Abstract
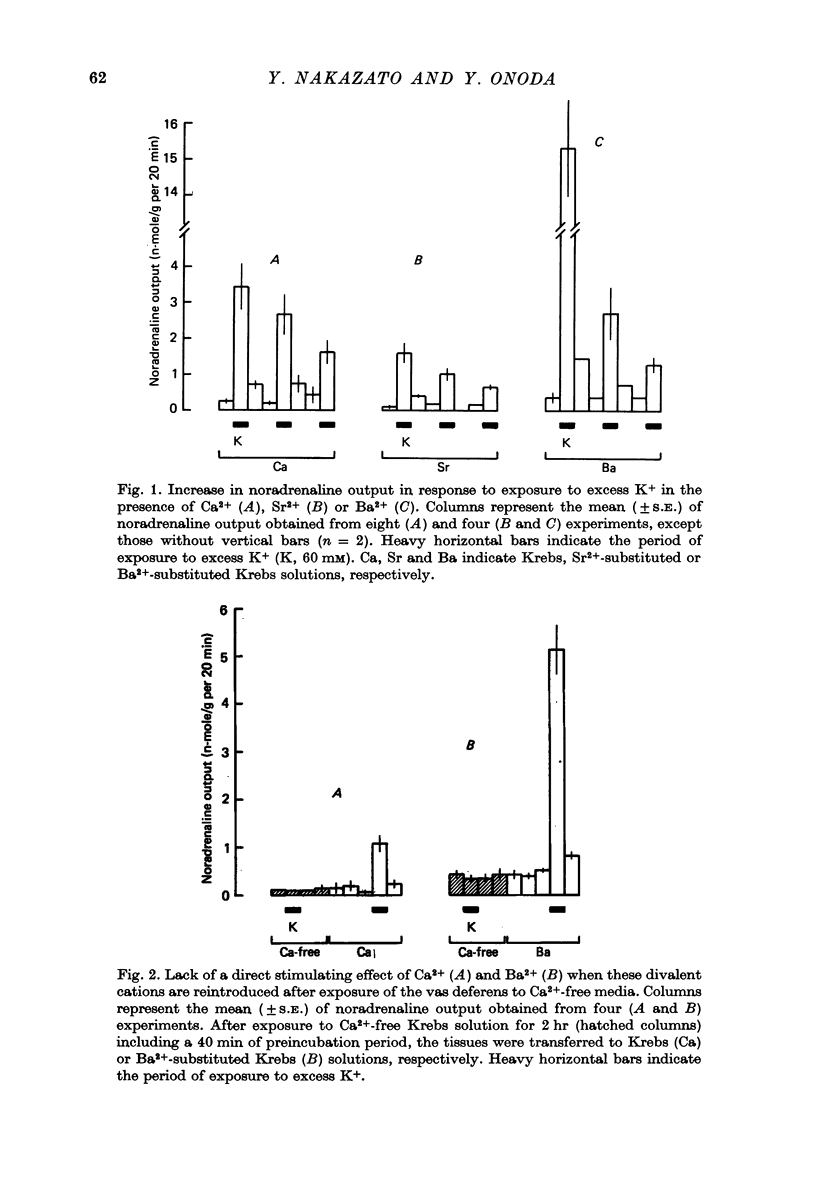
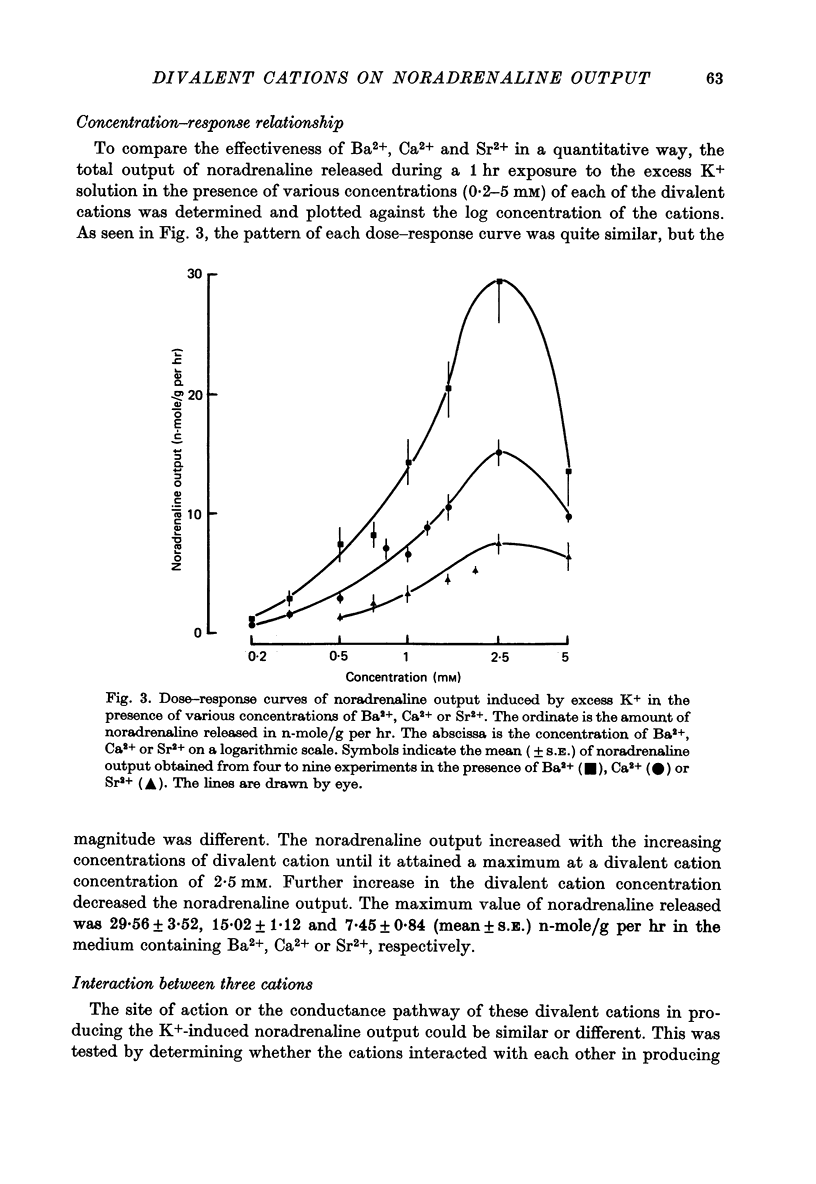
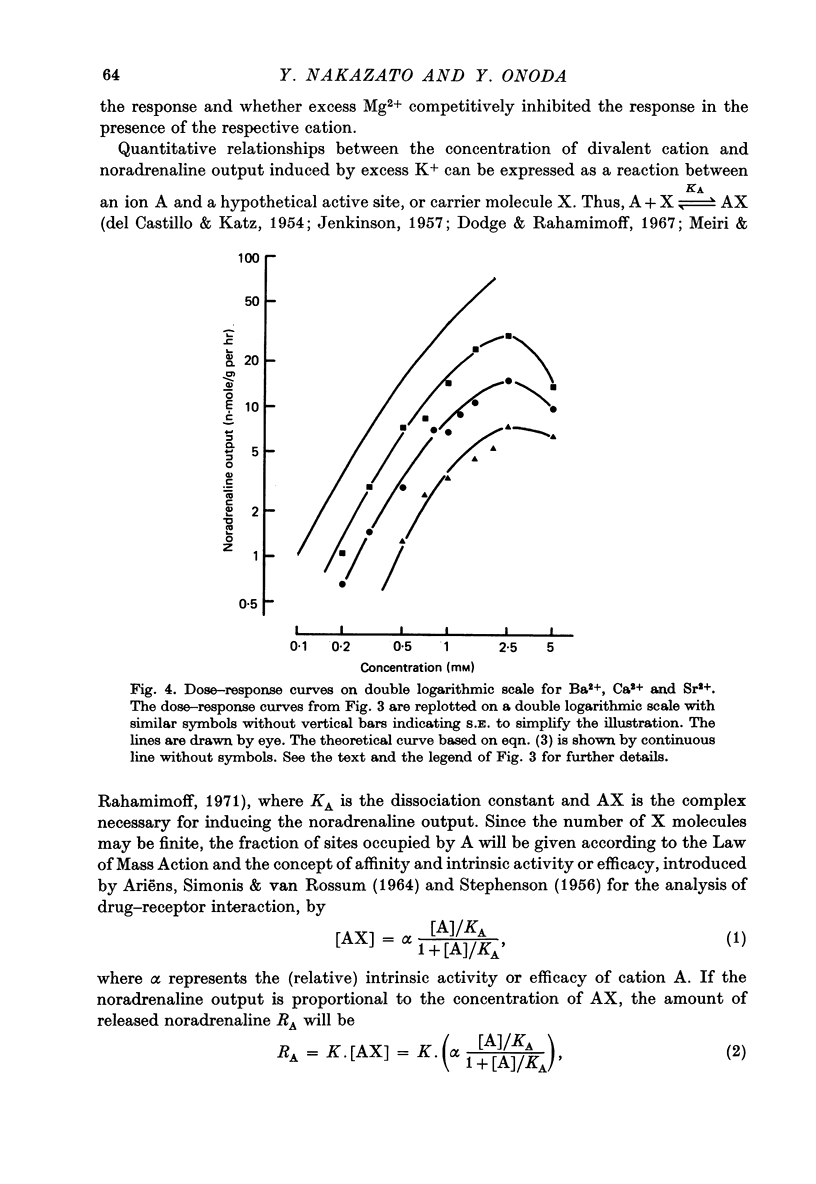
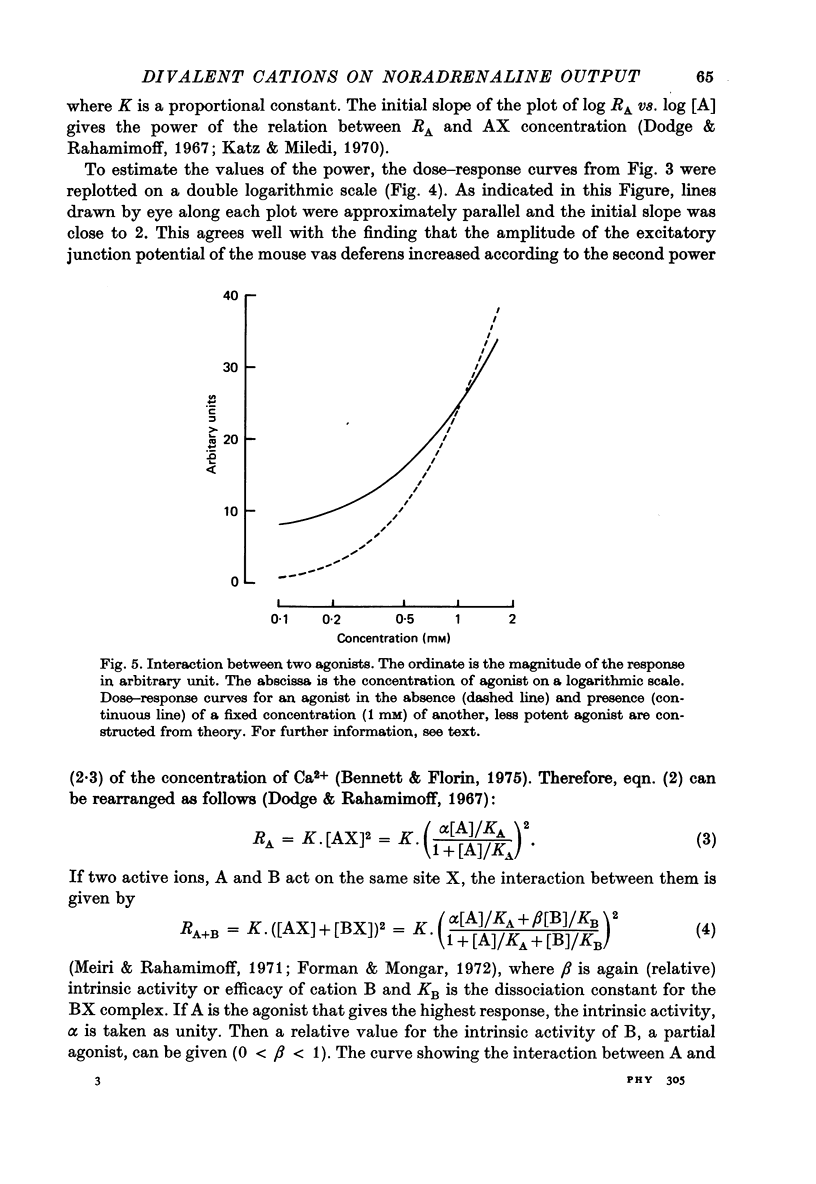
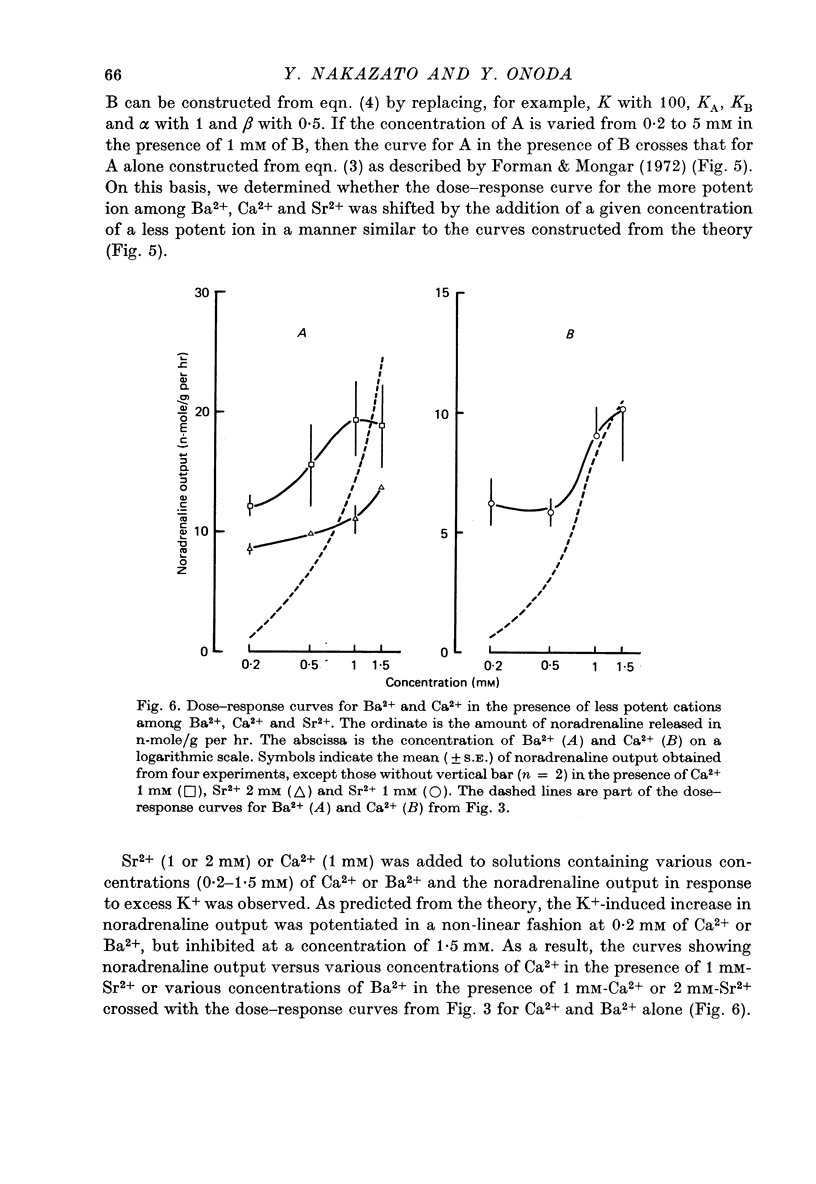
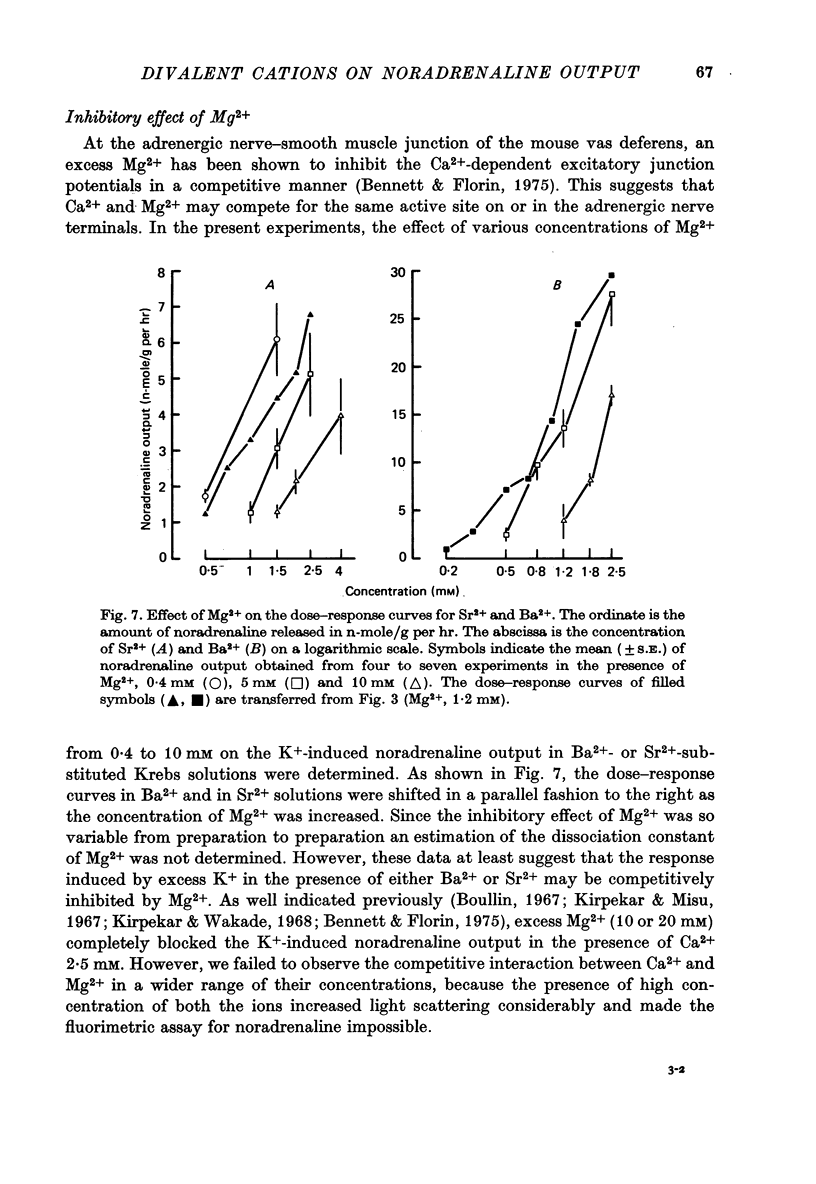
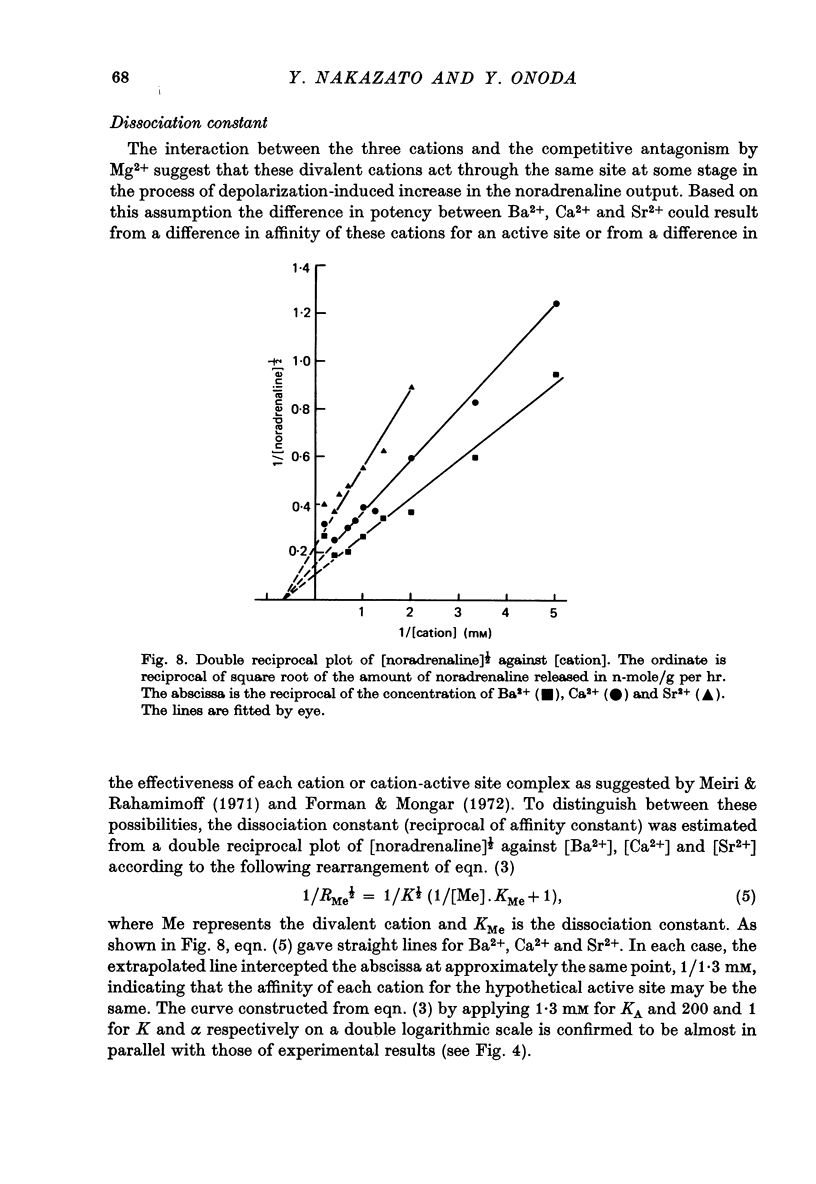
1. The ability of Ba2+ and Sr2+ to substitute for Ca2+ in the noradrenaline output induced by excess K+ was examined using isolated guinea-pig vas deferens. 2. When the vas deferens was repeatedly exposed to excess K+ (60 mM) at 40 min intervals, the noradrenaline output increased at least three-fold in incubation medium which contained either Ca2+, Ba2+ or Sr2+. The response decreased on repetition. The order of effectiveness was roughly Ba2+ > Ca2+ > Sr2+. 3. In the absence of excess K+, these cations had no significant stimulating effect on the noradrenaline output even when added after exposure to Ca2+-free solution. 4. As the concentration of divalent cation was increased from 0.2 to 2.5 mM the noradrenaline output induced by excess K+ increased. The maximum noradrenaline output was achieved at a divalent cation concentration of 2.5 mM and was 29.56 +/- 3.52, 15.02 +/- 1.12 and 7.45 +/- 0.84 (mean +/- S.E. of mean) n-mole/g per hr in the presence of either Ba2+, Ca2+ or Sr2+, respectively. Further increase in the concentration of the cations reduced the response. 5. The addition of either Sr2+ (2 mM) or Ca2+ (1 mM) to a solution containing various concentrations of Ba2+ facilitated the K+-induced increase in the noradrenaline output when the Ba2+ concentration was low, but inhibited release of noradrenaline when higher concentrations of Ba2+ were used. The addition of Sr2+ (1 mM) to Ca2+-containing solutions had a similar effect. 6. Mg2+ competitively inhibited the K+-induced increase in the noradrenaline output in the presence of either Ba2+ or Sr2+ and blocked that in the presence of CA2+. 7. The results indicate that both Ba2+ and Sr2+ can substitute for Ca2+ in the cations act though the same site at some stage in the process of K+-induced transmitter release.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTON A. H., SAYRE D. F. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962 Dec;138:360–375. [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Calcium entry in response to maintained depolarization of squid axons. J Physiol. 1973 Jun;231(3):527–548. doi: 10.1113/jphysiol.1973.sp010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Effects of manganese and other agents on the calcium uptake that follows depolarization of squid axons. J Physiol. 1973 Jun;231(3):511–526. doi: 10.1113/jphysiol.1973.sp010246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F. Transport and metabolism of calcium ions in nerve. Prog Biophys Mol Biol. 1972;24:177–223. doi: 10.1016/0079-6107(72)90007-7. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Florin T. An electrophysiological analysis of the effect of Ca ions on neuromuscular transmission in the mouse vas deferens. Br J Pharmacol. 1975 Sep;55(1):97–104. doi: 10.1111/j.1476-5381.1975.tb07616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blioch Z. L., Glagoleva I. M., Liberman E. A., Nenashev V. A. A study of the mechanism of quantal transmitter release at a chemical synapse. J Physiol. 1968 Nov;199(1):11–35. doi: 10.1113/jphysiol.1968.sp008637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boullin D. J. The action of extracellular cations on the release of the sympathetic transmitter from peripheral nerves. J Physiol. 1967 Mar;189(1):85–99. doi: 10.1113/jphysiol.1967.sp008156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. The effect of magnesium on the activity of motor nerve endings. J Physiol. 1954 Jun 28;124(3):553–559. doi: 10.1113/jphysiol.1954.sp005128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., LYWOOD D. W., STRAUB R. W. The stimulant effect of barium on the release of acetylcholine from the superior cervical ganglion. J Physiol. 1961 May;156:515–522. doi: 10.1113/jphysiol.1961.sp006690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. STIMULANT ACTION OF BARIUM ON THE ADRENAL MEDULLA. Nature. 1964 Jul 18;203:305–307. doi: 10.1038/203305a0. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. THE EFFECTS OF ALKALINE EARTHS AND OTHER DIVALENT CATIONS ON ADRENAL MEDULLARY SECRETION. J Physiol. 1964 Dec;175:231–241. doi: 10.1113/jphysiol.1964.sp007514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol. 1961 Nov;159:40–57. doi: 10.1113/jphysiol.1961.sp006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Miledi R., Rahamimoff R. Strontium and quantal release of transmitter at the neuromuscular junction. J Physiol. 1969 Jan;200(1):267–283. doi: 10.1113/jphysiol.1969.sp008692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L. The role of the alkaline earth ions in anaphylactic histamine secretion. J Physiol. 1972 Aug;224(3):753–769. doi: 10.1113/jphysiol.1972.sp009921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia A. G., Kirpekar S. M. Release of noradrenaline from slices of cat spleen by pre-treatment with calcium, strontium and barium. J Physiol. 1973 Dec;235(3):693–713. doi: 10.1113/jphysiol.1973.sp010411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Nakazato Y., Ohga A. The effect of the ionophores X-537A and A23187 on the noradrenaline output from peripheral adrenergic neurones in the presence of various divalent cations. Br J Pharmacol. 1978 Jan;62(1):91–98. doi: 10.1111/j.1476-5381.1978.tb07010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno T., Cochrane D. E., Douglas W. W. Exocytosis (secretory granule extrusion) induced by injection of calcium into mast cells. Can J Physiol Pharmacol. 1973 Dec;51(12):1001–1004. doi: 10.1139/y73-153. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Further study of the role of calcium in synaptic transmission. J Physiol. 1970 May;207(3):789–801. doi: 10.1113/jphysiol.1970.sp009095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpekar S. M., Misu Y. Release of noradrenaline by splenic nerve stimulation and its dependence on calcium. J Physiol. 1967 Jan;188(2):219–234. doi: 10.1113/jphysiol.1967.sp008135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpekar S. M., Wakade A. R. Release of noradrenaline from the cat spleen by potassium. J Physiol. 1968 Feb;194(3):595–608. doi: 10.1113/jphysiol.1968.sp008427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M. The effects of strontium and barium ions at synapses in sympathetic ganglia. J Physiol. 1977 May;267(2):497–518. doi: 10.1113/jphysiol.1977.sp011823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiri U., Rahamimoff R. Activation of transmitter release by strontium and calcium ions at the neuromuscular junction. J Physiol. 1971 Jul;215(3):709–726. doi: 10.1113/jphysiol.1971.sp009493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellow A. M., Phillips T. E., Silinsky E. M. On the conductance pathway traversed by strontium in mediating the asynchronous release of acetylcholine by motor nerve impulses. Br J Pharmacol. 1978 Jun;63(2):229–232. doi: 10.1111/j.1476-5381.1978.tb09750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. Transmitter release induced by injection of calcium ions into nerve terminals. Proc R Soc Lond B Biol Sci. 1973 Jul 3;183(1073):421–425. doi: 10.1098/rspb.1973.0026. [DOI] [PubMed] [Google Scholar]
- Nakazato Y., Ohga A., Onoda Y. The effect of ouabain on noradrenaline output from peripheral adrenergic neurones of isolated guinea-pig vas deferens. J Physiol. 1978 May;278:45–54. doi: 10.1113/jphysiol.1978.sp012291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEPHENSON R. P. A modification of receptor theory. Br J Pharmacol Chemother. 1956 Dec;11(4):379–393. doi: 10.1111/j.1476-5381.1956.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M. Can barium support the release of acetylcholine by nerve impulses? Br J Pharmacol. 1977 Jan;59(1):215–217. doi: 10.1111/j.1476-5381.1977.tb06997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M., Mellow A. M., Phillips T. E. Conventional calcium channel mediates asynchronous acetylcholine release by motor nerve impulses. Nature. 1977 Dec 8;270(5637):528–530. doi: 10.1038/270528a0. [DOI] [PubMed] [Google Scholar]
- Silinsky E. M. On the role of barium in supporting the asynchronous release of acetylcholine quanta by motor nerve impulses. J Physiol. 1978 Jan;274:157–171. doi: 10.1113/jphysiol.1978.sp012141. [DOI] [PMC free article] [PubMed] [Google Scholar]


