Abstract
The usefulness of isoniazid (INH), a key component of short-course chemotherapy of tuberculosis, is threatened by the emergence of drug-resistant strains of Mycobacterium tuberculosis with mutations in the katG gene. It is shown here that the most commonly occurring KatG mutation, where Ser 315 is replaced by Thr (S315T), is associated with clinically significant levels of INH resistance. In contrast to another resistant isolate, in which Pro replaces Thr 275, the S315T mutant produces active catalase-peroxidase and is virulent in the mouse model of the disease, indicating that a significant loss of bacterial fitness does not result from this frequent mutation. The implications of this finding for the transmission and reactivation of multidrug-resistant strains of M. tuberculosis are severe.
The emergence of multidrug-resistant (MDR) strains of Mycobacterium tuberculosis poses a significant threat to the global control of tuberculosis. Although individual cases of MDR tuberculosis (MDR-TB) have occurred since the initial introduction of combination chemotherapy for tuberculosis, microepidemics of MDR-TB appeared in the United States during the 1980s (8, 35). Massive investment in appropriate health care interventions appears to have brought these epidemics under control (10), but recent worldwide surveillance has demonstrated that drug-resistant strains are now widespread and reaching alarmingly high levels in certain countries (9). MDR-TB is a potentially untreatable, transmissible disease associated with a high mortality (15).
The appearance of drug-resistant strains of bacteria has prompted the question of whether the more prudent use or complete cessation of use of an antibiotic would lead to the elimination of these strains. Attempts to mathematically model these scenarios indicate that the cost of the resistance-conferring mutations, in terms of bacterial fitness, and the ability of the bacteria to genetically compensate for such costs are key parameters in determining if resistance-conferring mutations will be maintained within a bacterial population in the absence of the antibiotic (18). Experimental work conducted in various model systems has established that chromosomal mutations conferring antibiotic resistance are almost invariably associated with a significant cost and in the absence of drug are adapted for by accessory compensatory mutations rather than by reversion to the drug-sensitive high-fitness genotype (2). This predicts that drug-resistant alleles are likely to become fixed in these bacterial populations in the absence, or following the diminished use, of the antibiotic in question, with obvious implications for the control of pathogenic organisms.
Little is known about the effects of antibiotic-resistance-conferring mutations on the bacterial fitness of M. tuberculosis (3, 4). We were particularly interested in the paradox posed by isoniazid (INH) resistance. Clinically significant INH resistance is most commonly due to missense or null mutations in the katG gene, which codes for a catalase-peroxidase enzyme (12, 43) that converts INH to its bioactive form (16). KatG is also a virulence factor, and strains of M. tuberculosis lacking katG are heavily attenuated in a variety of animal models (17, 25, 30, 42). KatG is thought to be required for protection against oxygen free radicals within the macrophage (21), though some studies have failed to demonstrate this (1, 26, 27). How is it then that the transmission of INH-resistant strains occurs even in the context of an MDR phenotype, which is likely to be associated with other fitness-reducing mutations? One possibility is the presence of compensatory mutations. Mutations have been described previously for the promoter region of ahpC, resulting in the upregulation of AhpC (34), an alkyl hydroperoxidase normally only feebly expressed in M. tuberculosis (36). It has been suggested that this is a mechanism for rebalancing the protective mechanisms against oxidative stress caused by the loss of a functional KatG. However, studies with different animal models have failed to conclusively demonstrate that AhpC overexpression acts as a compensatory virulence mechanism (14, 36, 41).
An alternative explanation has been suggested by biochemical analysis of mutant KatG proteins (33, 40). These revealed that one such mutant, with a serine-to-threonine alteration at codon 315 (S315T), appeared to have a diminished capacity to activate INH while retaining significant catalase activity. This mutation might therefore be selected for in vivo because it confers INH resistance (32), while leading to only a minimal reduction in fitness, a hypothesis supported by the observation that S315T is the most frequently encountered INH resistance-conferring mutation (29). To test this hypothesis, we constructed a panel of isogenic strains of M. tuberculosis with different katG alleles and characterized them in an animal model of tuberculosis. Unlike most resistance-conferring mutations, S315T was found to result in near-normal catalase-peroxidase activities and levels of virulence while also conferring resistance to INH. Contrary to previous perceptions, this mechanism of INH resistance will not usually be associated with a large reduction in virulence and is an exception to the rule that antibiotic-resistance-conferring mutations carry a significant fitness cost. This has important implications for the transmission and control of MDR-TB.
MATERIALS AND METHODS
Cloning.
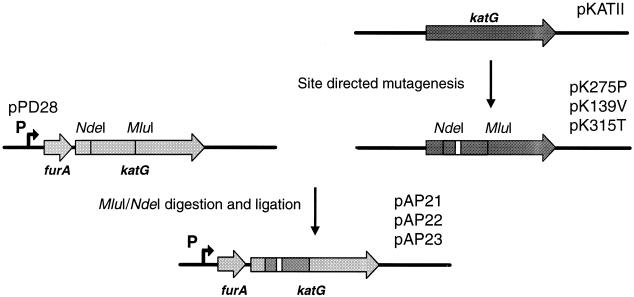
The Chameleon double-stranded site-directed mutagenesis kit (Stratagene) was applied to an Escherichia coli expression vector containing the M. tuberculosis katG gene (pKAT11) (33) to introduce single-base-pair mutations into either codon 139 (GCC to GTC), 275 (ACC to CCC), or 315 (AGC to ACC). An 1,100-bp NdeI-MluI fragment from each of the three resulting plasmids was then ligated into the equivalent restriction sites of the katG gene of plasmid pPD28 (30) (Fig. 1). This produced the plasmids pAP21 to pAP23 (Table 1) with the respective mutant katG genes located downstream of the M. tuberculosis furA gene, in exact concordance with the normal genomic organization of furA and katG in M. tuberculosis. We opted for this cloning strategy to ensure that the main katG promoter, located upstream of furA (23, 30), was used to drive katG expression. The presence of the point mutation in each plasmid was confirmed by nucleotide sequencing.
FIG. 1.
Schematic representation of the cloning strategy used to construct plasmids for complementing a ΔfurA-katG strain of M. tuberculosis with different katG alleles. Site-directed mutagenesis of katG was carried out with plasmid pKATII. An NdeI-MluI fragment spanning the point mutations was subsequently transferred to the equivalent sites in pPD28 to create pAP21 to pAP23. pPD28 is based on pKINT, a Bluescript-based mycobacterial integration vector. The boldface arrow marked P denotes the site of the main katG promoter in M. tuberculosis.
TABLE 1.
Principal bacterial strains and plasmids used in this study
| Bacterium or plasmid | Genotype or relevant features | Reference |
|---|---|---|
| Bacteria | ||
| INH34 | ΔfurA-katG strain of M. tuberculosis | 30 |
| H37Rv | Genome sequenced strain of M. tuberculosis | 5 |
| Plasmids | ||
| pKATII | E. coli expression vector containing katG of M. tuberculosis | 33 |
| pK275P | pKATII with T275P mutation in katG | 33 |
| pK139V | pKATII with A139V mutation in katG | This study |
| pK315T | pKATII with S315T mutation in katG | 33 |
| pKINT | Mycobacterial integration vector | 30 |
| pPD28 | pKINT with 4.8-kb fragment spanning the furA-katG locus of M. tuberculosis | 30 |
| pAP01 | pPD28 with deletional interruption of katG | 30 |
| pAP21 | pPD28 with T275P mutation in katG | This study |
| pAP22 | pPD28 with A139V mutation in katG | This study |
| pAP23 | pPD28 with S315T mutation in katG | This study |
Transformation of M. tuberculosis and determination of INH sensitivity.
Middlebrook 7H9 medium (Difco) supplemented with 4 ml of glycerol, 5 g of bovine albumin (fraction V), and 2 g of dextrose per liter and Tween 80 to 0.05% was used for all experiments requiring liquid medium. Electrocompetent cells for INH-resistant strain INH34 (30) (ΔfurA-katG) were generated from 400 ml of a 10-day-old culture. Bacilli were harvested by centrifugation at 3,000 × g for 20 min at 16°C, washed with H2O at room temperature, and resuspended in 1 to 2 ml of room-temperature 10% glycerol after recentrifugation at 3,000 × g. Two hundred fifty microliters of bacilli and approximately 5 μg of purified plasmid were mixed and electroporated with a Bio-Rad Gene Pulser with settings of 2.5 kV, 25 μF, and 1,000 Ω. After electroporation bacilli were resuspended in 2 ml of culture medium and left overnight at 37°C. Transformants were selected for by plating out on Middlebrook 7H11 agar (Difco) supplemented with oleic acid-albumin-dextrose-catalase (Difco) and 25 μg of kanamycin (Sigma)/ml. Kanamycin-resistant colonies, appearing after 3 weeks, were analyzed for the presence of the integrated vector by PCR, with primers specific for the kanamycin resistance cassette. The MIC of INH was determined by inoculating dilutions of 7-day-old cultures into a 48-well cell culture plate containing 1 ml of fresh medium per well. Each well was supplemented with one of eight concentrations of INH ranging from 0 to 10 μg ml−1, and the plates were read after 1 and 2 weeks to ascertain the MIC.
Enzymatic assays and immunoblotting.
Bacilli from 14-day-old 50-ml cultures were concentrated by centrifugation at 3,000 × g for 15 min, washed twice with 50 mM sodium phosphate buffer (pH 7.0), and resuspended in 500 μl of the same buffer. Cells were then lysed by being shaken in a Mickle apparatus for 10 min with 500 μl of acid-washed 600-μm-diameter glass beads. The supernatant, obtained by centrifugation at 10,000 × g for 30 min, was aliquoted and stored at −20°C, after protein quantification with a Bio-Rad protein assay. Catalase and peroxidase activities were detected on nondenaturing polyacrylamide gels (10%) (13) with 25 μg of protein for each sample. Gels for catalase activity were immersed in a solution containing 5 mM H2O2 for 20 min, and activity was revealed by adding 2% ferric chloride and 2% potassium ferricyanide. Resolution of peroxidase activity gels was achieved by immersion in a solution containing 3 mM H2O2 and 0.5 mg of p-diaminobendizine/ml. Catalase activity in the supernatants was determined spectrophotometrically by measuring the decrease in H2O2 concentration at 240 nm (ɛ240 = 0.0435 mM−1 cm−1). The reaction mixture (1 ml) contained 50 mM sodium phosphate buffer, pH 7.0, and 10 mM H2O2 (6). Peroxidase activity was determined by measuring the rate of oxidation of 0.1 mM O-dianisidine at 460 nm (ɛ460 = 11.3 mM−1 cm−1), in the presence of 23 mM t-butyl hydroperoxide in 50 mM sodium acetate buffer, pH 5.5 (20). Immunoblotting was carried out after sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transfer of proteins to a nitrocellulose membrane (Hybond C+; Amersham), followed by incubation with an anti-KatG polyclonal antibody (33) (diluted 1:2,500). Detection of bound immunoglobulin was achieved with an enhanced chemiluminescence-peroxidase system (Amersham).
Virulence studies.
Fifty-milliliter cultures of the individual strains were grown in parallel in Middlebrook 7H9 medium supplemented with 0.05% Tween 80 and 25 μg of kanamycin ml−1, for 7 to 10 days. Bacteria were harvested by centrifugation for 10 min at 3,000 × g and washed once with 50 mM sodium phosphate buffer (pH 7.0) before resuspension in 1 to 5 ml of the same buffer. The bacteria were then sonicated briefly and allowed to stand for 2 h to allow residual aggregates to settle. The bacterial suspensions were then aliquoted and frozen for 48 h at −80°C. A single defrosted aliquot was used to quantify the viable counts (CFU) prior to inoculation. Six-week-old female BALB/cByJIco mice (Iffa Credo, Les Oncins, France) were infected intravenously via the lateral tail vein, with 106 CFU in 250 μl of normal saline. Organs from the sacrificed mice were homogenized for 5 min in 500 μl of 50 mM sodium phosphate buffer, by using a Mickle apparatus and 2.5-mm-diameter glass beads. Serial fivefold dilutions in phosphate-buffered saline were plated on 7H11 agar, and CFU were ascertained after 2 to 3 weeks of growth at 37°C.
RESULTS
Rationale and mutant selection.
Three mutants were chosen for detailed study, the first of which, the S315T strain, harbors the most frequently occurring mutation in INH-resistant clinical isolates while the second, the T275P strain, has a mutation found occasionally in resistant strains from tuberculosis patients. Modeling has shown both of these substitutions to occur near the catalytic site of the enzyme (12, 33). The A139V mutant has not been described previously but was created to test whether replacing this alanine with an amino acid with a bulkier side chain might interfere with substrate access to the active site of KatG and, therefore, with catalytic activity, as predicted from structural comparisons between KatG and the highly similar cytochrome c peroxidase from Saccharomyces cerevisiae (12, 33). Characterization of this purified A139V mutant after expression in E. coli demonstrated that there was no significant difference in catalytic activities between the mutant and the wild-type protein (data not shown). This mutant was therefore included in the subsequent experiments to act as an additional positive control.
As described in Materials and Methods, a panel of isogenic M. tuberculosis strains with differing katG alleles was assembled by transforming mutant katG genes (on the mycobacterial integration vector pKINT) into INH34, a ΔfurA-katG clinical isolate (Table 1). Previous studies have shown that the main katG promoter is located upstream of furA (23, 30). We therefore adopted a combinatorial strategy in which katG is expressed in tandem with furA (from a single chromosomally located vector) from its normal promoter (Fig. 1). INH34 has a promoter mutation resulting in the upregulation of ahpC (30). The use of this strain ensured that any phenotypic differences detected among the INH34 transformants could not be due to the emergence of this putative compensatory mutation. For all experiments pAP01 (30) was used as a control, since this construct is identical to the other katG-bearing plasmids (with furA upstream of katG), except for a large deletion internal to katG.
Expression of KatG variants in M. tuberculosis.
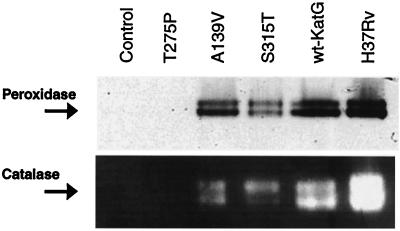
Verification that physiological levels of KatG expression were obtained by using the various constructions was achieved by immunoblotting with an anti-KatG polyclonal antibody. Levels of expression for the complemented strains of INH34 harboring the wild-type katG and the S315T or A139V mutation were comparable with that of the M. tuberculosis reference strain H37Rv, confirming that the cloning strategy effectively produces normal levels of KatG expression (Fig. 2). However, for the T275P mutant we were able to detect only low levels of expression, indicating that this protein is unstable in vivo, as was found when it was expressed in E. coli (33).
FIG. 2.
Western blot analysis of whole-cell protein fractions extracted from M. tuberculosis strain INH34 (ΔfurA-katG) complemented with a control plasmid (pAP01) or plasmids carrying the katG alleles T275P (pAP21), A139V (pAP22), and S315T (pAP23) or wild-type katG (pPD28) and also extracted from M. tuberculosis strain H37Rv. Extracts were immunoblotted with an anti-KatG polyclonal antibody following sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transfer to a nitrocellulose membrane. The positions of molecular mass markers are shown.
Having established that the expression levels for the S315T and A139V mutants were comparable to that of H37Rv, catalase and peroxidase activities of whole-cell protein extracts were assessed by using activity gels (Fig. 3). These confirmed that the S315T as well as the A139V mutation resulted in functional catalase-peroxidase enzymes. No enzymatic activity was detected in the extracts from the T275P recombinant, indicating either that the intracellular levels were insufficient to produce detectable catalytic activity or that the threonine-to-proline alteration renders KatG inactive as well as unstable. Catalase and peroxidase activities in the protein extracts were also determined spectrophotometrically, a more quantitative measure of these enzymatic activities than activity gels (Table 2). This established that there was only a small reduction in the catalase and peroxidase activities (12 and 17%, respectively) in the protein extracts from the S315T recombinant compared to those from the recombinant with the wild-type gene, the katG+ recombinant. The levels of catalase activity obtained with the katG+ recombinant were less than those seen with M. tuberculosis H37Rv. The katG deletion in INH34 spans four open reading frames other than furA and katG (30). It is conceivable that the absence of these genes in the recombinant strains could account for these differences in catalase activity or that this stems from integration of the genes in the attB site of the chromosome rather than at the katG locus itself. When the MIC was assessed, complementation with both katG+ and A139V restored sensitivity to INH whereas the S315T-complemented strain remained highly resistant to INH (Table 2).
FIG. 3.
Catalase and peroxidase activity gel analysis of whole-cell protein fractions from M. tuberculosis strain INH34 (ΔfurA-katG) complemented with a control plasmid (pAP01) or plasmids carrying the katG alleles T275P (pAP21), A139V (pAP22), and S315T (pAP23) or wild-type katG (pPD28) and also from M. tuberculosis strain H37Rv.
TABLE 2.
Sensitivity to INH and catalase-peroxidase activities of M. tuberculosis strain INH34 transformed with plasmids bearing different katG alleles and of the reference strain M. tuberculosis H37Rv
| M. tuberculosis strain | katG genotype | Catalase activitya | Peroxidase activitya | MIC of INH (μg/mL) |
|---|---|---|---|---|
| H37Rv | WTb | 3.81 ± 0.17 | 0.11 ± 0.005 | 0.05 |
| INH34-pAP01 | ΔkatG | NDc | ND | >10 |
| INH34-pPD28 | WT | 1.87 ± 0.26 | 0.054 ± 0.002 | 0.1 |
| INH34-pAP21 | T275P | ND | ND | >10 |
| INH34-pAP22 | A139V | 1.96 ± 0.44 | 0.043 ± 0.003 | 0.1 |
| INH34-pAP23 | S315T | 1.66 ± 0.18 | 0.046 ± 0.001 | 5 |
Mean activity in whole-cell protein extracts in units per minute per milligram ± standard deviation from three experiments.
WT, wild type.
ND, not detected.
Effect of katG mutations on virulence of M. tuberculosis.
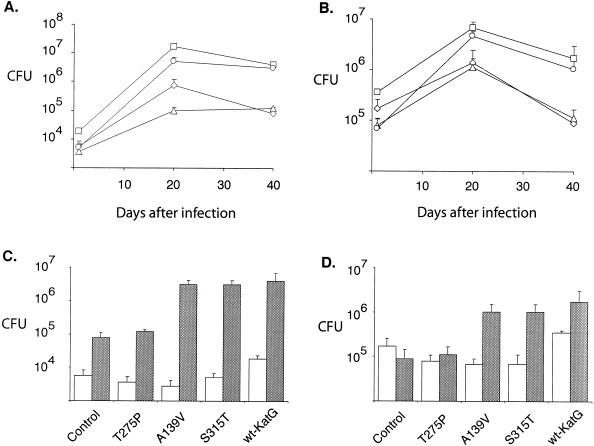
The virulence of the various strains was assessed by measuring growth rates in the spleen and lungs of intravenously infected mice, and as anticipated, the katG+-complemented strain grew more briskly in both organs than the ΔfurA-katG strain complemented with the control plasmid (Fig. 4A and B). From this experiment it can be concluded that KatG-proficient strains, including the S315T recombinant, grew more rapidly during the first 20 days postinfection than did their isogenic KatG-deficient counterparts. Furthermore, after day 20, when cell-mediated immune responses have been induced, M. tuberculosis strains producing normal or near-wild-type amounts of catalase-peroxidase activity appeared more resistant to killing in both spleen and lung than those producing little or no enzyme. These findings were replicated in an experiment in which an additional positive control (the A139V strain) was included (Fig. 4C and D). KatG-proficient strains reached higher numbers, in both spleens and lungs, than did the ΔfurA-katG parent or its T275P recombinant derivative. Again, it was particularly noteworthy that the S315T recombinant grew much more vigorously than the matched negative controls, particularly in the lung (Fig. 4C), suggesting that its virulence was not impaired.
FIG. 4.
(A and B) Growth in lungs (A) and spleen (B) of M. tuberculosis strain INH34 (ΔfurA-katG) complemented with control plasmid pAP01 (diamonds) or plasmids carrying the katG allele T275P (triangles) or S315T (circles) or wild-type katG (pPD28) (squares) in BALB/c mice following intravenous infection with 106 CFU. (C and D) Results of an experiment in which we monitored the growth in BALB/c mice of strain INH34 complemented with the plasmid (pAP22) carrying the katG allele A139V compared to that of INH34 complemented with the other plasmids, pAP01, pAP21, pAP23, and pPD28. The white bars correspond to the CFU after 1 day, and the gray bars correspond to the CFU after 40 days, in the lungs (C) and spleen (D). Each time point represents the mean result of three or four mice, and the error bars correspond to the standard deviation.
DISCUSSION
In this study we have used a panel of isogenic M. tuberculosis strains to assess the in vivo physiological costs of the most frequently encountered INH resistance-conferring mutation, katG S315T. We demonstrate that the strain with this mutation retains virulence in a mouse model of tuberculosis while displaying a clinically significant level of resistance to INH. This is compatible with the observation that the katG S315T mutant produces a functional catalase-peroxidase with enzymatic activities comparable to the wild-type protein and is in broad agreement with the findings of biochemical studies carried out on recombinant KatG S315T proteins purified after expression in E. coli (33, 40). In contrast, production of the less stable T275P enzyme by M. tuberculosis is associated with undetectable catalase-peroxidase activity, high-level INH resistance, and greatly reduced virulence in the mouse model of disease.
In the absence of a crystal structure for KatG, it is difficult to explain with precision how the S315T substitution leads to loss of activation of INH but retention of catalase-peroxidase activity. The equivalent serine of the structurally similar cytochrome c peroxidase is close to the ligand access channel, and substitutions of this residue in KatG could plausibly alter substrate binding and specificity (12, 33), and indeed subtle alterations in the binding of INH to KatG have been reported elsewhere with the S315T mutant (39). Although various schemata have been proposed for the oxidation of INH by KatG, the free radical species thought to be the bioactive form of INH have not yet been experimentally defined. Recently, it has been suggested that KatG-dependent INH oxidation can proceed by either a peroxidative or a superoxide-dependent pathway and that the S315T substitution results in selective loss of the latter. This could account for the decreased activation of INH but persisting peroxidatic activity (38). Whatever the structural basis for this peculiarly optimal adaptation to INH toxicity, it is unfortunate for the treatment and control of MDR-TB that M. tuberculosis can so successfully balance the competing demands of resistance to host killing and resistance to INH.
At least 40% of all INH-resistant clinical isolates of M. tuberculosis harbor the S315T mutation, and a much higher percentage do so if one considers only strains with clinically significant levels of resistance (7, 11, 22, 28, 29, 37). The high frequency and lack of attenuation associated with the S315T substitution mean that the majority of INH-resistant clinical isolates will be virulent, and this premise is supported by a recent molecular epidemiological study (37) which demonstrated that, although INH-resistant strains are in general less transmissible in humans, the transmission of S315T mutants is no different from that of drug-sensitive strains. Thus, the conventional view that INH-resistant strains of M. tuberculosis are of low virulence, prevalent since Middlebrook's original description of attenuated, catalase-negative, INH-resistant isolates of M. tuberculosis (24, 25), needs to be modified.
It is also pertinent that the S315T substitution is particularly associated with MDR strains of M. tuberculosis (22, 28), including the notorious W strain (31). MDR strains will have acquired mutations in genes mediating resistance to multiple antimycobacterial drugs, which will exert a cumulative effect on virulence. Mutations such as katG S315T, which minimize the cost of antibiotic resistance, may therefore favor the emergence of transmissible MDR strains.
The fitness of a strain of M. tuberculosis will also affect the long-term outcome of a tuberculosis infection. Modeling of bacterial population dynamics in a host undergoing antimycobacterial treatment has demonstrated that the fitness cost of resistance has a major effect on the predicted outcomes of treatment in differing circumstances (19). The fitness of an M. tuberculosis strain also impinges on the likelihood of reactivation of latent tuberculosis. A third of the world's population is alleged to be latently infected with M. tuberculosis, and a significant proportion of these individuals will be harboring MDR-TB, given the widespread nature of these strains. It was previously thought that the cost of resistance would significantly reduce the likelihood of reactivation. However, our demonstration that the principal mutation conferring INH resistance will have minimal effects on virulence indicates that future reactivation of latent MDR-TB cases should be anticipated.
Acknowledgments
We gratefully acknowledge the financial support of the Institut Pasteur, the Association Française Raoul Follereau, the Wellcome Trust, and the Biomed Program of the European Union (BMH4-CT97-2277). A.S.P. was in receipt of a Wellcome Trust Research Fellowship in Clinical Tropical Medicine.
We also acknowledge the support of C. Gilks, P. Winstanley, M. J. Colston, and B. Heym.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Adams, L. B., M. C. Dinauer, D. E. Morgenstern, and J. L. Krahenbuhl. 1997. Comparison of the roles of reactive oxygen and nitrogen intermediates in the host response to Mycobacterium tuberculosis using transgenic mice. Tuber. Lung Dis. 78:237-246. [DOI] [PubMed] [Google Scholar]
- 2.Andersson, D. I., and B. R. Levin. 1999. The biological cost of antibiotic resistance. Curr. Opin. Microbiol. 2:489-493. [DOI] [PubMed] [Google Scholar]
- 3.Billington, O. J., T. D. McHugh, and S. H. Gillespie. 1999. Physiological cost of rifampin resistance induced in vitro in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 43:1866-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Böttger, E. C., M. Springer, M. Pletschette, and P. Sander. 1998. Fitness of antibiotic-resistant microorganisms and compensatory mutations. Nat. Med. 4:1343-1344. [DOI] [PubMed] [Google Scholar]
- 5.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, B. G. Barrell, et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 6.Devi, B. G., M. S. Shaila, T. Ramakrishnan, and K. P. Gopinathan. 1975. The purification and properties of peroxidase in Mycobacterium tuberculosis H37Rv and its possible role in the mechanism of action of isonicotinic acid hydrazide. Biochem. J. 149:187-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobner, P., S. Rusch-Gerdes, G. Bretzel, K. Feldmann, M. Rifai, T. Loscher, and H. Rinder. 1997. Usefulness of Mycobacterium tuberculosis genomic mutations in the genes katG and inhA for the prediction of isoniazid resistance. Int. J. Tuberc. Lung Dis. 1:365-369. [PubMed] [Google Scholar]
- 8.Edlin, B. R., J. I. Tokars, M. H. Grieco, J. T. Crawford, J. Williams, E. M. Sordillo, K. R. Ong, J. O. Kilburn, S. W. Dooley, K. G. Castro, et al. 1992. An outbreak of multidrug-resistant tuberculosis among hospitalized patients with the acquired immunodeficiency syndrome. N. Engl. J. Med. 326:1514-1521. [DOI] [PubMed] [Google Scholar]
- 9.Espinal, M. A., A. Laszlo, L. Simonsen, F. Boulahbal, S. J. Kim, A. Reniero, S. Hoffner, H. L. Rieder, N. Binkin, C. Dye, R. Williams, M. C. Raviglione, et al. 2001. Global trends in resistance to antituberculosis drugs. N. Engl. J. Med. 344:1294-1303. [DOI] [PubMed] [Google Scholar]
- 10.Frieden, T. R., P. I. Fujiwara, R. M. Washko, and M. A. Hamburg. 1995. Tuberculosis in New York City—turning the tide. N. Engl. J. Med. 333:229-233. [DOI] [PubMed] [Google Scholar]
- 11.Haas, W. H., K. Schilke, J. Brand, B. Amthor, K. Weyer, P. B. Fourie, G. Bretzel, V. Sticht-Groh, and H. J. Bremer. 1997. Molecular analysis of katG gene mutations in strains of Mycobacterium tuberculosis complex from Africa. Antimicrob. Agents Chemother. 41:1601-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heym, B., P. M. Alzari, N. Honore, and S. T. Cole. 1995. Missense mutations in the catalase-peroxidase gene, katG, are associated with isoniazid resistance in Mycobacterium tuberculosis. Mol. Microbiol. 15:235-245. [DOI] [PubMed] [Google Scholar]
- 13.Heym, B., and S. T. Cole. 1992. Isolation and characterization of isoniazid-resistant mutants of Mycobacterium smegmatis and Mycobacterium aurum. Res. Microbiol. 143:721-730. [DOI] [PubMed] [Google Scholar]
- 14.Heym, B., E. Stavropoulos, N. Honore, P. Domenech, B. Saint-Joanis, T. M. Wilson, D. M. Collins, M. J. Colston, and S. T. Cole. 1997. Effects of overexpression of the alkyl hydroperoxide reductase AhpC on the virulence and isoniazid resistance of Mycobacterium tuberculosis. Infect. Immun. 65:1395-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iseman, M. D. 1993. Treatment of multidrug-resistant tuberculosis. N. Engl. J. Med. 329:784-791. [DOI] [PubMed] [Google Scholar]
- 16.Johnsson, K., W. A. Froland, and P. G. Schultz. 1997. Overexpression, purification, and characterization of the catalase-peroxidase KatG from Mycobacterium tuberculosis. J. Biol. Chem. 272:2834-2840. [DOI] [PubMed] [Google Scholar]
- 17.Li, Z., C. Kelley, F. Collins, D. Rouse, and S. Morris. 1998. Expression of katG in Mycobacterium tuberculosis is associated with its growth and persistence in mice and guinea. pigs. J. Infect. Dis. 177:1030-1035. [DOI] [PubMed] [Google Scholar]
- 18.Lipsitch, M. 2001. The rise and fall of antimicrobial resistance. Trends Microbiol. 9:438-444. [DOI] [PubMed] [Google Scholar]
- 19.Lipsitch, M., and B. R. Levin. 1998. Population dynamics of tuberculosis treatment: mathematical models of the roles of non-compliance and bacterial heterogeneity in the evolution of drug resistance. Int. J. Tuberc. Lung Dis. 2:187-199. [PubMed] [Google Scholar]
- 20.Loprasert, S., S. Negoro, and H. Okada. 1988. Thermostable peroxidase from Bacillus stearothermophilus. J. Gen. Microbiol. 134:1971-1976. [DOI] [PubMed] [Google Scholar]
- 21.Manca, C., S. Paul, C. E. Barry III, V. H. Freedman, and G. Kaplan. 1999. Mycobacterium tuberculosis catalase and peroxidase activities and resistance to oxidative killing in human monocytes in vitro. Infect. Immun. 67:74-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marttila, H. J., H. Soini, E. Eerola, E. Vyshnevskaya, B. I. Vyshnevskiy, T. F. Otten, A. V. Vasilyef, and M. K. Viljanen. 1998. A Ser315Thr substitution in KatG is predominant in genetically heterogeneous multidrug-resistant Mycobacterium tuberculosis isolates originating from the St. Petersburg area in Russia. Antimicrob. Agents Chemother. 42:2443-2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Master, S., T. C. Zahrt, J. Song, and V. Deretic. 2001. Mapping of Mycobacterium tuberculosis katG promoters and their differential expression in infected macrophages. J. Bacteriol. 183:4033-4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Middlebrook, G. 1954. Isoniazid-resistance and catalase activities of tubercle bacilli. A preliminary report. Am. Rev. Tuberc. 69:471-472. [DOI] [PubMed] [Google Scholar]
- 25.Middlebrook, G., and M. L. Cohn. 1953. Some observations on the pathogenicity of isoniazid-resistant variants of tubercle bacilli. Science 118:297-299. [DOI] [PubMed] [Google Scholar]
- 26.O'Brien, S., and P. W. Andrew. 1991. Guinea-pig alveolar macrophage killing of Mycobacterium tuberculosis, in vitro, does not require hydrogen peroxide or hydroxyl radical. Microb. Pathog. 11:229-236. [DOI] [PubMed] [Google Scholar]
- 27.O'Brien, S., P. S. Jackett, D. B. Lowrie, and P. W. Andrew. 1991. Guinea-pig alveolar macrophages kill Mycobacterium tuberculosis in vitro, but killing is independent of susceptibility to hydrogen peroxide or triggering of the respiratory burst. Microb. Pathog. 10:199-207. [DOI] [PubMed] [Google Scholar]
- 28.Piatek, A. S., A. Telenti, M. R. Murray, H. El-Hajj, W. R. Jacobs, Jr., F. R. Kramer, and D. Alland. 2000. Genotypic analysis of Mycobacterium tuberculosis in two distinct populations using molecular beacons: implications for rapid susceptibility testing. Antimicrob. Agents Chemother. 44:103-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pym, A. S., and S. T. Cole. 2002. Tuberculosis chemotherapy—from conception to genomics, p. 355-403. In R. Wax, K. Lewis, and H. Taber (ed.), Bacterial resistance to antimicrobials: mechanisms, genetics, medical practice and public health. Marcel Dekker, Inc., New York, N.Y.
- 30.Pym, A. S., P. Domenech, N. Honore, J. Song, V. Deretic, and S. T. Cole. 2001. Regulation of catalase-peroxidase (KatG) expression, isoniazid sensitivity and virulence by furA of Mycobacterium tuberculosis. Mol. Microbiol. 40:879-889. [DOI] [PubMed] [Google Scholar]
- 31.Ramaswamy, S., and J. M. Musser. 1998. Molecular genetic basis of antimicrobial agent resistance in Mycobacterium tuberculosis: 1998 update. Tuber. Lung Dis. 79:3-29. [DOI] [PubMed] [Google Scholar]
- 32.Rouse, D. A., J. A. DeVito, Z. Li, H. Byer, and S. L. Morris. 1996. Site-directed mutagenesis of the katG gene of Mycobacterium tuberculosis: effects on catalase-peroxidase activities and isoniazid resistance. Mol. Microbiol. 22:583-592. [DOI] [PubMed] [Google Scholar]
- 33.Saint-Joanis, B., H. Souchon, M. Wilming, K. Johnsson, P. M. Alzari, and S. T. Cole. 1999. Use of site-directed mutagenesis to probe the structure, function and isoniazid activation of the catalase/peroxidase, KatG, from Mycobacterium tuberculosis. Biochem. J. 338:753-760. [PMC free article] [PubMed] [Google Scholar]
- 34.Sherman, D. R., K. Mdluli, M. J. Hickey, T. M. Arain, S. L. Morris, C. E. Barry III, and C. K. Stover. 1996. Compensatory ahpC gene expression in isoniazid-resistant Mycobacterium tuberculosis. Science 272:1641-1643. [DOI] [PubMed] [Google Scholar]
- 35.Small, P. M., R. W. Shafer, P. C. Hopewell, S. P. Singh, M. J. Murphy, E. Desmond, M. F. Sierra, and G. K. Schoolnik. 1993. Exogenous reinfection with multidrug-resistant Mycobacterium tuberculosis in patients with advanced HIV infection. N. Engl. J. Med. 328:1137-1144. [DOI] [PubMed] [Google Scholar]
- 36.Springer, B., S. Master, P. Sander, T. Zahrt, M. McFalone, J. Song, K. G. Papavinasasundaram, M. J. Colston, E. Boettger, and V. Deretic. 2001. Silencing of oxidative stress response in Mycobacterium tuberculosis: expression patterns of ahpC in virulent and avirulent strains and effect of ahpC inactivation. Infect. Immun. 69:5967-5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Soolingen, D., P. E. de Haas, H. R. van Doorn, E. Kuijper, H. Rinder, and M. W. Borgdorff. 2000. Mutations at amino acid position 315 of the katG gene are associated with high-level resistance to isoniazid, other drug resistance, and successful transmission of Mycobacterium tuberculosis in the Netherlands. J. Infect. Dis. 182:1788-1790. [DOI] [PubMed] [Google Scholar]
- 38.Wengenack, N. L., and F. Rusnak. 2001. Evidence for isoniazid-dependent free radical generation catalyzed by Mycobacterium tuberculosis KatG and the isoniazid-resistant mutant KatG(S315T). Biochemistry 40:8990-8996. [DOI] [PubMed] [Google Scholar]
- 39.Wengenack, N. L., S. Todorovic, L. Yu, and F. Rusnak. 1998. Evidence for differential binding of isoniazid by Mycobacterium tuberculosis KatG and the isoniazid-resistant mutant KatG(S315T). Biochemistry 37:15825-15834. [DOI] [PubMed] [Google Scholar]
- 40.Wengenack, N. L., J. R. Uhl, A. L. St. Amand, A. J. Tomlinson, L. M. Benson, S. Naylor, B. C. Kline, F. R. Cockerill III, and F. Rusnak. 1997. Recombinant Mycobacterium tuberculosis KatG(S315T) is a competent catalase-peroxidase with reduced activity toward isoniazid. J. Infect. Dis. 176:722-727. [DOI] [PubMed] [Google Scholar]
- 41.Wilson, T., G. W. de Lisle, J. A. Marcinkeviciene, J. S. Blanchard, and D. M. Collins. 1998. Antisense RNA to ahpC, an oxidative stress defence gene involved in isoniazid resistance, indicates that AhpC of Mycobacterium bovis has virulence properties. Microbiology 144:2687-2695. [DOI] [PubMed] [Google Scholar]
- 42.Wilson, T. M., G. W. de Lisle, and D. M. Collins. 1995. Effect of inhA and katG on isoniazid resistance and virulence of Mycobacterium bovis. Mol. Microbiol. 15:1009-1015. [DOI] [PubMed] [Google Scholar]
- 43.Zhang, Y., B. Heym, B. Allen, D. Young, and S. Cole. 1992. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature 358:591-593. [DOI] [PubMed] [Google Scholar]