-5 to 10-3 M), or a Ca chelator EGTA (10-4 or 10-3 M), ACh-induced secretion was only 30% of the control value, whereas the magnitude of the ACh-induced hyperpolarization was unchanged.
3. With 10-5 M-verapamil, but not with 10-3 M-verapamil or EGTA-containing solutions, the inhibition of ACh-induced flow was completely reversed either by replacing the test with control solution or by increasing the Ca concentration of the test solution twofold.
4. In addition, EGTA (10-4 M) solution containing the normal extracellular Ca2+ concentration inhibited secretion by 30%.
5. During perfusion with verapamil or EGTA test solutions the mean resting membrane potential of superficial, probably acinar, cells was depolarized by 15%.
6. It is concluded that although extracellular Ca is required for the major portion of ACh-induced secretion, the magnitude of the ACh-induced hyperpolarization of cells, which are most likely acinar, is independent of extracellular Ca.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. H., van Lennep E. W., Young J. A. Water and electrolyte secretion by the exorbital lacrimal gland of the rat studied by micropuncture and catheterization techniques. Pflugers Arch. 1972;337(4):299–309. doi: 10.1007/BF00586647. [DOI] [PubMed] [Google Scholar]
- Arenson M. S., Wilson H. The parasympathetic secretory nerves of the lacrimal gland of the cat. J Physiol. 1971 Aug;217(1):201–212. doi: 10.1113/jphysiol.1971.sp009566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botelho S. Y., Fuenmayor N., Hisada M. Flow and potentials during perfusion of lacrimal gland with electrolyte solutions. Am J Physiol. 1978 Jul;235(1):C8–12. doi: 10.1152/ajpcell.1978.235.1.C8. [DOI] [PubMed] [Google Scholar]
- Botelho S. Y., Hisada M., Fuenmayor N. Functional innervation of the lacrimal gland in the cat. Origin of secretomotor fibers in the lacrimal nerve. Arch Ophthalmol. 1966 Oct;76(4):581–588. doi: 10.1001/archopht.1966.03850010583019. [DOI] [PubMed] [Google Scholar]
- Botelho S. Y., Martinez E. V. Electrolytes in lacrimal gland fluid and in tears at various flow rates in the rabbit. Am J Physiol. 1973 Sep;225(3):606–609. doi: 10.1152/ajplegacy.1973.225.3.606. [DOI] [PubMed] [Google Scholar]
- Botelho S. Y., Martinez E. V., Pholpramool C., Prooyen H. C., Janssen J. T., De Palau A. Modification of stimulated lacrimal gland flow by sympathetic nerve impulses in rabbit. Am J Physiol. 1976 Jan;230(1):80–84. doi: 10.1152/ajplegacy.1976.230.1.80. [DOI] [PubMed] [Google Scholar]
- DREISBACH R. H. CALCIUM TRANSFER IN RAT SALIVARY AND LACRIMAL GLANDS. Int Ser Monogr Oral Biol. 1964;3:237–251. doi: 10.1016/b978-1-4832-2871-6.50022-9. [DOI] [PubMed] [Google Scholar]
- Dreifuss J. J., Grau J. D., Nordmann J. J. Effects on the isolated neurohypophysis of agents which affect the membrane permeability to calcium. J Physiol. 1973 Jun;231(2):96P–98P. [PMC free article] [PubMed] [Google Scholar]
- Eto S., Wood J. M., Hutchins M., Fleischer N. Pituitary 45 Ca ion uptake and release of ACTH, GH, and TSH: effect of verapamil. Am J Physiol. 1974 Jun;226(6):1315–1320. doi: 10.1152/ajplegacy.1974.226.6.1315. [DOI] [PubMed] [Google Scholar]
- Hisada M., Botelho S. Y. Membrane potentials of in situ lacrimal gland in the cat. Am J Physiol. 1968 Jun;214(6):1262–1267. doi: 10.1152/ajplegacy.1968.214.6.1262. [DOI] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Intracellular Ca2+ injection causes membrane hyperpolarization and conductance increase in lacrimal acinar cells. Pflugers Arch. 1978 Nov 14;377(2):185–187. [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Membrane potential, resistance, and intercellular communication in the lacrimal gland: effects of acetylcholine and adrenaline. J Physiol. 1978 Feb;275:507–520. doi: 10.1113/jphysiol.1978.sp012204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno T. The electrogenic sodium pump in the hyperpolarizing and secretory effects of pancreozymin in the pancreatic acinar cell. J Physiol. 1975 Mar;245(3):599–616. doi: 10.1113/jphysiol.1975.sp010864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keryer G., Rossignol B. Effect of carbachol on 45Ca uptake and protein secretion in rat lacrimal gland. Am J Physiol. 1976 Jan;230(1):99–104. doi: 10.1152/ajplegacy.1976.230.1.99. [DOI] [PubMed] [Google Scholar]
- Kondo S., Schulz I. Calcium ion uptake in isolated pancreas cells induced by secretagogues. Biochim Biophys Acta. 1976 Jan 8;419(1):76–92. doi: 10.1016/0005-2736(76)90373-4. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Herchuelz A., Levy J., Sener A. Calcium antagonists and islet function--III. The possible site of action of verapamil. Biochem Pharmacol. 1977 Apr 15;26(8):735–740. doi: 10.1016/0006-2952(77)90217-9. [DOI] [PubMed] [Google Scholar]
- Parod R. J., Putney J. W., Jr The role of calcium in the receptor mediated control of potassium permeability in the rat lacrimal gland. J Physiol. 1978 Aug;281:371–381. doi: 10.1113/jphysiol.1978.sp012428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H. Electrophysiological studies on gland cells. Experientia. 1974 Feb 15;30(2):130–134. doi: 10.1007/BF01927689. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Poulsen J. H., Thorn N. A. Secretory potentials, secretory rate and water permeability of the duct system in the cat submandibular gland during perfusion with calcium-free Locke's solution. Acta Physiol Scand. 1967 Oct-Nov;71(2):203–210. doi: 10.1111/j.1748-1716.1967.tb03726.x. [DOI] [PubMed] [Google Scholar]
- Pholpramool C., Korppaibool S. Ionic dependence of the resting membrane potential of rabbit lacrimal gland in vitro. Biochim Biophys Acta. 1977 Aug 1;468(3):353–363. doi: 10.1016/0005-2736(77)90287-5. [DOI] [PubMed] [Google Scholar]
- Pholpramool C., Tangkrisanavinont V. A calcium ionophore-induced secretion in rabbit lacrimal gland in vivo. Life Sci. 1976 Aug 1;19(3):381–388. doi: 10.1016/0024-3205(76)90042-4. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr, Parod R. J., Marier S. H. Control by calcium of protein discharge and membrane permeability to potassium in the rat lacrimal gland. Life Sci. 1977 Jun 1;20(11):1905–1911. doi: 10.1016/0024-3205(77)90227-2. [DOI] [PubMed] [Google Scholar]


