Abstract
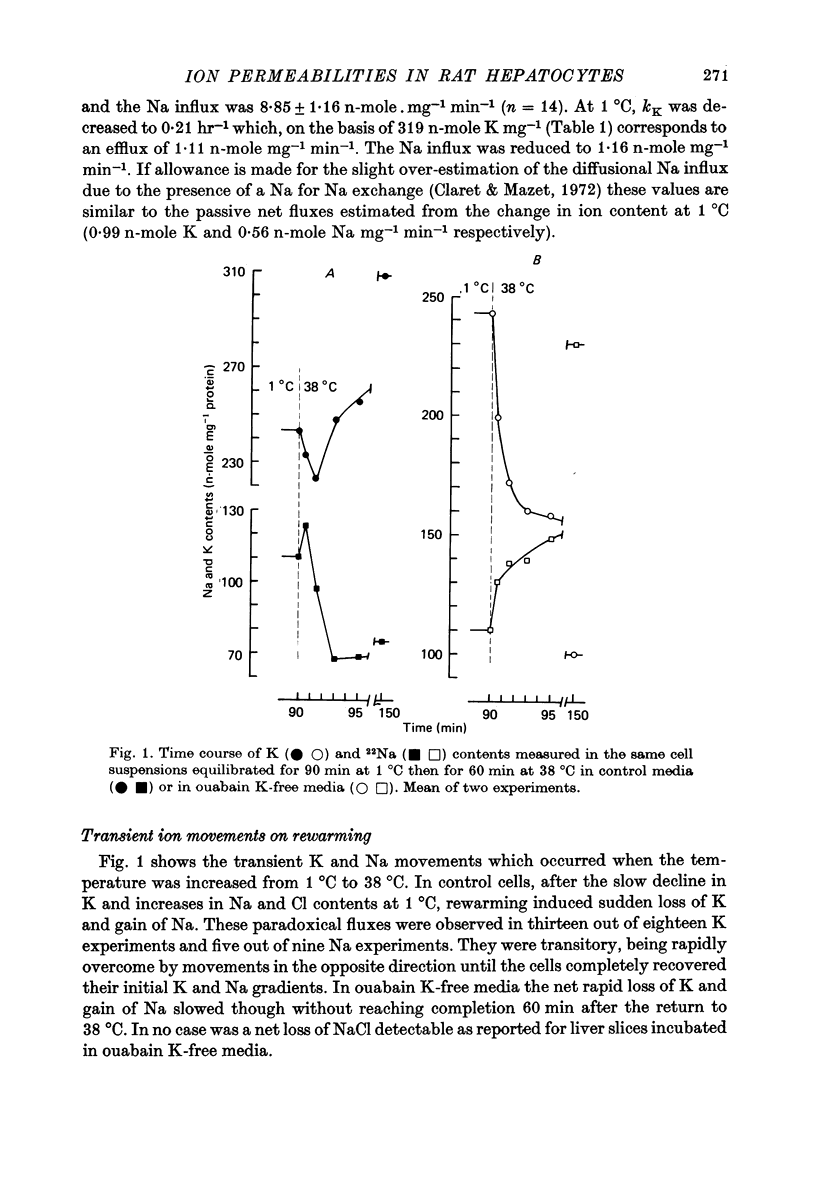
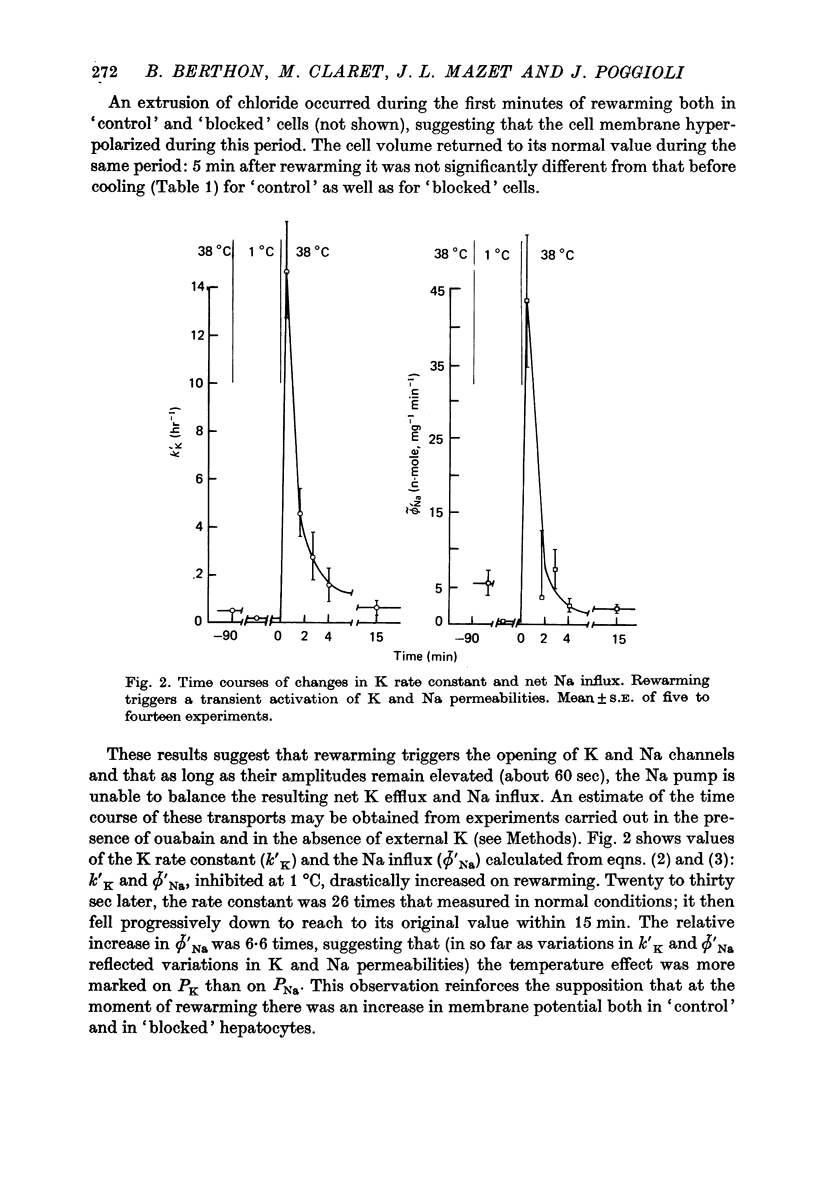
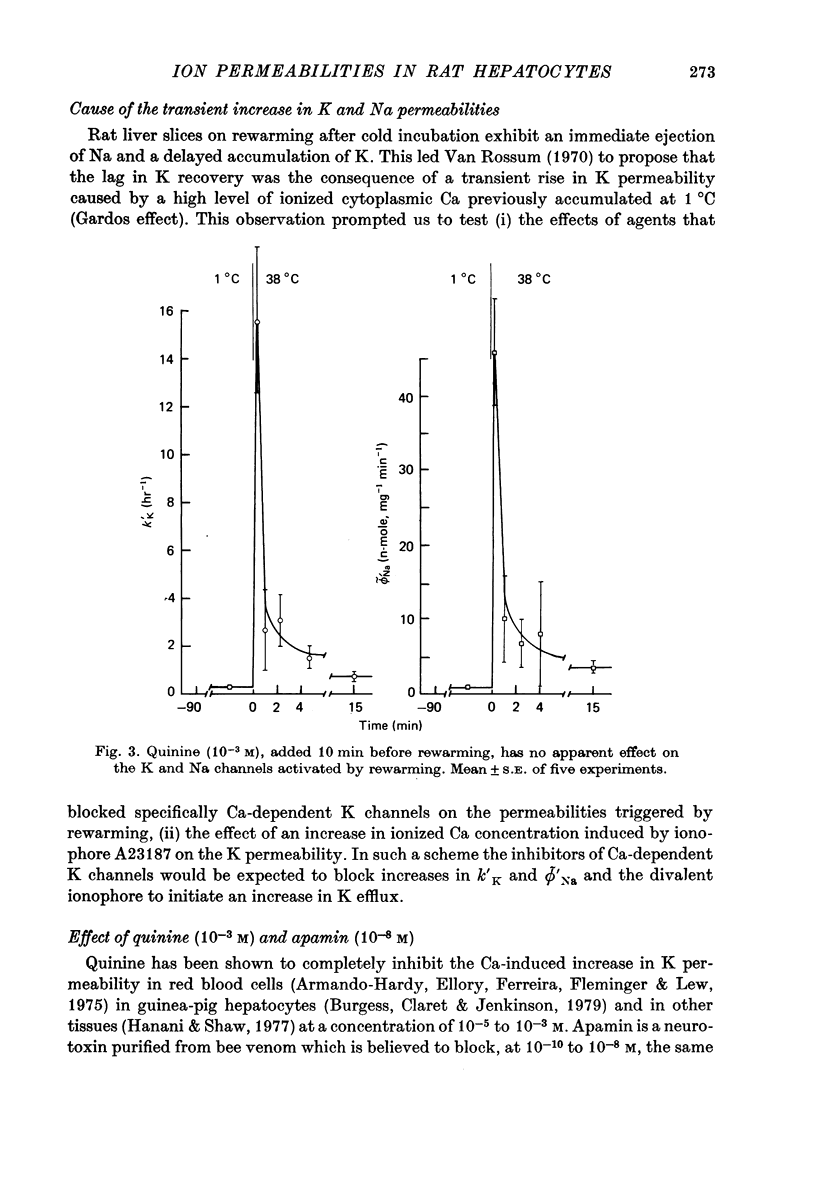
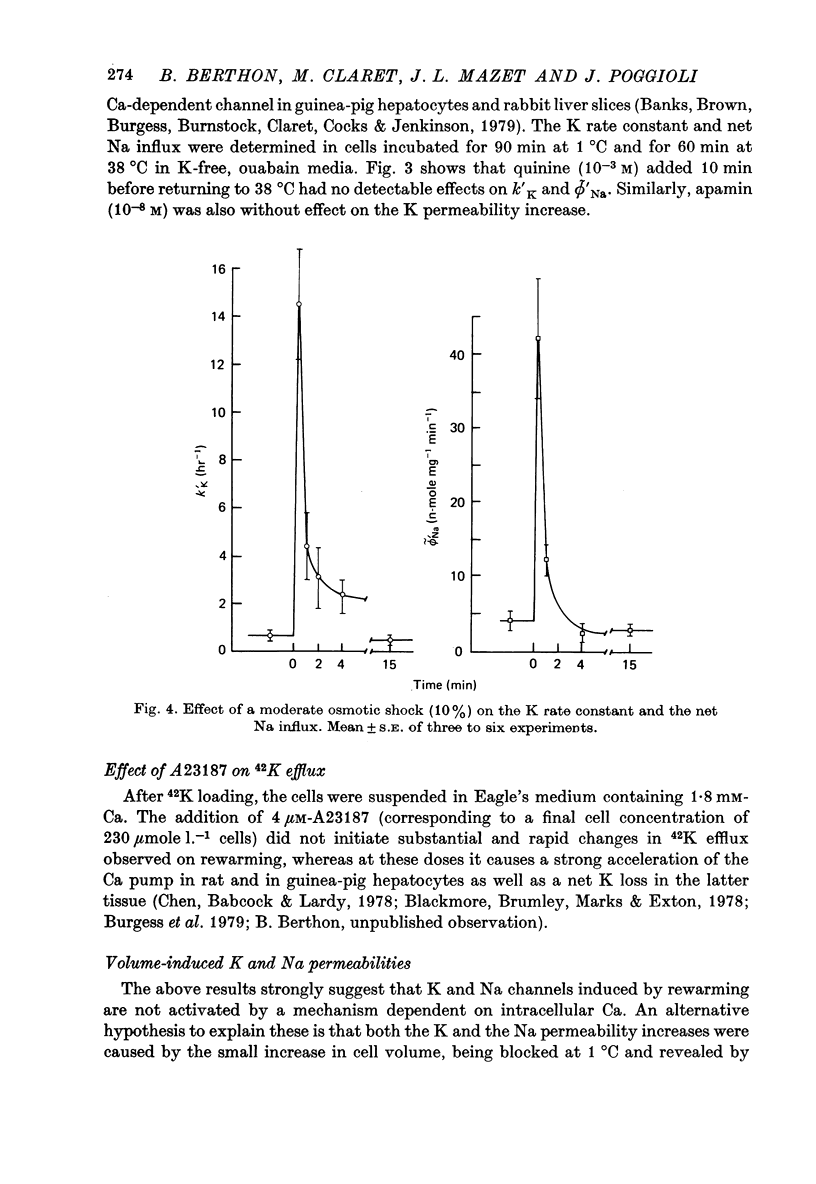
1. Water, K, Na and Cl contents and fluxes of K and Na were determined in isolated rat hepatocytes incubated at 1 degrees C (90 min) then at 38 degrees C (60 min). At 1 degrees C cells progressively gained Na and Cl, lost K and increased their volume by 17%. 2. Rewarming triggered a net loss of K and gain of Na. They were transitory (about 60 sec) being overcome rapidly by movements in the opposite direction until cells recovered their initial K and Na gradients. 3. Determination of time courses of the K rate constant (kappa' K) and net Na influx (phi Na) in cells incubated in ouabain K-free media indicated that these paradoxical movements were due to a temporary shunting of the Na pump by sudden increases in K and Na permeabilities. 4. Increases in kappa' K and phi' Na were not sensitive to inhibitors of Ca-activated K channels such as quinine (10(-3) M) of apamin (10(-8) M), suggesting they were not dependent on internal ionized Ca. 5. In control media containing 1.8 mM-Ca divalent ionophore A23187, though stimulating the Ca pump (Ca efflux), presumably by increasing internal ionized Ca concentration, did not cause substantial and rapid changes in K permeability. This supports the hypothesis that Ca-sensitive K channels are lacking in rat hepatocytes. 6. A 10% increase in cell volume provoked by a hypo-osmotic shock triggered increases in both kappa' K and phi' Na with time courses very similar to those brought about by rewarming. 7. It is proposed that transient changes in K and Na permeabilities are the consequence of the cell swelling, induced by cooling. These volume-dependent permeabilities are blocked at 1 degrees C and revealed by rewarming.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armando-Hardy M., Ellory J. C., Ferreira H. G., Fleminger S., Lew V. L. Inhibition of the calcium-induced increase in the potassium permeability of human red blood cells by quinine. J Physiol. 1975 Aug;250(1):32P–33P. [PubMed] [Google Scholar]
- Banks B. E., Brown C., Burgess G. M., Burnstock G., Claret M., Cocks T. M., Jenkinson D. H. Apamin blocks certain neurotransmitter-induced increases in potassium permeability. Nature. 1979 Nov 22;282(5737):415–417. doi: 10.1038/282415a0. [DOI] [PubMed] [Google Scholar]
- Blackmore P. F., Brumley F. T., Marks J. L., Exton J. H. Studies on alpha-adrenergic activation of hepatic glucose output. Relationship between alpha-adrenergic stimulation of calcium efflux and activation of phosphorylase in isolated rat liver parenchymal cells. J Biol Chem. 1978 Jul 25;253(14):4851–4858. [PubMed] [Google Scholar]
- Burgess G. M., Claret M., Jenkinson D. H. Effects of catecholamines, ATP and ionophore A23187 on potassium and calcium movements in isolated hepatocytes. Nature. 1979 Jun 7;279(5713):544–546. doi: 10.1038/279544a0. [DOI] [PubMed] [Google Scholar]
- Chen J. L., Babcock D. F., Lardy H. A. Norepinephrine, vasopressin, glucagon, and A23187 induce efflux of calcium from an exchangeable pool in isolated rat hepatocytes. Proc Natl Acad Sci U S A. 1978 May;75(5):2234–2238. doi: 10.1073/pnas.75.5.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claret B., Claret M., Mazet J. L. Ionic transport and membrane potential of rat liver cells in normal and low-chloride solutions. J Physiol. 1973 Apr;230(1):87–101. doi: 10.1113/jphysiol.1973.sp010176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claret M., Mazet J. L. Ionic fluxes and permeabilities of cell membranes in rat liver. J Physiol. 1972 Jun;223(2):279–295. doi: 10.1113/jphysiol.1972.sp009847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claret M., Mazet J. L., Poggioli J. Proceedings: Effects of pyruvate and ouabain on isolated rat hepatocytes. J Physiol. 1976 Jun;258(2):93P–94P. [PubMed] [Google Scholar]
- Ferreira H. G., Lew V. L. Use of ionophore A23187 to measure cytoplasmic Ca buffering and activation of the Ca pump by internal Ca. Nature. 1976 Jan 1;259(5538):47–49. doi: 10.1038/259047a0. [DOI] [PubMed] [Google Scholar]
- Hanani M., Shaw C. A potassium contribution to the response of the barnacle photoreceptor. J Physiol. 1977 Aug;270(1):151–163. doi: 10.1113/jphysiol.1977.sp011943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUDAH J. D., McLEAN A. E. Action of antihistamine drugs in vitro. II. Ion movements and phosphoproteins in whole cells. Biochem Pharmacol. 1962 Jul;11:593–602. doi: 10.1016/0006-2952(62)90120-x. [DOI] [PubMed] [Google Scholar]
- Macknight A. D., Leaf A. Regulation of cellular volume. Physiol Rev. 1977 Jul;57(3):510–573. doi: 10.1152/physrev.1977.57.3.510. [DOI] [PubMed] [Google Scholar]
- Macknight A. D., Pilgrim J. P., Robinson B. A. The regulation of cellular volume in liver slices. J Physiol. 1974 Apr;238(2):279–294. doi: 10.1113/jphysiol.1974.sp010524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roti Roti L. W., Rothstein A. Adaptation of mouse leukemic cells (L5178Y) to anisotonic media. I. Cell volume regulation. Exp Cell Res. 1973 Jun;79(2):295–310. doi: 10.1016/0014-4827(73)90448-5. [DOI] [PubMed] [Google Scholar]
- Russo M. A., van Rossum G. D., Galeotti T. Observations on the regulation of cell volume and metabolic control in vitro; changes in the composition and ultrastructure of liver slices under conditions of varying metabolic and transporting activity. J Membr Biol. 1977 Mar 8;31(3):267–299. doi: 10.1007/BF01869409. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of rat liver cells. I. Effect of Ca 2+ on enzymatic dispersion of isolated, perfused liver. Exp Cell Res. 1972 Oct;74(2):450–454. doi: 10.1016/0014-4827(72)90400-4. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of rat liver cells. II. Effects of ions and chelators on tissue dispersion. Exp Cell Res. 1973 Jan;76(1):25–30. doi: 10.1016/0014-4827(73)90414-x. [DOI] [PubMed] [Google Scholar]
- Shank B. B., Rosenberg H. M., Horowitz C. Ionic basis of volume regulation in mammalian cells following osmotic shock. J Cell Physiol. 1973 Oct;82(2):257–265. doi: 10.1002/jcp.1040820214. [DOI] [PubMed] [Google Scholar]
- Yeh J. Z., Narahashi T. Mechanism of action of quinidine on squid axon membranes. J Pharmacol Exp Ther. 1976 Jan;196(1):62–70. [PubMed] [Google Scholar]



