Abstract
1. Repetitive firing in space-clamped squid axons bathed in low Ca and stimulated by a just suprathreshold step of current can be annihilated by a brief depolarizing or hyperpolarizing pulse of the proper magnitude applied at the proper phase. 2. In response to such perturbations, membrane potential and ionic currents show damped oscillations toward a steady state. 3. For other, non-annihilating, perturbations repetitive firing resumes with unaltered frequency but with phase resetting. 4. Experimental findings are compared with calculations for the space-and current-clamped Hodgkin-Huxley equations. Annihilation of repetitive firing to a steady state corresponds to a solution trajectory perturbed off a stable limit cycle and into the domain of attraction of a coexistent stable singular point. 5. Experimentally and theoretically the nerve exhibits hysteresis with two different stable modes of operation for a just suprathreshold range of bias current: the oscillatory repetitive firing state and the time-independent steady state. 6. Analogy is made to a brief synaptic input (excitatory or inhibitory) which may start or stop a biological pace-maker.
Full text
PDF


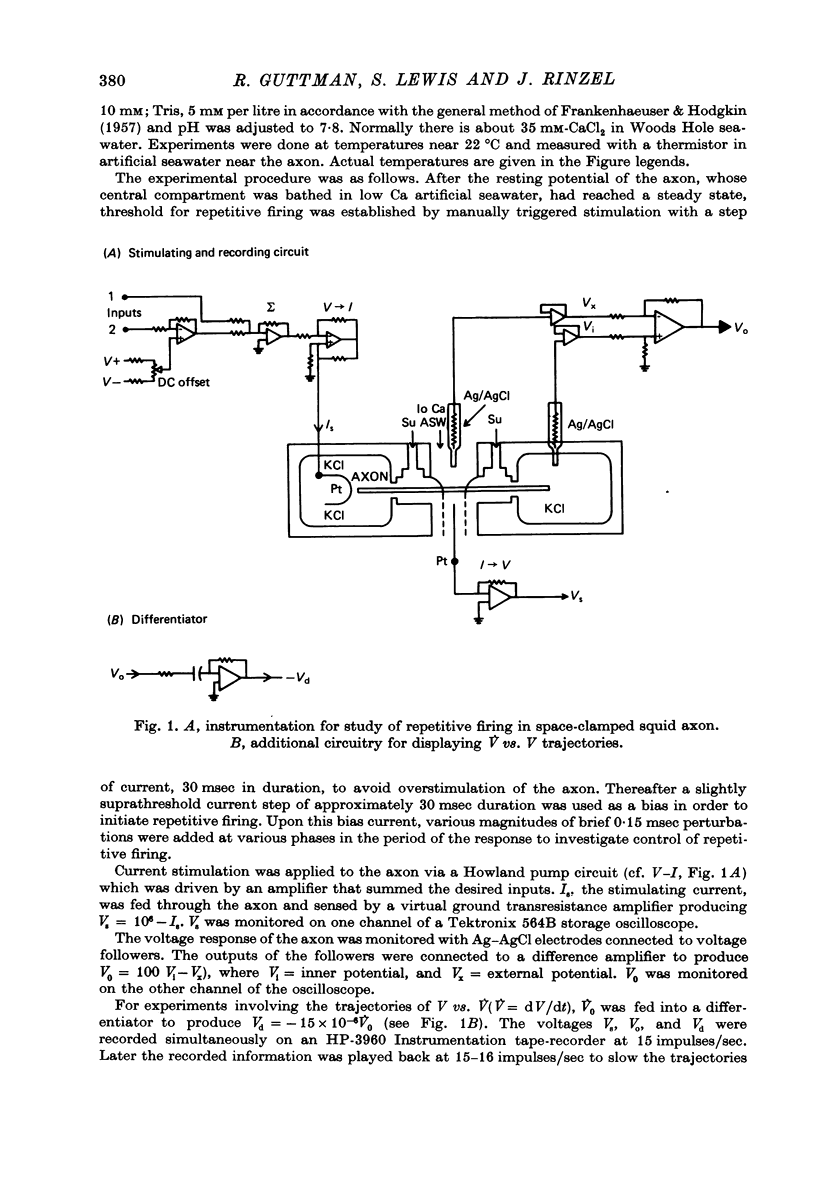



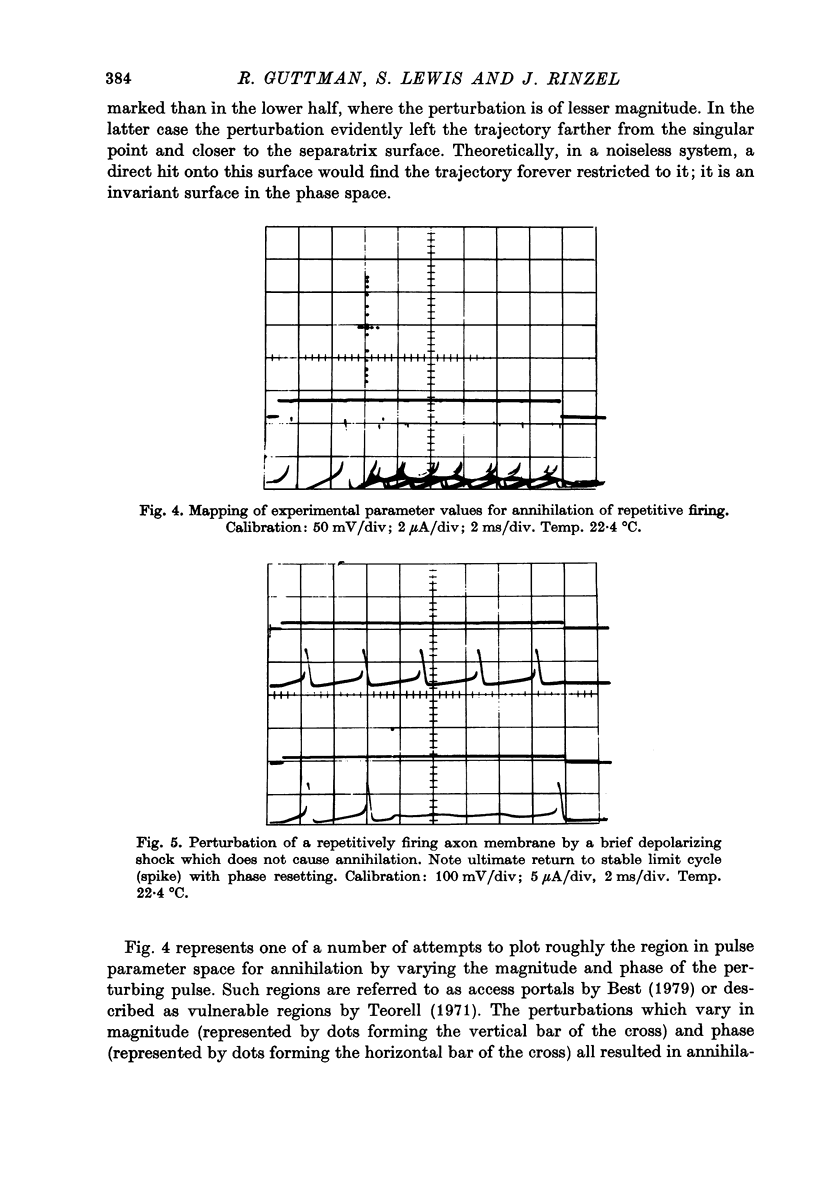





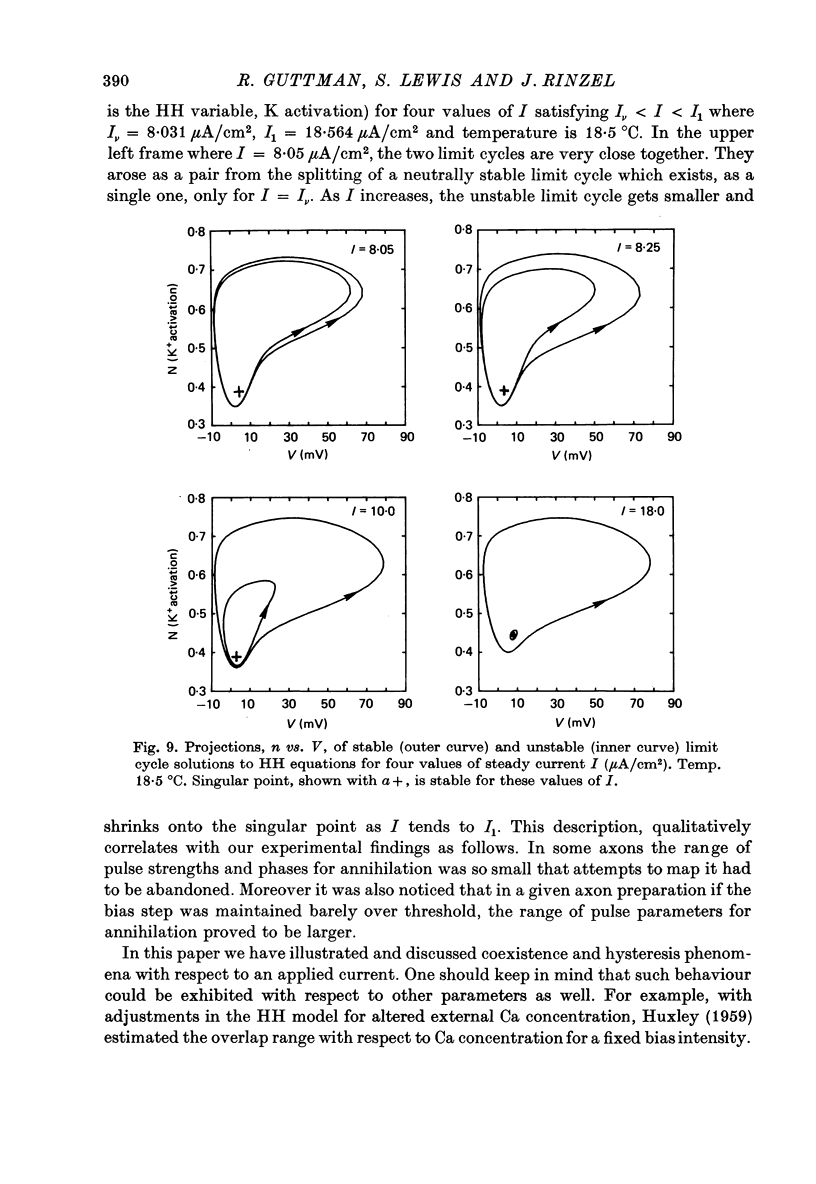





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Best E. N. Null space in the Hodgkin-Huxley Equations. A critical test. Biophys J. 1979 Jul;27(1):87–104. doi: 10.1016/S0006-3495(79)85204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Prediction of repetitive firing behaviour from voltage clamp data on an isolated neurone soma. J Physiol. 1971 Feb;213(1):31–53. doi: 10.1113/jphysiol.1971.sp009366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory J. E., Harvey R. J., Proske U. A 'late supernormal period' in the recovery of excitability following an action potential in muscle spindle and tendon organ receptors. J Physiol. 1977 Oct;271(2):449–472. doi: 10.1113/jphysiol.1977.sp012008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman R., Barnhill R. Oscillation and repetitive firing in squid axons. Comparison of experiments with computations. J Gen Physiol. 1970 Jan;55(1):104–118. doi: 10.1085/jgp.55.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Ion movements during nerve activity. Ann N Y Acad Sci. 1959 Aug 28;81:221–246. doi: 10.1111/j.1749-6632.1959.tb49311.x. [DOI] [PubMed] [Google Scholar]
- JENERICK H. PHASE PLANE TRAJECTORIES OF THE MUSCLE SPIKE POTENTIAL. Biophys J. 1963 Sep;3:363–377. doi: 10.1016/s0006-3495(63)86827-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalife J., Antzelevitch C. Phase resetting and annihilation of pacemaker activity in cardiac tissue. Science. 1979 Nov 9;206(4419):695–697. doi: 10.1126/science.493975. [DOI] [PubMed] [Google Scholar]
- Knight B. W. Dynamics of encoding in a population of neurons. J Gen Physiol. 1972 Jun;59(6):734–766. doi: 10.1085/jgp.59.6.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlidis T., Pinsker H. M. Oscillator theory and neurophysiology: introduction. Fed Proc. 1977 Jun;36(7):2033–2035. [PubMed] [Google Scholar]
- Rall W. Distinguishing theoretical synaptic potentials computed for different soma-dendritic distributions of synaptic input. J Neurophysiol. 1967 Sep;30(5):1138–1168. doi: 10.1152/jn.1967.30.5.1138. [DOI] [PubMed] [Google Scholar]
- Rinzel J. On repetitive activity in nerve. Fed Proc. 1978 Dec;37(14):2793–2802. [PubMed] [Google Scholar]
- TASAKI I. Initiation and abolition of the action potential of a single node of Ranvier. J Gen Physiol. 1956 Jan 20;39(3):377–395. doi: 10.1085/jgp.39.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERZUOLO C. A., WASHIZU Y. Relation between stimulus strength, generator potential and impulse frequency in stretch receptor of Crustacea. J Neurophysiol. 1962 Jan;25:56–66. doi: 10.1152/jn.1962.25.1.56. [DOI] [PubMed] [Google Scholar]
- WEIDMANN S. Effect of current flow on the membrane potential of cardiac muscle. J Physiol. 1951 Oct 29;115(2):227–236. doi: 10.1113/jphysiol.1951.sp004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson H. R., Cowan J. D. Excitatory and inhibitory interactions in localized populations of model neurons. Biophys J. 1972 Jan;12(1):1–24. doi: 10.1016/S0006-3495(72)86068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfree A. T. Phase control of neural pacemakers. Science. 1977 Aug 19;197(4305):761–763. doi: 10.1126/science.887919. [DOI] [PubMed] [Google Scholar]



