Abstract
A virulent, low-passage culture of a tick-derived strain of Borrelia garinii was subjected to serial in vitro passages, from which inoculations were made into C3H/HeN mice. A full display of pathogenicity was observed through passage 4, as measured by cultures of ear punch biopsy samples and internal organs and determination of tibiotarsal joint swelling. Decreased dissemination through skin and infection of internal organs were observed beginning at passage 6. These losses correlated with both the selection of clones harboring 21% less flagella than the parent strain, as seen by electron microscopy, and loss of the motility of the higher passages, as demonstrated by a swarm assay. However, during the chronic phase (3 months after infection), spirochetes were cultured from the bladder and kidney of a mouse inoculated with passage 12. The kidney isolate had the same number of flagella and motility as the original low-passage isolate. Although we can't exclude the possibility that other subtle variations may be arising given the uncloned nature of the isolate, we have found a strong association between loss of flagella and decreased invasiveness. Arthritogenicity progressively decreased with passages, so that only 12.5% of chronically infected mice inoculated with passage 29 still presented with joint swelling, concurrent with a decrease in the staining intensity in a Southern blot with a vlsE-based probe. These results suggest a multifactorial model in which the number of flagella drives the invasiveness of this agent, while plasmid-associated factors are responsible for triggering arthritogenicity.
Lyme disease is a tick-transmitted illness caused by Borrelia burgdorferi sensu lato (4, 8, 13, 27). This multisystemic process starts at the site of the tick bite and progresses from a localized skin rash, erythema migrans, to a variety of disorders that involve several organ systems (49).
The plasmid loss that occurs during serial in vitro culture of low-passage, infectious strains has been described as the cause of the concurrent loss of pathogenicity of the higher passages (38, 43, 52, 53). However, this correlation is not absolute, as plasmids whose loss coincides with diminishing infectivity are not always present in wild, low-passage, infectious isolates. Other authors have described an inherent plasmid instability in this species and variations in plasmid profiles in the range of the expected variability (6, 15, 32, 39, 45, 46, 52, 53), which could not be related to changes in pathogenicity (33).
vlsE and its associated silent vls cassettes of linear plasmid (lp) 28-1 (55) (lp21 in Borrelia garinii [51]) mediate antigenic switches, similar to those found in the relapsing fever borrelia and some parasites (5, 34). A recent report has described an additional infectivity-associated plasmid (lp25) (40), whose presence leads to an infectious phenotype. Labandeira-Rey and Skare (29) have recently corroborated that both lp25 and lp28-1 are needed for full virulence, that clones lp25+ and lp28-1− are capable of infecting just the joints, and that clones lp25− and lp28-1+ are not able to infect any organ.
From the site of inoculation in the skin, the organism has to disseminate through a viscous environment. To achieve this, two factors are needed: a plasmin binding system that assists the organism to degrade the extracellular matrix (17) and a motility apparatus. The genome lists 37 motility and chemotaxis genes (about 3% of its genome) for B. burgdorferi (21, 24), underscoring the potential importance for movement in this species (22). Periplasmic flagella, which play an essential role in motility and cell morphology (36, 41), allow the organisms to progress through semisolid environments, such as connective tissue (26, 28), and the extracellular matrix, for organ colonization. Without flagella, it is unlikely that these organisms could be virulent (23, 28, 41).
The purpose of this study was to analyze the effect of in vitro passage of an infectious strain (PV6) of B. garinii on the loss of pathogenicity in C3H mice. To accomplish this, we inoculated groups of mice with passage 2 (p2) to p29, cultured the internal organs, and calculated the percentage of mice that developed arthritis. Each passage and each isolate from organs were characterized by determining the plasmid and protein profiles, the presence of vlsE, and the content of flagella. The results showed that high numbers of flagella strongly correlated with greater invasiveness and that a progressive loss of vlsE-carrying lp25 correlated with a lower percentage of mice that developed arthritis.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Low-passage tick-derived B. garinii strain PV6, which was previously determined to be pathogenic to C3H mice (20), was subjected to serial in vitro passages in Barbour-Stoenner-Kelly II (BSKII) medium (9) supplemented with 10% rabbit serum (Sigma-Aldrich, St. Louis, Mo.) (BSK-RS) by transferring 100 μl of each passage to a 4.5-ml tube with fresh medium.
Experimental design.
The C3H/HeN Lyme disease mouse model (7) was used to assess the pathogenicity of the different passages in this study. Twenty mice were injected intradermally in the lower back with 104 spirochetes derived from p2, p12, and p29 of strain PV6. The percentage of mice that developed arthritis after injection was determined for each group by monitoring signs of inflammation of the tibiotarsal joints (TTJ) for 12 weeks. The level of spirochete dissemination through the skin was determined on day 15 by culturing 3-mm-diameter ear punch biopsy (EPB) samples of all mice of each group in BSK-RS. On days 30 and 90, livers, kidneys, hearts, brains, spleens, and urinary bladders from four mice that showed signs of inflammation were cultured in BSK-RS. Two mice were also selected on the basis of the level of antibodies to the homologous strain from the groups of mice that did not show signs of inflammation. Citrated blood samples from each mouse were also cultured to exclude the possibility that tissue isolates were not derived from blood. Once the passage at which strain PV6 lost its pathogenicity was determined, groups of six mice were inoculated with the intermediate passages (p4 and p6) and processed in the same manner.
Determination of the plasmid profile and Southern blotting.
Plasmid content and conformation for each passage and isolate of PV6 were determined by both pulsed-field gel electrophoresis (PFGE) and field-inverted gel electrophoresis (FIGE) as described previously (14, 20, 47). FIGE consisted of a run of 28 h at 4.5 V/cm with a repeated sequence of forward-reverse pulses of 0.6 to 0.2 s, in addition to an initial run of 15 min without pulses and a final forward pulse of 2 s by using a power unit PC 750 pulse controller (Hoeffer Scientific Instruments, San Francisco, Calif.). The ramp factor was 0.1. Gels were stained with 0.5 μg of ethidium bromide per ml, visualized by UV illumination, and photographed in a Fluor-S multimager (Bio-Rad Laboratories, Hercules, Calif.). Size markers were 8.3- to 48.5-kb fragments (Gibco/BRL, Gaithersburg, Md.) and 48.5- to 1,000-kb fragments (Boehringer Mannheim, Indianapolis, Ind.) of digested lambda phage DNA. Samples subjected to FIGE were run in duplicate with and without previous irradiation by UV light for 10 min to determine plasmid conformation, as described previously (53). Consequently, bands that disappeared in the irradiated sample were thought to be circular plasmids in their supercoiled conformation, given that their open-circle counterparts would not be able to enter the gel since they would be trapped in the agarose at the given voltage. The bands that did not change after UV treatment were identified as linear plasmids.
DNA from PFGE gels was transferred overnight onto Immobilon Ny+ membranes (Millipore Co., Bedford, Mass.) and fixed as described previously (42). A 133-bp PCR-generated probe was constructed by using primers F4064, nucleotides (nt) 835 to 857 (55), and vlsE-R (this study; 5′-CTTCACAGCAAACTTTCCAT-3′, nt 968 to 949), which specifically probed the region between the variable VR-5 and the invariable IR-6 regions of vlsE. The amplification reaction was carried out for 30 cycles in a DNA thermal cycler (PTC-100; MJ Research, Inc., Watertown, Mass.) with an amplification profile of denaturation at 95°C for 30 s, annealing at 50°C for 90 s, and extension at 72°C for 2 min, with an initial cycle of 3 min at 95°C and a final extension at 72°C for 3 min. The probe was labeled by the NEBLOT Phototope kit (New England BioLabs, Beverly, Mass.) in accordance with the manufacturer's instructions. The rest of the hybridization procedure was done as described elsewhere (42). The Phototope-Star detection kit (New England BioLabs) was used for developing, and X-OMAT films (Eastman Kodak Co., Rochester, N.Y.) were used for autoradiography.
Relative intensity of staining in the vlsE Southern analysis was measured by densitometry in a Fluor-S multimager (Bio-Rad).
Gel electrophoresis of proteins and Western blotting.
For protein analysis, whole-cell sonicates of cultured spirochetes of each passage and isolate were separated by using the NuPAGE (bis[2-hydroxyethyl]imino-Tris[hydroxymethyl]methane)-HCl system (Novex, San Diego, Calif.). Gels were stained with Coomassie brilliant blue R-250 (Merck AG, Darmstadt, Germany). Prestained molecular weight standards (Novex) were used to determine the relative molecular masses of major proteins.
Sera from mice inoculated with p2 and p29 of PV6 were tested for reactivity to strips with p2 of the same strain as the antigen by Western blotting, as previously described (1).
Triton X-114 phase partitioning and treatment with proteinase K.
Purification of Triton X-114 was carried out as described previously (10, 12), and p2 and p29 of strain PV6 were subjected to phase separation as previously described for B. burgdorferi (18).
Surface proteolysis with proteinase K (Boehringer Mannheim) of live spirochetes was also done as described previously (37).
ELISA.
Monoclonal antibody CB1, directed to FlaB (18), was tested for its reactivity with whole-cell sonicates of p2 and p29 of PV6 by enzyme-linked immunosorbent assay (ELISA), as described previously (1).
Electron microscopy.
Electron microscopy photography was done as described previously (2).
Swarm assay.
A swarm assay was done as described previously (36) with minor modifications. Briefly, spirochetes were grown in 4.5-ml tubes until the stationary phase, pelleted, resuspended in Hanks' balanced salt solution (Gibco/BRL), and counted, and 5 × 106 organisms were resuspended in 25 μl of medium. Five microliters was then introduced with the help of a pipette tip at approximately 5 mm deep into platting BSK medium supplemented with 0.35% SeaKem LE agarose (FMC Bioproducts, Rockland, Maine). After 4 days of incubation at 34°C in 5% CO2, pictures were taken with a digital camera. B31-derived nonflagellated mutant MC-1 (36), kindly provided by N. W. Charon, Department of Microbiology and Immunology, Health Sciences Center, West Virginia University, Morgantown, W.Va., was used as a negative control in the assay.
Statistical analyses.
Kruskal-Wallis (48) and Mann-Whitney (31) tests were used for evaluation of the data with Statview, version 5.0, software for Macintosh computers (SAS Institute Inc., Cary, N.C.).
RESULTS
Low-passage infections result in greater recovery of spirochetes, inflammation, and antibody response.
p2 and p4 of strain PV6 of B. garinii were recovered in culture from EPB samples as well as from four of six internal organs (urinary bladder, spleen, kidney, and heart) (Table 1) during the acute phase of the infection. No isolates were obtained with any of the successive passages (p6, p12, and p29) from any organ. At 3 months after inoculation, only mice inoculated with p2 (mice chronically infected with p2 [CM2]) and p12 (CM12) yielded isolates (from EPB samples, bladder, and heart with p2 and from bladder and kidney with p12) (Table 1).
TABLE 1.
Recovery of organisms in culture and percentage of arthritis in mice inoculated with different passages of the PV6 strain of B. garinii
| Passage | No. of positive mice/no. tested (%)
|
No. of positive organs
|
||||
|---|---|---|---|---|---|---|
| EPB
|
Arthritis
|
|||||
| Acutea | Chronicb | Acutec | Chronic | Acute | Chronic | |
| 2 | 15/20 (75) | 16/16 (100) | 13/20 (65) | 11/16 (68.7) | 5 | 2 |
| 4 | 6/6 (100) | 0 | 2/6 (33.3) | 1/4 (25) | 5 | 0 |
| 6 | 0/6 | 0 | 0/6 | 1/4 (25) | 0 | 0 |
| 12 | 0/20 | 0 | 0/20 | 3/16 (18.7) | 0 | 2 |
| 29 | 0/20 | 0 | 0/20 | 2/16 (12.5)d | 0 | 0 |
15 days after infection.
3 months after infection.
1 month after infection.
Arthritis was seen in mice inoculated with p29 only from weeks 8 to 10.
During the acute phase, swelling of the TTJ was noted only in mice inoculated with p2 and p4. However, during the chronic phase, TTJ swelling was observed with all the passages assayed but with a progressive decrease in the percentage of mice affected (range, 68.7 to 12.5%) (Table 1), so there was less inflammation at higher passages.
When sera from mice infected with p2, p6, and p29 were assayed by Western blotting against an antigen from p2, a strong decrease of antibody synthesis was noted at the last two passages (Fig. 1), showing that decreased invasiveness of the inoculum correlated with lower antibody synthesis.
FIG. 1.
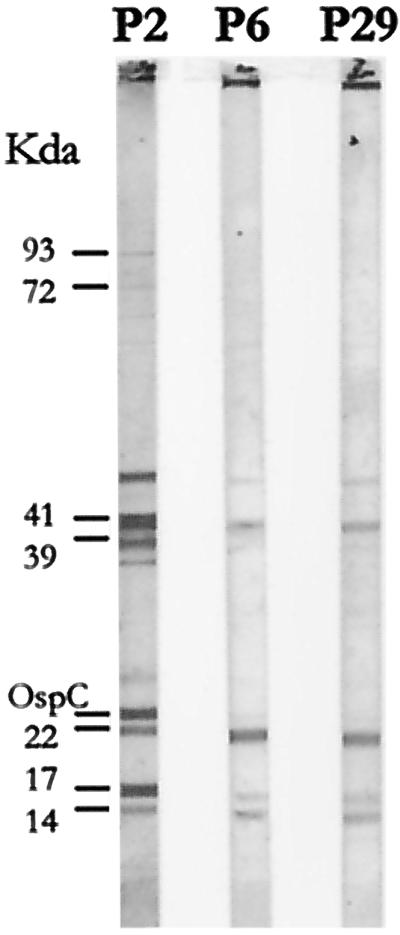
Serum reactivity of mice inoculated with p2, p6, and p29 of strain PV6, with p2 as the antigen.
Plasmid and protein profiles do not correlate with invasiveness.
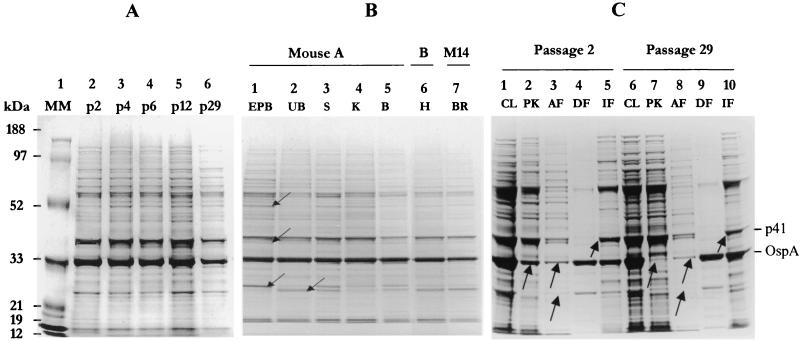
Protein profiles of all the passages and isolates from organs were studied by one-dimensional gel electrophoresis. No differences among the passages assayed were observed (Fig. 2A), although some differences among isolates from organs in the low-molecular-mass range were observed (Fig. 2B).
FIG. 2.
Protein profile analyses. (A) Analysis of different passages of strain PV6. MM, molecular mass standards. (B) Analysis of isolates from different organs. UB, urinary bladder; S, spleen; K, kidney; B, blood; H, heart, BR, brain. (C) Comparison of fractions of Triton X-114 phase partitioning from p2 and p29. CL, whole-cell lysate; PK, organisms treated with proteinase K; AF, aqueous fraction; DF, detergent fraction; IF, insoluble fraction. Arrows, bands that showed differences between isolates and subcellular fractions
p2 and p29 were additionally studied by treatment of intact, live spirochetes with proteinase K, as well as by Triton X-114 phase partitioning (Fig. 2C). Differences between the two passages were seen, mainly in OspA, which in p2 resisted treatment with proteinase K (Fig. 2C, lane 2) but which in p29 was digested with the same treatment (Fig. 2C, lane 7). However, in both passages, OspA coprecipitated in the detergent and insoluble phases (Fig. 2C, lanes 4, 5, 9, and 10). A slight difference in intensity in protein p41 in the insoluble phase between the two passages was noted (Fig. 2C, lanes 5 and 10).
An analysis of the plasmid profile by FIGE and PFGE did not reveal any potential correlation between plasmid profile and invasiveness (data not shown).
vlsE signal decreases with increases in passage.
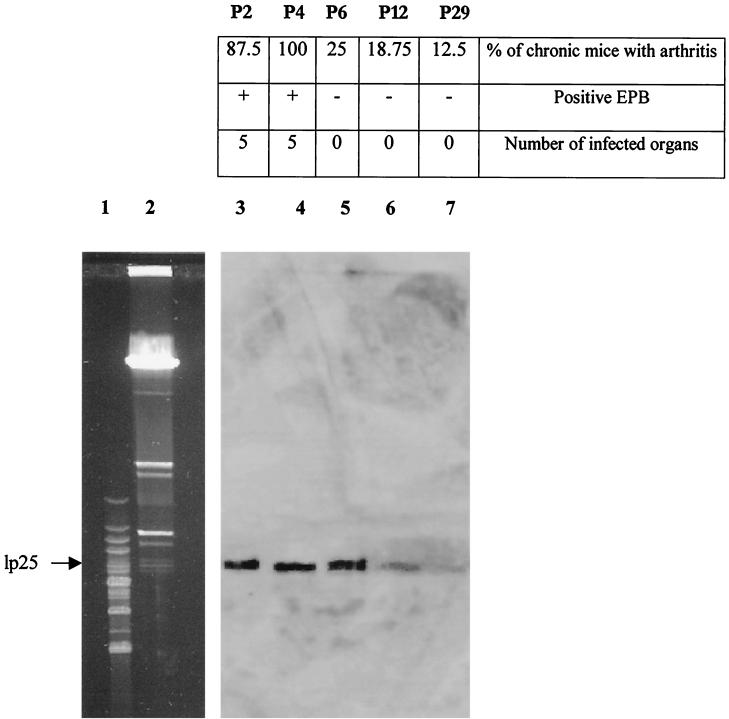
The sequences of the primers used to construct the vlsE probe allowed us to amplify a region of the gene that comprised the segment between VR-5 and IR-6 of vlsE. When this vlsE-based probe was hybridized with a PFGE gel of undigested DNA, a signal was obtained in all passages assayed, although a progressive decrease in the intensity of staining was seen with the passages: a strong signal in p2, p4, and p6 (Fig. 3, lanes 3 to 5), a weak signal in p12 (Fig. 3, lane 6), and a barely visible signal in p29 (Fig. 3, lane 7). Densitometric analysis revealed that, considering the p2 intensity as 100%, p4, p6, p12, and p29 presented intensities of 100, 91.4, 42.8, and 21.4%, respectively.
FIG. 3.
Southern hybridization with a vlsE probe and summary of the pathogenicity assay. Lane 1, size markers (8.2 to 48.5 kb); lane 2, PFGE gel of p2 of PV6; lanes 3 to 7, Southern hybridization with vlsE probe of p2, p4, p6, p12, and p29 of PV6
Loss of flagella in serial passages results in decreased invasiveness.
We performed an ELISA to quantify the reaction of monoclonal antibody CB1, directed against FlaB (18), with whole-cell sonicates from p2 and p29. p2 had an optical density and cutoff of 0.259 and 0.110, respectively, and p29 had an optical density and cutoff of 0.205 and 0.110, respectively, which represented a decrease of 21%.
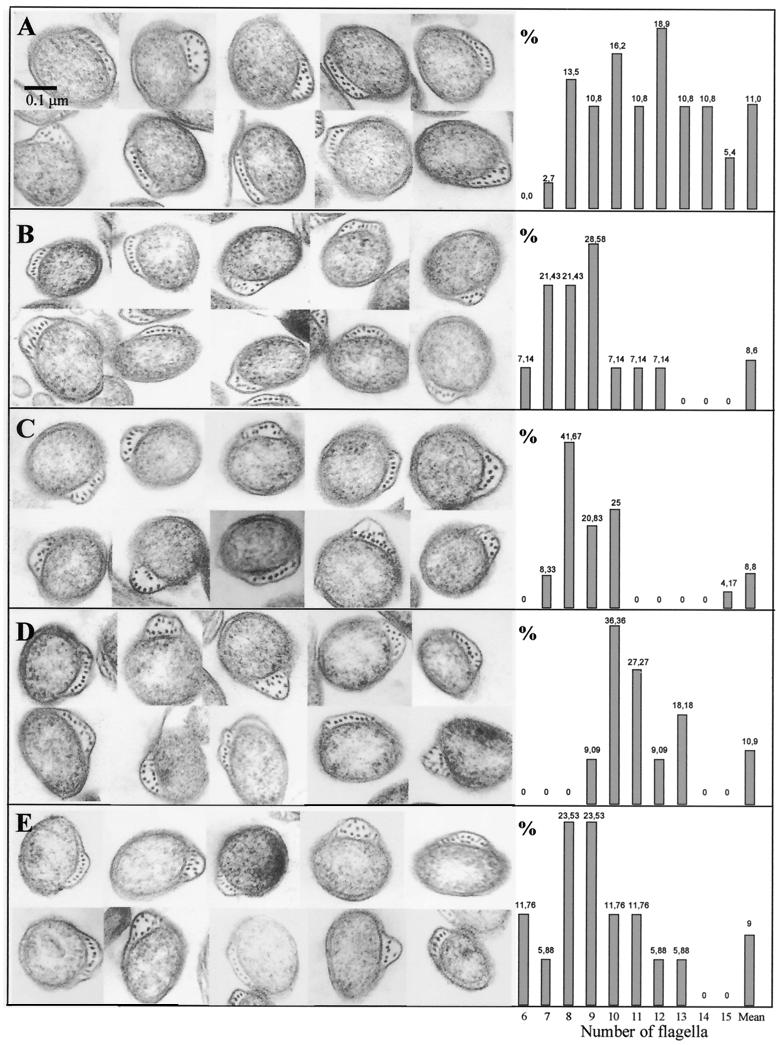
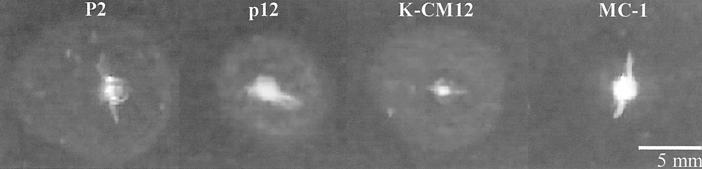
Identical results were obtained from the numbers of flagella of a mean of 36 spirochetes per passage, counted in photographs of cross-sections by transmission electron microscopy (Fig. 4; Table 2). The highest frequencies of numbers of flagella were for 12, 9, and 8 flagella at p2, p6, p12, respectively, and for 10 and 8 flagella for the isolates from kidney and bladder of CM12, respectively. The mean numbers of flagella in each sample were 11, 8.6, 8.8, 11, and 9, for the p2, p6, p12, kidney, and bladder samples, respectively. These data were shown to be statistically significant by the Kruskal-Wallis test (P < 0.005) and correlated with the behavior of p2 and p12 in a swarm assay. In 4 days, passage 2 spread to a circle of 11.9 mm, while p12 reached only 8.3 mm. These values correlated with the decrease in the number of flagella (Fig. 5). However, in the same assay, the isolate from kidney of CM12 spread over the agar plate more than the parental strain (10.2 mm; Fig. 5), showing a recovery of its motility.
FIG. 4.
Transmission electron microscopy and flagellum counts of different passages and isolates of B. garinii PV6. (A) p2; (B) p6; (C) p12; (D) isolate from kidney of CM12; (E) isolate from bladder of CM12. y axes indicate the percentages of borrelias with each number of flagella (indicated above histograms).
TABLE 2.
Distribution of the number of flagella for each passage and isolate
| Bacterium | Mean no. of flagella | Frequency (%) of borrelias with the indicated no. of flagella
|
||
|---|---|---|---|---|
| ≥8 | ≥10 | ≥12 | ||
| p2 | 11 | 97.3 | 73 | 45.9 |
| p6 | 8.6 | 71.4 | 21.4 | 7.1 |
| p12 | 8.8 | 91.4 | 2.9 | 2.9 |
| Isolate from kidney | 10.9 | 100 | 90.9 | 27.3 |
| Isolate from bladder | 9 | 82.3 | 35.3 | 11.8 |
FIG. 5.
Swarm agar plate assay. p2 and p12, p2 and p12 of PV6; K-CM12: isolate from kidney of CM12; MC-1, nonflagellated mutant derived from strain B31 of B. burgdorferi (44).
DISCUSSION
The serial in vitro culture of a low-passage, tick-derived isolate of B. garinii pathogenic for C3H mice (20) led to a progressive selection of clones harboring less flagella. Correlating with this, the capacity of dissemination through the skin and colonization of internal organs of this strain during the acute phase (first month after infection) disappeared from p6, coincident with a decrease in motility in a swarm assay. Even considering that we can't exclude the possibility that other subtle variations may be arising given the uncloned nature of the isolate, we have found a strong association between loss of flagella and decreased invasiveness. In this study, 12 flagella seemed to be the lowest number needed for this strain to be able to disseminate to organs. The complicated structure of this organelle (16) could account for this way of saving energy under suboptimal conditions. The importance of this structure for B. burgdorferi is suggested by the high percentage of the chromosome dedicated to the chemotaxis and motility functions (21, 22). Moreover, the description of a flagellum-less B. burgdorferi mutant (41) which was unable to infect organs in a mouse model confirmed the importance of flagella for the virulence of B. burgdorferi. Recently, skeletal and motility functions for the flagella of B. burgdorferi have been described (36). As suspected, the motility of the organism seems to play an important role in its pathogenic mechanisms, since we have found that the number of flagella strongly correlates with invasiveness of B. garinii as well as with its swarm capabilities.
The isolate recovered from the kidney of CM12 3 months after the infection (no organs from CM12 yielded isolates during the acute phase) had a mean number of flagella of 11, compared to the homologous inoculum's 8.8 (p12). Considering that a minority of organisms with high number of flagella were still present in p12, clones with a mean of 11 flagella would be able to reach the kidney after 3 months and would be recovered in culture. The isolate from the bladders of the same mice consisted of a more variable population of clones (range of flagella, 6 to 13 [mean, 9], compared to 9 to 13 for the isolate from kidney). The bladder has been described as an organ preferentially infected (44), so we can assume that less flagella would be necessary to reach it. It is important to stress that, given the low frequency of borrelias with ≥12 flagella in both p6 and p12 (7.1 and 2.9%, respectively), the fact that spirochetes could be isolated from one mouse inoculated with p12 and but not from any mice inoculated with p6 could be due to the number of mice included in each experiment (6 mice were inoculated with p6, and 20 were inoculated with p12).
Flagella have been proposed as a virulence factor in other bacteria. For example, in Legionella spp. they have been identified as a positive predictive marker for virulence (11). Mutations in flaA of Vibrio anguillarum lead to partially motile organisms with an increase of up to 104-fold in the 50% lethal dose (35). Interestingly, the activity of flagellar regulatory protein FlrC of Vibrio cholerae has been demonstrated to contribute not only to motility but also to colonization (19). The flagellar export apparatus of Yersinia enterocolitica has been demonstrated to function also as a secretion system for the transport of virulence factors (54). Proteins similar to flagellar and virulence factor export proteins of other bacteria have been described for B. burgdorferi and have been found to be well conserved in the Eubacteria (25). Moreover, the binding of plasminogen, a vital mechanism in B. burgdorferi dissemination (17), has been found in Escherichia coli to be associated with flagella (30).
We have not found a clear correlation between plasmid or protein profile and invasiveness. This is in agreement with previous reports in which several authors have described a decreased invasiveness during in vitro culture or among different clones without discernible differences in the plasmid or protein profile from the corresponding parent strains (3, 33, 38, 50), suggesting that other genetic and protein-related factors seem to be important in the virulence and pathogenicity of this organism (38).
Recent reports have demonstrated that both lp25 and lp28-1 are needed for a full range of pathogenicity in B. burgdorferi and that the presence of the vlsE-carrying lp28-1 does not necessarily imply virulence (29, 40). The strain of B. garinii utilized in this study apparently lacks lp28-1, and vlsE is located in a 25-kb plasmid. We observed a decrease in the intensity of a Southern blot with a vlsE-based probe in p12 and p29, suggesting that the number of clones harboring the corresponding plasmid could decrease with increasing in vitro passages. However, the decrease in invasiveness was observed also in p6, which still efficiently hybridized with the vlsE probe but whose arthritogenicity was already diminished with respect to that of p2 and p4. Consequently, we did not find a correlation between the passage from which a decreased pathogenicity was noted and the presence of vlsE, although it can be assumed that p6, being an intermediate between pathogenic and nonpathogenic clones, could still have some of the characteristics that help in constructing the pathogenic phenotype. Alternatively, and due to the lack of selective pressure within the joint (29), lp28-1 (lp25 in this strain) was not needed in constructing the arthritogenicity phenotype. Another possible interpretation would be that in the populations selected in vitro the arthritogenicity could be the consequence of other subtle genetic differences and that the presence of clones harboring the vmp-like sequence locus had no effect on the arthritogenicity.
In this work we analyzed the events that lead to a loss of invasiveness during serial in vitro passages of a strain of B. garinii and demonstrated that a decrease in the content of flagella and in the swarm capabilities strongly correlates with the loss of invasiveness, as measured by the number of organs infected and by a decreased serologic response. The use of a polyclonal population in the original inoculum, more similar to the mixed populations that a tick injects in the mammalian hosts, allowed us to determine one of the reasons of the deleterious effect of the in vitro culture of B. garinii on pathogenicity, which seems to be related to the overgrowth of less-energy-consuming clones harboring smaller numbers of flagella.
Acknowledgments
This work was supported by a grant from Fondo de Investigación Sanitaria (98/0026-01). Ricela E. Sellek participated in this study while supported by a Beca de Iniciación of the Instituto de Salud Carlos III (97/4181). Raquel Escudero and Horacio Gil were financed by a fellowship from the Fondo de Investigación Sanitaria (98/0026-01).
We thankfully acknowledge N. W. Charon, from the Department of Microbiology and Immunology, Health Sciences Center, West Virginia University, for providing us with the B31 aflagellated mutant MC-1 and M. A. Motaleb for his help in setting the swarm assay. We are also grateful to Angel del Pozo for the photographic work.
Editor: V. J. DiRita
REFERENCES
- 1.Anda, P., I. Rodríguez, A. de la Loma, M. V. Fernández, and A. Lozano. 1993. A serological survey and review of clinical Lyme borreliosis in Spain. Clin. Infect. Dis. 16:310-319. [DOI] [PubMed] [Google Scholar]
- 2.Anda, P., W. Sánchez-Yebra, M. M. Vitutia, E. Pérez-Pastrana, I. Rodríguez, N. S. Miller, P. B. Backenson, and J. L. Benach. 1996. A new Borrelia species isolated from patients with relapsing fever in Spain. Lancet 348:162-165. [DOI] [PubMed] [Google Scholar]
- 3.Anguita, J., S. Samanta, B. Revilla, K. Suk, S. Das, S. W. Barthold, and E. Fikrig. 2000. Borrelia burgdorferi gene expression in vivo and spirochete pathogenicity. Infect. Immun. 68:1222-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baranton, G., D. Postic, I. Saint Girons, P. Boerlin, J. C. Piffaretti, M. Assous, and P. A. Grimont. 1992. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int. J. Syst. Bacteriol. 42:378-383. [DOI] [PubMed] [Google Scholar]
- 5.Barbour, A. G., and B. I. Restrepo. 2000. Antigenic variation in vector-borne pathogens. Emerg. Infect. Dis. 6:449-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbour, A. G. 1988. Plasmid analysis of Borrelia burgdorferi, the Lyme disease agent. J. Clin. Microbiol. 26:475-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barthold, S. W., D. S. Beck, G. M. Hansen, G. A. Terwilliger, and K. D. Moody. 1990. Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 162:133-138. [DOI] [PubMed] [Google Scholar]
- 8.Benach, J. L., E. M. Bosler, J. P. Hanrahan, J. L. Coleman, G. S. Habicht, T. F. Bast, D. J. Cameron, J. L. Ziegler, A. G. Barbour, W. Burgdorfer, R. Edelman, and R. A. Kaslow. 1983. Spirochetes isolated from the blood of two patients with Lyme disease. N. Engl. J. Med. 308:740-742. [DOI] [PubMed] [Google Scholar]
- 9.Benach, J. L., H. B. Fleit, G. S. Habicht, J. L. Coleman, E. M. Bosler, and B. P. Lane. 1984. Interactions of phagocytes with the Lyme disease spirochete: role of the Fc receptor. J. Infect. Dis. 150:497-507. [DOI] [PubMed] [Google Scholar]
- 10.Bordier, C. 1981. Phase separation of integral membrane proteins in Triton X-114 solution. J. Biol. Chem. 256:1604-1607. [PubMed] [Google Scholar]
- 11.Bosshardt, S. C., R. F. Benson, and B. S. Fields. 1997. Flagella are a positive predictor for virulence in Legionella. Microb. Pathog. 23:107-112. [DOI] [PubMed] [Google Scholar]
- 12.Brusca, J. S., and J. D. Radolf. 1994. Isolation of integral membrane proteins by phase partitioning with Triton X-114. Methods Enzymol. 228:182-193. [DOI] [PubMed] [Google Scholar]
- 13.Burgdorfer, W., A. G. Barbour, S. F. Hayes, J. L. Benach, E. Grunwaldt, and J. P. Davies. 1982. Lyme disease—a tick-borne spirochetosis? Science 216:1317-1319. [DOI] [PubMed] [Google Scholar]
- 14.Carle, G. F., F. Frank, and M. V. Olson. 1986. Electrophoretic separations of large DNA molecules by periodic inversion of the field. Science 232:65-68. [DOI] [PubMed] [Google Scholar]
- 15.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. M. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490-516. [DOI] [PubMed] [Google Scholar]
- 16.Charon, N. W., E. P. Greeberg, M. B. Koopman, and R. J. Limberger. 1992. Spirochete chemotaxis, motility, and the structure of the spirochetal periplasmic flagella. Res. Microbiol. 143:587-603. [DOI] [PubMed] [Google Scholar]
- 17.Coleman, J. L., J. A. Gebbia, J. Piesman, J. L. Degen, T. H. Bugge, and J. L. Benach. 1997. Plasminogen is required for efficient dissemination of B. burdorferi in ticks and for enhancement of spirochetemia in mice. Cell 89:1111-1119. [DOI] [PubMed] [Google Scholar]
- 18.Coleman, J. L., and J. L. Benach. 1989. Identification and characterization of an endoflagellar antigen of Borrelia burgdorferi. J. Clin. Investig. 84:322-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Correa, N. E., C. M. Lauriano, R. McGee, and K. E. Klose. 2000. Phosphorylation of the flagellar regulatory protein FlrC is necessary for Vibrio cholerae motility and enhanced colonization. Mol. Microbiol. 35:743-755. [DOI] [PubMed] [Google Scholar]
- 20.Escudero, R., M. Barral, A. Pérez, I. Rodríguez, A. L. García-Pérez, S. Jiménez, R. Sellek, and P. Anda. 2000. Molecular and pathogenic characterization of Borrelia burgdorferi sensu lato isolates from Spain. J. Clin. Microbiol. 38:4026-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J.-F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. Weidman, T. Utterback, L. Watthey, L. McDonald, P. Artiach, C. Bowman, S. Garland, C. Fujii, M. D. Cotton, K. Horst, K. Roberts, B. Hatch, H. O. Smith, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 22.Ge, Y., and N. W. Charon. 1997. An unexpected flaA homolog is present and expressed in Borrelia burgdorferi. J. Bacteriol. 179:552-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ge, Y., C. Li, L. Corum, C. A. Slaughter, and N. W. Charon. 1998. Structure and expression of the FlaA periplasmic flagellar protein of Borrelia burgdorferi. J. Bacteriol. 180:2418-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ge, Y., I. G. Old, I. Saint Girons, and N. W. Charon. 1997. Molecular characterization of a large Borrelia burgdorferi motility operon which is initiated by a consensus σ70 promoter. J. Bacteriol. 179:2289-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ge, Y., I. Old, I. Saint Girons, D. B. Yelton, and N. W. Charon. 1996. FliH and FliI of Borrelia burgdorferi are similar to flagellar and virulence factor export proteins of other bacteria. Gene 168:73-75. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein, S. F., N. W. Charon, and J. A. Kreiling. 1994. Borrelia burgdorferi swims with a planar waveform similar to that of eukaryotic flagella. Proc. Natl. Acad. Sci. USA 91:3433-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson, R. C., G. P. Schmid, F. W. Hyde, A. G. Steigerwalt, and D. J. Brenner. 1984. Borrelia burgdorferi sp. nov.: etiologic agent of Lyme disease. Int. J. Syst. Bacteriol. 34:496-497. [Google Scholar]
- 28.Kimsey, R. B., and A. Spielman. 1990. Motility of Lyme disease spirochetes in fluids as viscous as the extracellular matrix. J. Infect. Dis. 162:1205-1208. [DOI] [PubMed] [Google Scholar]
- 29.Labandeira-Rey, M., and J. T. Skare. 2001. Decreased infectivity in Borrelia burgdorferi strain B31 is associated with loss of either linear plasmid 25 or 28-1. Infect. Immun. 69:446-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lahteenmaki, K., B. Westerlund, P. Kuusela, and T. K. Korhonen. 1993. Immobilization of plasminogen on Escherichia coli flagella. FEMS Microbiol. Lett. 106:309-314. [DOI] [PubMed] [Google Scholar]
- 31.Mann, H. B., and D. R. Whitney. 1947. On a test whether one of two random variables is stochastically larger than the other. Ann. Math. Statist. 18:50-60. [Google Scholar]
- 32.Marconi, R. T., S. Casjens, U. G. Munderloh, and D. S. Samuels. 1996. Analysis of linear plasmid dimers in Borrelia burgdorferi sensu lato isolates: implications concerning the potential mechanism of linear plasmid replication. J. Bacteriol. 178:3357-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDowell, J. V., S. Y. Sung, M. Labandeira-Rey, J. T. Skare, and R. T. Marconi. 2001. Analysis of mechanisms associated with loss of infectivity of clonal populations of Borrelia burgdorferi B31M1. Infect. Immun. 69:3670-3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meier, J. T., M. I. Simon, and A. G. Barbour. 1985. Antigenic variation is associated with DNA rearrangements in a relapsing fever Borrelia. Cell 41:403-409. [DOI] [PubMed] [Google Scholar]
- 35.Milton, D. L., R. O'Toole, P. Hörstedt, and H. Wolf-Watz. 1996. Flagellin A is essential for the virulence of Vibrio anguillarum. J. Bacteriol. 178:1310-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Motaleb, M. A., L. Corum, J. L. Bono, A. F. Elias, P. A. Rosa, D. S. Samuels, and N. W. Charon. 2000. Borrelia burgdorferi periplasmic flagella have both skeletal and motility functions. Proc. Natl. Acad. Sci. USA 97:10899-10904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norris, S. J., C. J. Carter, J. K. Howell, and A. G. Barbour. 1992. Low-passage-associated proteins of Borrelia burgdorferi B31: characterization and molecular cloning of OspD, a surface-exposed, plasmid-encoded lipoprotein. Infect. Immun. 60:4662-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norris, S. J., J. K. Howell, S. A. Garza, M. S. Ferdows, and A. G. Barbour. 1995. High- and low-infectivity phenotypes of clonal populations of in vitro-cultured Borrelia burgdorferi. Infect. Immun. 63:2206-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palmer, N., C. Fraser, and S. Casjens. 2000. Distribution of twelve linear extrachromosomal DNAs in natural isolates of Lyme disease spirochetes. J. Bacteriol. 182:2476-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Purser, J. E., and S. J. Norris. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 97:13865-13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sadziene, A., P. A. Thompson, and A. G. Barbour. 1996. A flagella-less mutant of Borrelia burgdorferi as a live attenuated vaccine in the murine model of Lyme disease. J. Infect. Dis. 173:1184-1193. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed., vol. 2. Cold Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 43.Schwan, T. G., W. Burgdorfer, and C. Garon. 1988. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect. Immun. 56:1831-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwan, T. G., W. Burgdorfer, M. E. Schrupf, and R. H. Karstens. 1988. The urinary bladder, a consistent source of Borrelia burgdorferi in experimentally infected white-footed mice (Peromiscus leucopus). J. Clin. Microbiol. 26:893-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simpson, W. J., C. F. Garon, and T. G. Schwan. 1990. Analysis of supercoiled circular plasmids in infectious and non-infectious Borrelia burgdorferi. Microb. Pathog. 8:109-118. [DOI] [PubMed] [Google Scholar]
- 46.Simpson, W. J., C. F. Garon, and T. G. Schwan. 1990. Borrelia burgdorferi contains repeated DNA sequences that are species specific and plasmid associated. Infect. Immun. 58:847-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sobral, B. W., and A. G. Atherly. 1989. Pulse time and agarose concentration affect the electrophoretic mobility of cccDNA during PFGE and FIGE. Nucleic Acids Res. 17:7359-7369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sokal, R. R., and F. J. Rohlf. 1969. Biometry. W. H. Freeman and Co., San Francisco, Calif.
- 49.Steere, A. C. 1989. Lyme disease. N. Engl. J. Med. 321:586-596. [DOI] [PubMed] [Google Scholar]
- 50.Thomas, V., J. Anguita, S. Samanta, P. A. Rosa, P. Stewart, S. W. Barthold, and E. Fikrig. 2001. Dissociation of infectivity and pathogenicity in Borrelia burgdorferi. Infect. Immun. 69:3507-3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang, G. Q., A. P. van Dam, and J. Dankert. 2001. Analysis of a VMP-like sequence (vls) locus in Borrelia garinii and Vls homologues among four Borrelia burgdorferi sensu lato species. FEMS Microbiol. Lett. 199:39-45. [DOI] [PubMed] [Google Scholar]
- 52.Xu, Y., and R. C. Johnson. 1995. Analysis and comparison of plasmid profiles of Borrelia burgdorferi sensu lato strains. J. Clin. Microbiol. 33:2679-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu, Y., C. Kodner, L. Coleman, and R. C. Johnson. 1996. Correlation of plasmids with infectivity of Borrelia burgdorferi sensu stricto type strain B31. Infect. Immun. 64:3870-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Young, G. M., D. H. Schmiel, and V. L. Miller. 1999. A new pathway for the secretion of virulence factors by bacteria: the flagellar export apparatus functions as a protein-secretion system. Proc. Natl. Acad. Sci. USA 96:6456-6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang, J.-R., J. M. Hardham, A. G. Barbour, and S. J. Norris. 1997. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell 89:275-285. [DOI] [PubMed] [Google Scholar]