Abstract
Defining the role of Staphylococcus aureus adhesins in disease pathogenesis may depend on the use of bacteria grown in culture media that more closely reflect the human milieu than conventional broth. This study examined the functional effect on S. aureus adhesins following growth in an ex vivo medium containing a complex mixture of human proteins (used peritoneal dialysate) relative to growth in Todd-Hewitt broth. The adherence of S. aureus, cultured in dialysate, to fibronectin and fibrinogen was markedly reduced despite the expresion of full-length ClfA, ClfB, and fibronectin-binding proteins. Growth in dialysate resulted in the acquisition of a surface coat, as visualized by transmission electron microscopy, which was shown to contain fibronectin, fibrinogen, and immunoglobulins. Adherence of S. aureus to fibrinogen following growth in dialysate was significantly reduced by expression of protein A but was restored following growth in immunoglobulin-depleted dialysate. We conclude that bacterial adherence to solid-phase protein is critically dependent on the culture medium, that S. aureus adhesins may become saturated with target protein prior to contact with solid surfaces, and that there is an interaction between fibrinogen-binding proteins and immunoglobulin bound to protein A following contact with host proteins. These findings have important implications for future studies of S. aureus adhesins.
Bacterial adherence is likely to play a central role in host-to-host transmission and the maintenance of stable carriage of Staphylococcus aureus. Adhesion is also considered to be important in the disease pathogenesis of this predominantly extracellular pathogen. S. aureus is known to express a range of cell wall-associated proteins that promote adherence to host cells, extracellular matrix components, and/or soluble plasma proteins. These cell wall-anchored proteins include the collagen-binding protein Cna (33); the fibrinogen-binding proteins clumping factor A (ClfA) and B (ClfB) (23, 28); two fibronectin-binding proteins, FnBPA and FnBPB (15, 41); and protein A (20, 45), which can bind Von Willebrand factor and the Fc region of immunoglobulin G (IgG) (7, 12).
The study of S. aureus adhesin-ligand interactions in vitro and in vivo has relied primarily on comparison of end points for wild-type S. aureus versus isogenic mutants defective in one or more adhesin or, more recently, on using an expression system in a heterologous host such as Lactococcus lactis. These strategies have made important contributions, providing evidence for the involvement of Cna (13), ClfA (27, 37, 40, 44), and FnBPA (37) in the pathogenesis of experimental endocarditis and protein A in a subcutaneous-infection model (32). In addition, FnBPs have been shown to be important in the pathogenesis of intravenous-device-related infection (46, 47) and in the process of uptake by a range of cell lines (6, 8, 19, 21, 34, 42, 43). However, the experimental growth conditions used to prepare S. aureus normally depend on culture in laboratory media, and it seems unlikely that the resulting bacteria accurately mirror those in vivo during human infection. For example, bacterial adhesins may rapidly interact with soluble host proteins in vivo, and this may inhibit subsequent interactions with surface-expressed host protein. This has clear implications for in vitro systems but may also be important in animal models where large inocula of broth-grown bacteria injected into a blood vessel or the peritoneal cavity may not resemble S. aureus precoated with host proteins during colonization and invasion.
The purpose of this study was to explore the functions of cell wall-associated adhesins following growth under conditions more closely analogous to those in the human host than is achieved by either conventional media or broth supplemented with one or more host components. The growth medium used was peritoneal dialysate from individuals undergoing renal replacement therapy by continuous ambulatory peritoneal dialysis. Fresh dialysate is instilled into the abdominal cavity, where it remains for 6 h while dialysis occurs across the peritoneal membrane by a process of diffusion. When the fluid is removed, it contains an array of human proteins at a lower concentration than that in the circulation, including fibronectin (approximately 1 to 5% of the level in plasma), fibrinogen (0.5% of the level in plasma), and immunoglobulins (IgG at 1 to 2% of the level in serum) (3, 16, 25). This medium is readily available in large quantities and supports the growth of S. aureus (48). We have examined the functional effect on S. aureus adhesins following growth in used peritoneal dialysate relative to growth in conventional culture media.
MATERIALS AND METHODS
Chemicals and reagents.
All chemicals were obtained from Sigma-Aldrich or BDH Chemicals unless otherwise indicated.
Bacterial strains and plasmids.
The S. aureus strains and plasmids used in this study are listed in Table 1.
TABLE 1.
S. aureus strains used in this study
| Strain | Relevant genotype | Properties | Source or reference |
|---|---|---|---|
| 8325-4 | NCTC 8325 cured of prophages | 29 | |
| Newman | High level of fibrinogen-binding protein ClfA | 5 | |
| DU5873 | Δspa::Tcr | Mutant strain of Newman defective in protein A | 24 |
| DU5917 | cps::Tn917 Emr | Mutant strain of Newman defective in capsular polysaccharide | 39 |
| V8 | Strain from which serine protease was first isolated | 4 | |
| SP20 | Δspa::Tcr | Mutant strain of V8 defective in protein A | This study |
| Wood | Naturally deficient in protein A | 17 | |
| SP21 | Wood (pSPA) Cmr | Wood complemented with multicopy plasmid expressing protein A | This study |
Bacterial storage and growth conditions.
S. aureus was stored in trypticase soy broth with glycerol (15% [vol/vol]) at −80°C. Cultures were inoculated from stocks into 10 ml of medium contained in 35-ml glass universal containers. S. aureus was grown in Todd-Hewitt broth (THB; Difco) or used peritoneal dialysate for 15 to 18 h under constant rotation at 37°C in air. Escherichia coli strain DH5α was cultured in Luria-Bertani medium under constant rotation at 37°C in air. Antibiotics were incorporated into media, where appropriate, at the following concentrations: erythromycin, 10 μg/ml; tetracycline, 2 μg/ml; and chloramphenicol, 10 μg/ml.
Used peritoneal dialysis fluid (hereafter termed dialysate) was obtained on an anonymous basis from patients receiving outpatient care at the Oxford Regional Renal Unit. Sterile, antibiotic-free dialysate from five different patients was pooled, aliquoted, and stored at −20°C. This served as the stock throughout the study. Sterility was checked by plating 100 μl of dialysate onto 5% horse blood agar, which was incubated at 37°C in air for 24 h. The presence of antibiotics was determined by pipetting 20 μl of dialysate onto a lawn of S. aureus NCTC 6571 on Diagnostic Sensitest agar. The plate was incubated at 37°C in air for 24 h, and any inhibition of growth was taken to indicate the presence of antibiotics.
Removal of immunoglobulins from dialysate.
Immunoglobulins were removed from dialysate using a protein L column (Actigen), which binds all immunoglobulin classes and proteins complexed to them. The dialysate was passed twice through the column, and Western immunoblotting demonstrated depletion of immunoglobulin (data not shown).
Adhesion of S. aureus to purified human proteins.
THB or dialysate was inoculated with bacterial strains and incubated overnight under constant rotation at 37°C in air. Bacteria were harvested by centrifugation and washed three times in sterile phosphate-buffered saline (PBS). Adherence of S. aureus to purified human fibronectin or fibrinogen (Calbiochem) at a concentration of 10 μg/ml was assessed using a standardized microtiter plate assay, as previously described (35). The fibrinogen used was purified to remove contaminating fibronectin (22, 34). Adherent bacteria were detected by staining with crystal violet (0.5% [vol/vol]), and the optical density was measured using an enzyme-linked immunosorbent assay plate reader. Each isolate was tested in quadruplicate in an individual assay, and each experiment was performed three times. All assay plates included a positive control (S. aureus strain Newman for fibrinogen and 8325-4 for fibronectin adherence assays) and PBS without bacteria as a negative control. The optical density at 405 nm used in the analysis was the mean value for a given strain minus the background optical density at 405 nm taken from the reading for the negative control on the same plate. Data were analyzed using the Statview version 4.5 software package (Abacus, Berkeley, Calif.). Comparison of the mean count between bacterial strains or growth conditions was performed using an unpaired t test.
Transmission electron microscopy.
Bacterial strains were grown overnight in either THB or dialysate at 37°C in air, harvested by centrifugation, and washed three times with PBS. Transmission electron microscopy of bacterial isolates was performed as previously described (49).
SDS-PAGE and Western immunoblotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western immunoblotting were used to examine three types of sample: (i) cell wall-associated proteins released by lysostaphin treatment, as previously described (35); (ii) host proteins in 20× concentrated dialysate; and (iii) host proteins associated with the bacterial cell wall after overnight incubation in dialysate. The last were removed from a 10-ml culture by washing the pellet three times in PBS and then boiling it for 2 min in an equal volume of SDS sample buffer. Proteins were separated by SDS-PAGE using a 7.5 to 10% acrylamide gradient and standard methods (18) and then transferred electrophoretically to polyvinylidene difluoride Western-blotting membrane (Boehringer Mannheim) by the semidry transblot system (Bio-Rad). Human proteins were detected using rabbit polyclonal antibody against fibronectin (F3648; Sigma), fibrinogen (catalog no. 341552; Calbiochem), or IgG, IgA, and IgM (A0190; Dako) followed by alkaline phosphatase-labeled goat anti-rabbit antibody (Chemicon) for fibronectin and immunoglobulin or alkaline phosphatase-labeled protein A (Dako) for fibrinogen. Antibody was visualized using the AP Conjugate Substrate kit (Bio-Rad) as instructed by the manufacturer. Bacterial FnBPs, ClfA, and ClfB were detected by immunoblotting using polyclonal antibodies as previously described (11, 21, 24, 28).
Transformation.
E. coli cells were transformed following CaCl2 treatment (38). S. aureus was electroporated as previously described (30) using S. aureus RN4220 as the initial recipient for plasmids prior to electroporation into the required background.
Manipulation of DNA.
DNA manipulations were performed by standard procedures (38). Chromosomal DNA was extracted using a Puregene DNA extraction kit (Gentra Systems), with the modification that 30 μg of lysostaphin (Ambi)/ml was added at the cell lysis step. Plasmid DNA for cloning was purified from E. coli using WizardPlus minipreps (Promega Corp.) and from S. aureus using Qiagen midipreps with the addition of lysostaphin (30 μg/ml) in the cell resuspension buffer. Restriction enzymes were purchased from New England BioLabs or from Boehringer Mannheim and were used as recommended by the suppliers.
PCR amplification of the spa gene from S. aureus.
Using a sequence in the GenBank database (accession number J01786), oligonucleotide primers were designed to amplify the full-length spa gene encoding S. aureus protein A. The nucleotide sequences of the primers used were as follows: 5′-CGGGATCCTCGAAATAGCGTGATTTTGC-3′ (forward) and 5′-CGGGATCCGCACTGAGCAACAAAAGATG-3′ (reverse); the underlined regions indicate the recognition sites for the restriction enzyme BamHI. The PCR mixtures contained 100 pmol of each primer, 10 ng of template DNA (strain 8325-4), 200 μM deoxynucleoside triphosphate, reaction buffer (1×), 1.5 mM MgCl2, and 2.5 U of Pfu polymerase in a volume of 50 μl. Amplifications were carried out in a DNA thermal cycler (Peltier Thermal Cycler) under the following conditions: 30 cycles of 94°C for 1 min, 65°C for 1 min, and 72°C for 3 min followed by a 10-min incubation at 72°C. Aliquots were analyzed on a 0.8% agarose gel, and the remaining DNA was extracted using the Wizard PCR purification kit (Promega) as instructed by the manufacturer.
Phage transduction.
The spa::Tcr mutation was transduced from DU5873 (S. aureus Newman spa::Tcr) to S. aureus strain V8 by phage 85-mediated transduction (1). Transductants resistant to 2 μg of tetracycline/ml were selected. The genetic background of transductants was verified by pulsed-field gel electrophoresis using established methodology (36). Genotypic verification was performed by the demonstration of an appropriate increase in PCR product size following amplification of the spa gene. The lack of adherence to myeloma IgG1 at 10 μg/ml was confirmed by microtiter adherence assay.
Construction of plasmid pSPA, expressing protein A.
The S. aureus-E. coli shuttle plasmid pCU1 (2) was used to clone the spa gene in E. coli and to subsequently express protein A in S. aureus. Plasmid DNA and the PCR-amplified spa gene were digested overnight at 37°C using BamHI and the purified DNA ligated together using T4 ligase. E. coli strain DH5α was transformed with the ligation product. This plasmid (pSPA) was electroporated into S. aureus RN4220 and then into strain Wood. Adherence of transformants to myeloma IgG1 at 10 μg/ml was confirmed by microtiter adherence assay.
RESULTS
Adherence of S. aureus to solid-phase fibronectin and fibrinogen is lost following growth in dialysate.
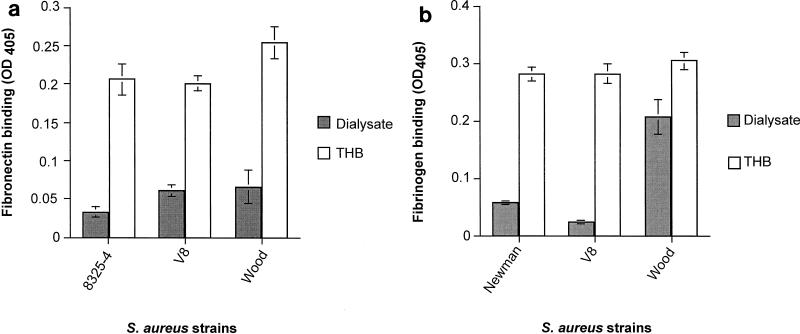
The adherence of S. aureus to fibronectin and fibrinogen was examined following overnight growth in THB and dialysate. This was performed using laboratory isolates known to have an appropriate adhesive phenotype for a given protein; hence the use of Newman in place of 8325-4 for the fibrinogen assay. The adherence of S. aureus strains 8325-4, V8, and Wood to fibronectin was markedly reduced following growth in dialysate compared with that following growth in THB (P < 0.001 for each strain) (Fig. 1a). Bacterial adherence to fibrinogen after growth in dialysate was markedly reduced for S. aureus strains Newman and V8 (P < 0.001 for both strains); the adherence of S. aureus strain Wood was decreased to a lesser extent (Fig. 1b).
FIG. 1.
Functional blocking of S. aureus adhesins following growth in dialysate. The adherence of S. aureus strains to fibronectin (a) and fibrinogen (b) was assessed by microtiter plate assay following growth in dialysate and THB. The data are shown as the mean ± standard error of the mean. OD405, optical density at 405 nm.
The loss of adherence to fibronectin and fibrinogen following growth in dialysate could be due to decreased expression or increased degradation of bacterial adhesins. Alternatively, the ligand binding sites of the FnBPs and fibrinogen-binding proteins could become blocked through saturation with their target protein or by steric hindrance from other proteins associated with the cell wall. The following strategies were used to define the mechanisms responsible.
FnBPs and fibrinogen-binding proteins are expressed on the surface of S. aureus following growth in dialysate.
Western immunoblotting of cell wall-associated proteins was used to examine the possibility that surface-expressed adhesins were affected through contact with or growth in dialysate. Equal numbers of bacterial cells were examined following growth in used dialysate and THB. Full-length ClfA, ClfB, and FnBPA were demonstrated in the cell wall extracts from Newman (ClfA and ClfB) and 8325-4 (FnBPA) grown under both culture conditions (Fig. 2). Thus, alteration in expression of ClfA, ClfB, and FnBPA or subsequent enzymatic degradation does not appear to explain the reduction in adherence to fibronectin and fibrinogen.
FIG. 2.
Surface expression of S. aureus adhesins. Western immunoblots demonstrate the cell wall-associated proteins ClfA, ClfB (strain Newman), and FnBPA (strain 8325-4) extracted from bacteria following growth in dialysate or THB.
S. aureus grown in THB but preincubated in dialysate fails to adhere to fibronectin and fibrinogen.
S. aureus was grown overnight in THB, washed, and then incubated in 10 ml of dialysate at 37°C for 1 h prior to adherence assays. The adherence of preincubated bacteria mirrored that following overnight growth in dialysate, with a significant reduction in the adherence of 8325-4, V8, and Wood to solid-phase fibronectin (P < 0.001 for all strains) and of Newman and V8 to fibrinogen (P < 0.001 for both strains) (data not shown). This suggests that reduction in adherence resulted from the interaction between bacterial adhesins and human proteins or other components of the dialysate.
S. aureus grown in dialysate becomes associated with host proteins.
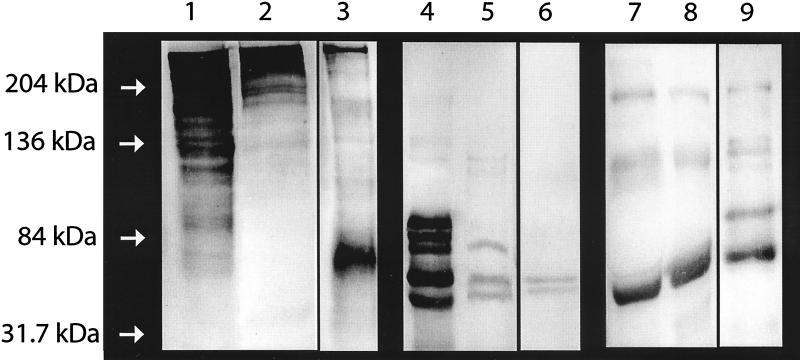
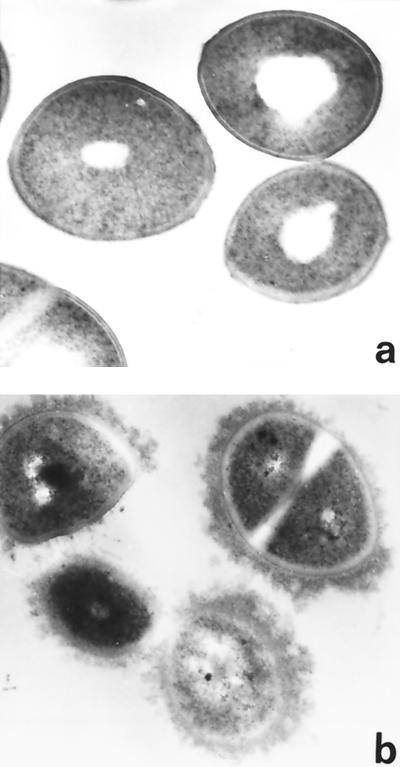
Western immunoblotting confirmed the presence of fibronectin, fibrinogen, and immunoglobulins in dialysate (Fig. 3), the fibrinogen observed appearing to have degraded to a lower apparent molecular mass. Strain Newman was examined by transmission electron microscopy following growth in THB and dialysate. Bacteria grown in dialysate acquired a surface coat that was not present on bacteria grown in THB (Fig. 4). This coat did not represent either enhanced visualization or production of capsular polysaccharide, since Newman DU5917, a strain deficient in capsular polysaccharide expression, also acquired a surface coat during growth in dialysate and its fibrinogen-binding properties were affected in a similar manner (data not shown). Possible explanations for the appearance of the coat include expression of a surface protein by bacteria grown in dialysate but not by those grown in THB or attachment of components derived from the dialysate. The latter is consistent with the observation that S. aureus failed to adhere to fibronectin and fibrinogen following pretreatment with dialysate, leading to the hypothesis that host proteins were major components of the surface coat. This was confirmed by Western immunoblots of material removed from the surface of 8325-4 by boiling in SDS following overnight growth in dialysate. Fibronectin, immunoglobulins, and fibrinogen were all detected (Fig. 3).
FIG. 3.
Fibronectin, fibrinogen, and immunoglobulins are present in dialysate and become associated with the surface of S. aureus. Western immunoblotting was used to detect the presence of human proteins in dialysate concentrated 20-fold and in the surface-associated layer acquired by S. aureus following growth in dialysate. Purified human proteins were used as positive controls. Lanes 1, 2, and 3, purified fibronectin, dialysate, and surface coat, respectively, probed with anti-fibronectin antibodies; lanes 4, 5, and 6, purified fibrinogen, dialysate, and surface coat, respectively, probed with anti-fibrinogen antibodies; lanes 7, 8, and 9, purified immunoglobulins (classes IgG, IgM, and IgA), dialysate, and surface coat, respectively, probed with antibodies to IgG, IgM, and IgA.
FIG. 4.
S. aureus grown in dialysate acquires a surface coat. Transmission electron microscopy of S. aureus strain Newman cultured overnight in THB (a) and dialysate (b). The bacteria acquire an electron-dense surface layer during growth in dialysate. Magnification, ×48,000.
Taken together, these results suggest that saturation of ligand binding sites by target proteins in dialysate was the most likely explanation for the reduction in bacterial adherence to fibronectin and fibrinogen. However, this does not explain the observation that adherence of strain Wood to fibrinogen following growth in dialysate was affected to a much lesser extent than that for other strains tested. Wood is naturally deficient in protein A (17), raising the possibility that the reduction in bacterial adherence to fibrinogen was due to an interaction between fibrinogen-binding proteins and protein A following contact with host proteins.
Protein A binds immunoglobulin in dialysate and prevents adherence of S. aureus to solid-phase fibrinogen.
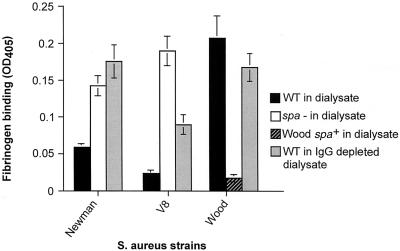
A panel of isogenic mutants (defective in protein A) and strain Wood (naturally deficient in protein A) complemented with a multicopy plasmid carrying the spa gene were constructed. Inactivation of spa in V8 and Newman resulted in an increase in adherence to fibrinogen compared with the wild type following growth in dialysate (P < 0.001 for both strains) (Fig 5). Complementation of Wood with the pSPA plasmid resulted in a reduced adherence to fibrinogen compared with the wild type following growth in dialysate (P < 0.001) (Fig. 5). We confirmed that adherence to solid-phase fibrinogen after growth in THB was not affected by the introduction of spa mutations or plasmid pSPA (data not shown). Dialysate depleted of immunoglobulin was used as a growth medium for Newman, V8, and Wood. Growth in this medium resulted in an increase in adherence to fibrinogen for Newman and V8 compared with that following growth in nondepleted dialysate (P < 0.001), while adherence for Wood was not significantly affected (P > 0.05) (Fig. 5). These data indicate that immunoglobulins bound to protein A prevent the interaction between the S. aureus fibrinogen-binding proteins and human fibrinogen.
FIG. 5.
Expression of protein A and its interaction with immunoglobulins interfere with bacterial adherence to fibrinogen following growth in dialysate. Shown are adherence of wild-type (WT) S. aureus strains Newman, V8, and Wood; Newman and V8 mutants defective in protein A (spa−); and Wood complemented with a multicopy plasmid expressing protein A (spa+) following growth in dialysate (wild-type Wood is naturally deficient in protein A). Also shown is the adherence of wild-type Newman, V8, and Wood following growth in IgG-depleted dialysate. The data are shown as the mean ± standard error of the mean. OD405, optical density at 405 nm.
DISCUSSION
The study of S. aureus following growth in laboratory broth in vitro has led to the identification of numerous putative virulence determinants. The challenge now is to determine how they function in vivo and to define which are important in human disease. The findings of this study add to existing evidence that in vitro expression and/or function of S. aureus factors, such as those involved in metabolism and the global regulator agr, may not mirror that in vivo or following growth in ex vivo media (9, 10, 51).
The rationale for using peritoneal dialysate from individuals with chronic renal failure as a growth medium for S. aureus rests primarily on the fact that many of the host components found in blood or plasma are present in dialysate at lower concentrations. Although this medium is relatively ill defined, its use makes it possible to study adhesins in a way that would prove difficult using blood or blood products which would cause S. aureus to become highly aggregated, while providing an environment that is closer to in vivo conditions than conventional media.
Bacterial adherence to solid-phase fibronectin was not preserved following growth in dialysate, a result that we have ascribed to saturation of FnBP ligand binding sites. Human fibronectins are present in soluble form in plasma and in many extracellular matrices, where they exist as polymers. It is possible, given our findings, that S. aureus in the bloodstream does not interact efficiently with extracellular matrix fibronectin and may therefore interact poorly with host cells such as endothelium in vivo. However, the acquisition of a surface coat of host proteins may confer other benefits. For example, immunological recognition and/or clearance of bacteria by phagocytes may be impeded. The idea that FnBPs interact with host defenses in vivo gains credibility from a study reporting that recombinant FnBPA interacts with integrin α5β1 via a fibronectin bridge to mediate adhesion and costimulatory signals to T lymphocytes (26).
Our observation that adherence to solid-phase fibrinogen was reduced after growth in dialysate can also be explained in part by functional blocking by fibrinogen acquired from dialysate. Fibrinogen is a soluble plasma protein, but unlike fibronectin, it is not a component of extracellular matrices. We speculate that the relevance of our observation to the in vivo situation may relate more to the biological imperative for S. aureus to become masked with host protein than the inability to bind further fibrinogen once coating has occurred. Binding of fibrinogen to the M protein of Streptococcus pyogenes blocks complement via the alternative pathway and inhibits the deposition of C3b, leading to reduced phagocytosis by polymorphonuclear leukocytes (14, 50). It is possible that S. aureus cell surface-associated fibrinogen is similarly antiphagocytic. It is also possible that S. aureus coated with fibrinogen can interact with platelets, since fibrinogen binds to platelets during the formation of thrombus. This could result in bacterial integration into a clot, which then becomes impacted in the microcirculation, where proliferation can occur prior to spread and seeding. S. aureus also appears to interact with platelets directly, since a mutant defective in ClfA has been shown to have reduced ability to bind human platelets in vitro (40) while extracellular fibrinogen-binding protein (Efb) has been demonstrated to inhibit platelet aggregation (31).
Our study also demonstrated an interaction between fibrinogen adherence and immunoglobulins bound to protein A. The mechanism for this is unclear, but it could be due to steric hindrance if the two adhesins are closely related on the bacterial cell surface. The biological relevance of this interaction also remains unclear. Our findings indicate that S. aureus adhesins may participate in complex interactions in the presence of host proteins, suggesting that the study of a single adhesin in isolation may represent an oversimplification of events in vivo, as may examining more than one adhesin in the absence of host proteins.
Acknowledgments
This work was funded by a Baxter Healthcare Extramural Grant.
We thank the patients and nurses at the Oxford Regional Renal Unit, who provided dialysate.
Ruth C. Massey and Shobana R. Dissanayeke contributed equally to this work.
Editor: E. I. Tuomanen
REFERENCES
- 1.Asheshov, E. H. 1966. Loss of antibiotic resistance in Staphylococcus aureus resulting from growth at high temperature. J. Gen. Microbiol. 42:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Augustin, J., R. Rosenstein, B. Wieland, U. Schnieder, N. Schnell, G. Engelke, K. D. Entian, and F. Gotz. 1992. Genetic analysis of epidermin biosynthetic genes and epidermin-negative mutants of Staphylococcus epidermidis. Eur. J. Biochem. 204:1149-1154. [DOI] [PubMed] [Google Scholar]
- 3.Davies, S. J., A. Animashaun, A. E. Taylor, G. A. Young, and J. H. Turney. 1987. Fibronectin and fibrinogen in the plasma and dialysate of patients on CAPD. Perit. Dial. Bull. 7:233-236. [Google Scholar]
- 4.Drapeau, G. R., Y. Boily, and J. Houmard. 1972. Purification and properties of an extracellular protease of Staphylococcus aureus. J. Biol. Chem. 247:6720-6726. [PubMed] [Google Scholar]
- 5.Duthie, E. S., and L. L. Lorenz. 1952. Staphylococcal coagulase: mode of action and antigenicity. J. Gen. Microbiol. 6:95-107. [DOI] [PubMed] [Google Scholar]
- 6.Dziewanowska, K., J. M. Patti, C. F. Deobald, K. W. Bayles, W. R. Trumble, and G. A. Bohach. 1999. Fibronectin-binding protein and host cell tyrosine kinase are required for internalization of Staphylococcus aureus by epithelial cells. Infect. Immun. 67:4673-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forsgren, A., V. Ghetie, R. Lindmark, and J. Sjoquist. 1983. Protein A and its exploitation, p. 429-480. In C. S. F. Easmon and C. Adlams (ed.), Staphylococci and staphylococcal infections. Academic Press, London, United Kingdom.
- 8.Fowler, T., E. R. Wann, D. Joh, S. Johansson, T. J. Foster, and M. Höök. 2000. Cellular invasion by Staphylococcus aureus involves a fibronectin bridge between the bacterial fibronectin-binding MSCRAMMs and host cell beta1 integrins. Eur. J. Cell Biol. 79:672-679. [DOI] [PubMed] [Google Scholar]
- 9.Goerke, C., S. Campana, M. G. Bayer, G. Doring, K. Botzenhart, and C. Wolz. 2000. Direct quantitative transcript analysis of the agr regulon of Staphylococcus aureus during human infection in comparison to the expression profile in vitro. Infect. Immun. 68:1304-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goerke, C., U. Fluckiger, A. Steinhuber, W. Zimmerli, and C. Wolz. 2001. Impact of the regulatory loci agr, sarA and sae of Staphylococcus aureus on the induction of alpha-toxin during device-related infection resolved by direct quantitative transcript analysis. Mol. Microbiol. 40:1439-1447. [DOI] [PubMed] [Google Scholar]
- 11.Hartford, O., P. Francois, P. Vaudaux, and T. J. Foster. 1997. The dipeptide repeat region of the fibrinogen-binding protein (clumping factor) is required for functional expression of the fibrinogen-binding domain on the Staphylococcus aureus cell surface. Mol. Microbiol. 25:1065-1076. [DOI] [PubMed] [Google Scholar]
- 12.Hartleib, J., N. Kohler, R. B. Dickinson, G. S. Chhatwal, J. J. Sixma, O. M. Hartford, T. J. Foster, G. Peters, B. E. Kehrel, and M. Herrmann. 2000. Protein A is the von Willebrand factor binding protein on Staphylococcus aureus. Blood 96:2149-2156. [PubMed] [Google Scholar]
- 13.Hienz, S. A., T. Schennings, A. Heimdahl, and J. I. Flock. 1996. Collagen binding of Staphylococcus aureus is a virulence factor in experimental endocarditis. J. Infect. Dis. 174: 83-88. [DOI] [PubMed] [Google Scholar]
- 14.Horstmann, R. D., H. J. Sievertsen, M. Leippe, and V. A. Fischetti. 1992. Role of fibrinogen in complement inhibition by streptococcal M protein. Infect. Immun. 60:5036-5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonsson, K., C. Signas, H. P. Muller, and M. Lindberg. 1991. Two different genes encode fibronectin-binding proteins in Staphylococcus aureus. The complete nucleotide sequence and characterization of the second gene. Eur. J. Biochem. 202:1041-1048. [DOI] [PubMed] [Google Scholar]
- 16.Khan, R. H., M. Klein, and S. Vas. 1987. Fibronectin in the normal peritoneal fluids of patients on chronic ambulatory peritoneal dialysis (CAPD) and during peritonitis. Perit. Dial. Int. 7:69-73. [Google Scholar]
- 17.Kronvall, G., J. H. Dossett, P. G. Quie, and R. C. Williams. 1971. Occurrence of protein A in staphylococcal strains: quantitative aspects and correlation to antigenic and bacteriophage types. Infect. Immun. 3:10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 19.Lammers, A., P. J. Nuijten, and H. E. Smith. 1999. The fibronectin binding proteins of Staphylococcus aureus are required for adhesion to and invasion of bovine mammary gland cells. FEMS Microbiol. Lett. 180:103-109. [DOI] [PubMed] [Google Scholar]
- 20.Lofdahl, S., B. Guss, M. Uhlen, L. Philipson, and M. Lindberg. 1983. Gene for staphylococcal protein A. Proc. Natl. Acad. Sci. USA 80:697-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massey, R. C., M. N. Kantzanou, T. Fowler, N. P. J. Day, K. Schofield, E. R. Wann, A. R. Berendt, M. Höök, and S. J. Peacock. 2001. Fibronectin-binding protein A of Staphylococcus aureus has multiple, substituting, binding regions that mediate adherence to fibronectin and invasion of endothelial cells. Cell. Microbiol. 3:839-851. [DOI] [PubMed] [Google Scholar]
- 22.McDevitt, D., P. Vaudaux, and T. J. Foster. 1992. Genetic evidence that bound coagulase of Staphylococcus aureus is not clumping factor. Infect. Immun. 60:1514-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDevitt, D., P. François, P. Vaudaux, and T. J. Foster. 1994. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol. Microbiol. 11:237-248. [DOI] [PubMed] [Google Scholar]
- 24.McDevitt, D., P. Francois, P. Vaudaux, and T. J. Foster. 1995. Identification of the ligand-binding domain of the surface-located fibrinogen receptor (clumping factor) of Staphylococcus aureus. Mol. Microbiol. 16:895-907. [DOI] [PubMed] [Google Scholar]
- 25.McGregor, S. J., J. H. Brock, J. D. Briggs, and B. J. Junor. 1987. Relationship of IgG, C3 and transferrin with opsonising and bacteriostatic activity of peritoneal fluid from CAPD patients and the incidence of peritonitis. Nephrol. Dial. Transplant. 2:551-556. [PubMed] [Google Scholar]
- 26.Miyamoto, Y. L., E. R. Wann, T. Fowler, E. Duffield, M. Höök, and B. W. McIntyre. 2001. Fibronectin binding protein A of Staphylococcus aureus can mediate human T lymphocyte adhesion and coactivation. J. Immunol. 166: 5129-5138. [DOI] [PubMed] [Google Scholar]
- 27.Moreillon, P., J. M. Entenza, P. Francioli, D. McDevitt, T. J. Foster, P. François, and P. Vaudaux. 1995. Role of Staphylococcus aureus coagulase and clumping factor in pathogenesis of experimental endocarditis. Infect. Immun. 63:4738-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ni Eidhin, D., S. Perkins, P. François, P. Vaudaux, M. Höök, and T. J. Foster. 1998. Clumping factor B (ClfB), a new surface-located fibrinogen-binding adhesin of Staphylococcus aureus. Mol. Microbiol. 30:245-257. [DOI] [PubMed] [Google Scholar]
- 29.Novick, R. 1967. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology 33:155-166. [DOI] [PubMed] [Google Scholar]
- 30.Oskouian, B., and G. C. Stewart. 1990. Repression and catabolite repression of the lactose operon of Staphylococcus aureus. J. Bacteriol. 172:3804-3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palma, M., O. Shannon, H. C. Quezada, A. Berg, and J. I. Flock. 2001. Extracellular fibrinogen-binding protein, Efb, from Staphylococcus aureus blocks platelet aggregation due to its binding to the alpha-chain. J. Biol. Chem. 276:31691-31697. [DOI] [PubMed] [Google Scholar]
- 32.Patel, A. H., P. Nowlan, E. D. Weavers, and T. J. Foster. 1987. Virulence of protein A-deficient and alpha-toxin-deficient mutants of Staphylococcus aureus isolated by allele replacement. Infect. Immun. 55:3103-3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patti, J. M., H. Jonsson, B. Guss, L. M. Switalski, K. Wiberg, M. Lindberg, and M. Höök. 1992. Molecular characterization and expression of a gene encoding a Staphylococcus aureus collagen adhesin. J. Biol. Chem. 267:4766-4772. [PubMed] [Google Scholar]
- 34.Peacock, S. J., T. J. Foster, B. J. Cameron, and A. R. Berendt. 1999. Bacterial fibronectin-binding proteins and endothelial cell surface fibronectin mediate adherence of Staphylococcus aureus to resting human endothelial cells. Microbiology 145:3477-3486. [DOI] [PubMed] [Google Scholar]
- 35.Peacock, S. J., N. P. Day, M. G. Thomas, A. R. Berendt, and T. J. Foster. 2000. Clinical isolates of Staphylococcus aureus exhibit diversity in fnb genes and adhesion to human fibronectin. J. Infect. 41:23-31. [DOI] [PubMed] [Google Scholar]
- 36.Prevost, G., B. Jaulhac, and Y. Piemont. 1992. DNA fingerprinting by pulsed-field gel electrophoresis is more effective than ribotyping in distinguishing among methicillin-resistant Staphylococcus aureus isolates. J. Clin. Microbiol. 30: 967-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Que, Y. A., P. Francois, J. A. Haefliger, J. M. Entenza, P. Vaudaux, and P. Moreillon. 2001. Reassessing the role of Staphylococcus aureus clumping factor and fibronectin-binding protein by expression in Lactococcus lactis. Infect. Immun. 79:6296-6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 39.Sau, S., N. Bhasin, E. R. Wann, J. C. Lee, T. J. Foster, and C. Y. Lee. 1997. The Staphylococcus aureus allelic genetic loci for serotype 5 and 8 capsule expression contain the type-specific genes flanked by common genes. Microbiology 143:2395-2405. [DOI] [PubMed] [Google Scholar]
- 40.Siboo, I. R., A. L. Cheung, A. S. Bayer, and P. M. Sullam. 2001. Clumping factor A mediates binding of Staphylococcus aureus to human platelets. Infect. Immun. 69:3120-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Signas, C., G. Raucci, K. Jonsson, P. E. Lindgren, G. M. Anantharamaiah, M. Höök, and M. Lindberg. 1989. Nucleotide sequence of the gene for a fibronectin-binding protein from Staphylococcus aureus: use of this peptide sequence in the synthesis of biologically active peptides. Proc. Natl. Acad. Sci. USA 86:699-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sinha, B., P. P. Francoise, O. NuBe, M. Foti, O. M. Hartford, P. Vaudaux, T. J. Foster, D. P. Lew, M. Herrmann, and K. H. Krause. 1999. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin α5β1. Cell. Microbiol. 1:101-117. [DOI] [PubMed] [Google Scholar]
- 43.Sinha, B., P. Francois, Y. A. Que, M. Hussain, C. Heilmann, P. Moreillon, D. Lew, K. H. Krause, G. Peters, and M. Herrman. 2000. Heterologously expressed Staphylococcus aureus fibronectin-binding proteins are sufficient for invasion of host cells. Infect. Immun. 68:6871-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stutzmann Meier, P., J. M. Entenza, P. Vaudaux, P. Francioli, M. P. Glauser, and P. Moreillon. 2001. Study of Staphylococcus aureus pathogenic genes by transfer and expression in the less virulent organism Streptococcus gordonii. Infect. Immun. 69:657-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uhlen, M., B. Guss, B. Nilsson, S. Gatenbeck, L. Philipson, and M. Lindberg. 1984. Complete sequence of the staphylococcal gene encoding protein A. A gene evolved through multiple duplications. J. Biol. Chem. 259:1695-1702. [PubMed] [Google Scholar]
- 46.Vaudaux, P., D. Pittet, A. Haeberli, E. Huggler, U. E. Nydegger, D. P. Lew, and F. A. Waldvogel. 1989. Host factors selectively increase staphylococcal adherence on inserted catheters: a role for fibronectin and fibrinogen or fibrin. J. Infect. Dis. 160: 865-875. [DOI] [PubMed] [Google Scholar]
- 47.Vaudaux, P., D. Pittet, A. Haeberli, P. G. Lerch, J. J. Morgenthaler, R. A. Proctor, F. A. Waldvogel, and D. P. Lew. 1993. Fibronectin is more active than fibrin or fibrinogen in promoting Staphylococcus aureus adherence to inserted intravascular catheters. J. Infect. Dis. 167: 633-641. [DOI] [PubMed] [Google Scholar]
- 48.Verbrugh, H. A., W. F. Keane, W. E. Conroy, and P. K. Peterson. 1984. Bacterial growth and killing in chronic ambulatory peritoneal dialysis fluids. J. Clin. Microbiol. 20:199-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Virji, M., H. Kayhty, D. J. Ferguson, C. Alexandrescu, J. E. Heckels, and E. R. Moxon. 1991. The role of pili in the interactions of pathogenic Neisseria with cultured human endothelial cells. Mol. Microbiol. 5:1831-1841. [DOI] [PubMed] [Google Scholar]
- 50.Whitnack, E., and E. H. Beachey. 1982. Antiopsonic activity of fibrinogen bound to M protein on the surface of group A streptococci. J. Clin. Investig. 69:1042-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wiltshire, M. D., and S. J. Foster. 2001. Identification and analysis of Staphylococcus aureus components expressed by a model system of growth in serum. Infect. Immun. 69:5198-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]