Abstract
Malaria merozoite surface protein 1 (MSP1) is cleaved in an essential step during erythrocyte invasion. The responses of children to natural malaria infection included antibodies that inhibit this cleavage and others that block the binding of these inhibitory antibodies. There was no correlation between the titer of the antibody to the 19-kDa fragment of MSP1 and its inhibitory activity. These findings have implications for the design of MSP1-based vaccines.
Malaria control is a major problem, and the need for the development of new effective measures, including vaccines, is urgent. The immune response to the parasite is complex, and various stages of the parasite's life cycle are being explored as potential targets for vaccine development (15). The clinical disease is associated with replication of the asexual blood stage parasite. Merozoite surface protein 1 (MSP1) is an ∼200-kDa precursor that is present on the surface of the merozoite as a multicomponent complex derived by proteolytic processing (12, 13). At the time of erythrocyte invasion, the 42-kDa C-terminal component (MSP142) is further cleaved to produce a soluble 33-kDa fragment (MSP133) and a 19-kDa fragment (MSP119) that remains on the merozoite surface during invasion (2, 3). This so-called secondary processing of MSP1 goes to completion during the successful invasion of a red blood cell, suggesting that it is a necessary step. Monoclonal antibodies (MAbs) that prevent invasion inhibit secondary processing of MSP1, suggesting that this is their mechanism of action (4). Blocking antibodies (10) are not inhibitory but interfere with inhibitory antibody activity by competing for binding to the merozoite surface. This suggests an immune evasion mechanism to avoid the action of protective antibodies (11). Here we present evidence that natural infection may induce both antibodies that inhibit MSP1 secondary processing and antibodies that block this inhibition.
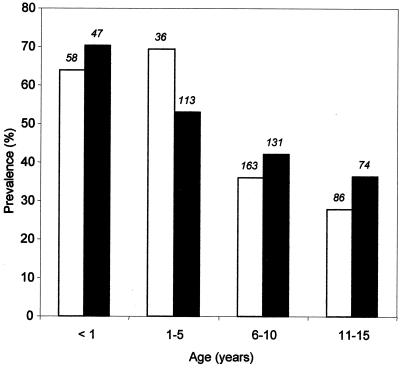
Prevalence of Plasmodium falciparum parasites.
This study was conducted at Igbo-Ora and Idere in southwestern Nigeria. After informed consent was obtained from their parents or guardians, children were recruited according to a protocol that was reviewed and approved by the Joint Ethical Committee of the College of Medicine and the University College Hospital, Ibadan, Nigeria. P. falciparum parasitemia was prevalent both at the end of the rainy season and in the middle of the dry season (Fig. 1), with no significant difference in the age distribution of the infected children between the two time points. Overall, the rate of parasitemia declined with age (data not shown).
FIG. 1.
Prevalence of malaria parasitemia among 343 children, 10 days to 15 years old, during the dry season (January to March 1999) and among 365 children with the same age distribution at the end of the rainy season (October to November 1999), at Idere and Igbo-Ora, rural towns in southwestern Nigeria. The prevalence was calculated as the percentage of malarial parasite-positive individuals. The actual number of parasitemic cases is shown at the top of each bar. Open bars, dry season; filled bars, wet season.
Antibodies to MSP119 measured by ELISA.
Plasma samples from 708 donors were analyzed by enzyme-linked immunosorbent assay (ELISA) for antibodies to recombinant MSP119 (6). The samples were diluted at a 1:25 ratio and then in twofold dilutions to 1:3,200; the reciprocal end point titer (the highest dilution that gave an absorbance value above that of the negative controls) was log transformed, and data were expressed as geometric mean log reciprocal titers. There were no differences in the geometric mean log reciprocal titers between those individuals who had parasitemia (2.58) and those who did not (2.56) or between sexes (P > 0.05, data not shown). In both the dry and the wet seasons, the mean log reciprocal titer for children under 12 months old (2.4) was the same as that for 12- to 60-month-old children. When samples collected during the dry season were compared, the antibody titers determined were higher for children of ≥6 years than for those of ≤5 years of age (P < 0.01); in contrast, there was a decline with age in antibody titers for the plasma samples collected during the rainy season, though the difference between the two groups was not significant (P > 0.05) (data not shown).
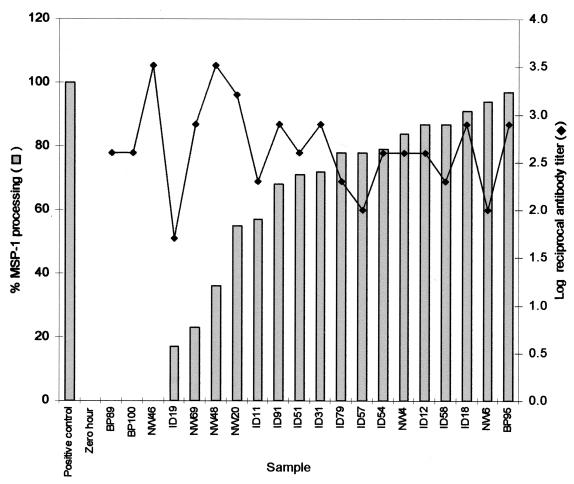
Antibody-mediated inhibition of MSP142 processing.
Plasma samples from 50 children, 1 month to 15 years of age, who were chosen randomly from the group of 343 children seen in the dry season were assayed for MSP1 processing-inhibitory activity. Merozoites were prepared according to the procedures of Blackman (1), and processing assays were performed essentially as described previously (4, 10). MSP142 and MSP133 were identified by enhanced chemiluminescence and exposure to autoradiographic film. The densities of the MSP142 and MSP133 bands were measured after a short exposure (2 to 5 s, in the linear density response range) with Scion (Frederick, Md.) image software. The relative proportion of MSP133 was calculated by use of the formula b/(a + b), where a is the amount of MSP142 remaining at the end of the assay and b is the amount of MSP133 produced. The percentage of MSP142 processing was calculated by the formula 100(X − B)/(A − B), where A, B, and X are the relative proportions of MSP133 produced, respectively, in the reaction buffer alone, in the zero-hour control (levels of MSP133 present at the start of the assay), and in the presence of MAb or the plasma sample being tested. Of the 50 plasma samples analyzed at random, the results for 20 are shown (Fig. 2). Three samples, BP89, BP100, and NW46 (from donors aged 13, 11 and 6 years, respectively), showed complete inhibition of secondary processing of MSP1. Three other samples, ID19, NW69, and NW48 (from donors aged 2 months and 6 and 7 years, respectively), showed significant inhibition of processing (64 to 83%). The remaining 44 samples did not show significant inhibition of MSP1 processing. Similar results were obtained with merozoites expressing either the Wellcome or MAD20 MSP1 types (data not shown). When the MSP119-specific antibody titers of these sera were examined, there was no significant correlation between specific antibody levels and processing-inhibitory activity (Fig. 2). Twenty-four of the samples (48%) appeared to show enhanced (up to threefold-higher) processing relative to that of the untreated positive control (data not shown). The biological significance of this is obscure, but it will be of interest to determine whether or not these antibodies contribute to the observed enhancement of parasite growth in vitro by human immunoglobulin G (5).
FIG. 2.
Some plasma samples contain MSP1 secondary-processing-inhibitory antibodies. Fifty samples (dry season set) chosen at random were analyzed for their ability to inhibit the processing of MSP142 in P. falciparum FCB-1 merozoite preparations. The samples comprised merozoites resuspended in (i) reaction buffer alone (positive control) (no Ab), (ii) reaction buffer followed by immediate solubilization in detergent (zero hour), (iii) reaction buffer after pretreatment with a 10% (vol/vol) plasma sample as indicated (remaining samples shown, individually labeled), (iv) reaction buffer after pretreatment with MAb 12.8 (data not shown), and (v) reaction buffer containing 1 mM phenylmethylsulfonyl fluoride (negative control, data not shown). After 1 h, the samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto nitrocellulose, and MSP142 and MSP133 were identified and quantified. The results (vertical bars, percent MSP1 processing) are shown for 20 plasma samples. The remainder of the 50 samples analyzed showed either no effect or enhanced MSP1 processing. The MSP119-specific antibody titers of the individual samples are also shown as log reciprocal antibody titers (⧫).
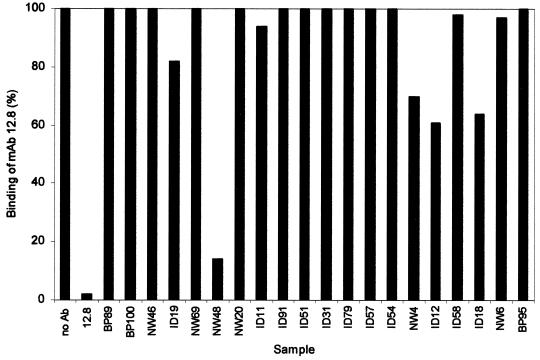
Antibodies that compete with inhibitory MAbs for binding to MSP1.
The ability of antibodies in the plasma samples to block the binding of processing-inhibitory MAbs 12.8 and 12.10 to MSP119 was examined with a competition ELISA. Figure 3 shows the results for the plasma samples assayed with MAb 12.8; similar results were obtained with MAb 12.10 (data not shown). Of the six samples that showed the most processing-inhibitory activity, only NW48 had antibodies that prevented the binding of MAb 12.8. The other five samples, BP100, BP89, NW46, ID19, and NW69, did not have antibodies that competed with the binding of MAb 12.8; thus, these samples contain antibodies that define new binding sites for MSP1 secondary-processing-inhibitory antibodies. Ten plasma samples (20% of the 50 randomly chosen samples) had antibodies that competed with MAbs 12.8 and 12.10 (Fig. 3 and data not shown); in the absence of significant processing inhibition, this implies that these samples contain largely blocking antibodies. The remaining 34 samples did not show significant MSP1 processing-inhibitory activity and did not compete with MAb 12.8 (or MAb 12.10) despite the presence of MSP119-specific antibodies detected by ELISA. The absence of either inhibitory or blocking activities is consistent with these samples containing neutral antibodies (11).
FIG. 3.
Human plasma samples compete with processing-inhibitory MAbs for binding to MSP119. A competitive ELISA was used to determine whether or not antibodies developed during natural infection with P. falciparum could competitively block the binding of MAbs 12.8 and 12.10. The results presented are for the same plasma samples as those described in the legend to Fig. 2 and arranged in the same order: (i) labeled antibody bound in the absence of a competitor (no Ab), (ii) labeled antibody bound in the presence of a competitor MAb (12.8), and (iii) labeled antibody bound in the presence of human plasma samples as indicated (remaining samples shown, individually labeled). The binding of MAb 12.8 to antigen is expressed as a percentage of the amount bound in the absence of a competitor; similar results were obtained for biotinylated MAb 12.10 (data not shown).
This is the first report of the induction of MSP1 processing-inhibitory antibodies by natural infection with P. falciparum. The natural immune response to MSP1 also includes the production of blocking antibodies, which may abolish these protective effects (11), providing an effective means for the malarial parasite to evade a host immune defense. It is possible that, after natural infection with P. falciparum, individuals produce both processing-inhibitory and blocking antibodies, with the outcome of exposure depending on the kinetics of the appearance of these two classes of antibody, their quantities, and their relative efficacies. Thus, the data provide support for the idea of developing MSP119 as a malaria vaccine and for the concept that an engineered protein may be more effective than the natural protein as a vaccine (17).
A major difficulty in the rational evaluation of malaria antigens for vaccine development has been the paucity of functional in vitro assays (14). Antibody-mediated inhibition of merozoite invasion has been used extensively as an in vitro test, for example, to examine the contribution of MSP119-specific antibodies in human sera (7, 9, 16). MSP119-specific MAbs that inhibited invasion also inhibited the processing of MSP142 (4), suggesting that this effect upon cleavage was the mechanism for inhibition of erythrocyte invasion. A second class of antibody may block the binding of these inhibitory antibodies, allowing processing and invasion to proceed in the presence of inhibitory antibodies (10). Despite the prevalence and level of MSP119-specific antibodies, only 12% of the 50 plasma samples analyzed had significant processing-inhibitory activity, suggesting that the fine specificity rather than the total level of specific antibody is important. This observation may explain why no evidence of association between antibody responses to MSP119 and clinical protection was found in a recent study (8). The contribution of passively acquired maternal antibody in young children and the importance of inhibitory antibodies for the clinical outcome of infection will need to be analyzed in a larger longitudinal study. Detailed evaluation of such assays for correlation with immune protection would be best achieved by using samples from MSP1 vaccine trials.
Acknowledgments
We acknowledge with deep gratitude the cooperation and endurance of the children who provided the blood samples for this study while attending the immunization program at the Primary Health Clinic at Idere and five nursery and primary schools at Igbo-Ora: Nawarudeen Primary School, Baptist Primary and Secondary School, His Grace Nursery and Primary School, Methodist Primary and Secondary School, and Adegoke Nursery and Primary School. We also thank the parents who gave permission for their children to participate in the study. We are grateful to O. O. Osossanya, A. A. Adeniran, and A. E. Echoga for their clinical assistance, to E. O. Koyejo for enrolling the children, to A. O. Olagoke for her cooperation in the use of the Idere Clinic, and to Muni Grainger for parasite culture.
This work was supported by grants from the Multilateral Initiative on Malaria Africa Research Capability Strengthening of the UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases.
Editor: J. M. Mansfield
REFERENCES
- 1.Blackman, M. J. 1994. Purification of Plasmodium falciparum merozoites for analysis of the processing of merozoite surface protein-1. Methods Cell. Biol. 45:213-220. [DOI] [PubMed] [Google Scholar]
- 2.Blackman, M. J., H.-G. Heidrich, S. Donachie, J. S. McBride, and A. A. Holder. 1990. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J. Exp. Med. 172:379-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackman, M. J., and A. A. Holder. 1992. Secondary processing of the Plasmodium falciparum merozoite surface protein-1 (MSP1) by a calcium-dependent membrane-bound serine protease: shedding of MSP-133 as a noncovalently associated complex with other fragments of the MSP1. Mol. Biochem. Parasitol. 50:307-316. [DOI] [PubMed] [Google Scholar]
- 4.Blackman, M. J., T. J. Scott-Finnigan, S. Shai, and A. A. Holder. 1994. Antibodies inhibit the protease-mediated processing of a malaria merozoite surface protein. J. Exp. Med. 180:389-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouharoun-Tayoun, H., P. Attanath, A. Sabchareon, T. Chongsuphajaisiddhi, and P. Druilhe. 1990. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J. Exp. Med. 172:1633-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burghaus, P. A., and A. A. Holder. 1994. Expression of the 19-kilodalton carboxy-terminal fragment of the Plasmodium falciparum merozoite surface protein-1 in Escherichia coli as a correctly folded protein. Mol. Biochem. Parasitol. 64:165-169. (Erratum, 67:343.) [DOI] [PubMed] [Google Scholar]
- 7.Chappel, J. A., A. F. Egan, E. M. Riley, P. Druilhe, and A. A. Holder. 1994. Naturally acquired human antibodies which recognize the first epidermal growth factor-like module in the Plasmodium falciparum merozoite surface protein 1 do not inhibit parasite growth in vitro. Infect. Immun. 62:4488-4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dodoo, D., T. G. Theander, J. A. Kurtzhals, K. Koram, E. Riley, B. D. Akanmori, F. K. Nkrumah, and L. Hviid. 1999. Levels of antibody to conserved parts of Plasmodium falciparum merozoite surface protein 1 in Ghanaian children are not associated with protection from clinical malaria. Infect. Immun. 67:2131-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egan, A. F., P. Burghaus, P. Druilhe, A. A. Holder, and E. M. Riley. 1999. Human antibodies to the 19kDa C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 inhibit parasite growth in vitro. Parasite Immunol. 21:133-139. [DOI] [PubMed] [Google Scholar]
- 10.Guevara Patino, J. A., A. A. Holder, J. S. McBride, and M. J. Blackman. 1997. Antibodies that inhibit malaria merozoite surface protein-1 processing and erythrocyte invasion are blocked by naturally acquired human antibodies. J. Exp. Med. 186:1689-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holder, A. A., J. A. Guevara Patino, C. Uthaipibull, S. E. Syed, I. T. Ling, T. Scott-Finnigan, and M. J. Blackman. 1999. Merozoite surface protein 1, immune evasion, and vaccines against asexual blood stage malaria. Parassitologia 41:409-414. [PubMed] [Google Scholar]
- 12.Holder, A. A., J. S. Sandhu, Y. Hillman, L. S. Davey, S. C. Nicholls, H. Cooper, and M. J. Lockyer. 1987. Processing of the precursor to the major merozoite surface antigens of Plasmodium faclciparum. Parasitology 94:199-208. [DOI] [PubMed] [Google Scholar]
- 13.McBride, J. S., and H.-G. Heidrich. 1987. Fragments of the polymorphic Mr185,000 glycoprotein from the surface of isolated Plasmodium falciparum merozoites form an antigenic complex. Mol. Biochem. Parasitol. 23:71-84. [DOI] [PubMed] [Google Scholar]
- 14.Miller, L. H., M. F. Good, and D. C. Kaslow. 1997. The need for assays predictive of protection in development of malaria bloodstage vaccines. Parasitol. Today 13:46-47. [DOI] [PubMed] [Google Scholar]
- 15.Miller, L. H., and S. L. Hoffman. 1998. Research toward vaccines against malaria. Nat. Med. 4:520-524. [DOI] [PubMed] [Google Scholar]
- 16.O'Donnell, R. A., T. F. de Koning-Ward, R. A. Burt, M. Bockarie, J. C. Reeder, A. F. Cowman, and B. S. Crabb. 2001. Antibodies against merozoite surface protein (MSP)-1(19) are a major component of the invasion-inhibitory response in individuals immune to malaria. J. Exp. Med. 193:1403-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uthaipibull, C., B. Aufiero, S. E. Syed, B. Hansen, J. A. Guevara Patino, E. Angov, I. T. Ling, K. Fegeding, W. D. Morgan, C. Ockenhouse, B. Birdsall, J. Feeney, J. A. Lyon, and A. A. Holder. 2001. Inhibitory and blocking monoclonal antibody epitopes on merozoite surface protein 1 of the malaria parasite Plasmodium falciparum. J. Mol. Biol. 307:1381-1394. [DOI] [PubMed] [Google Scholar]