Abstract
1. The Ca current seen in response to depolarization was investigated in Paramecium caudatum under voltage clamp. Inactivation of the current was measured with the double pulse method; a fixed test pulse of an amplitude sufficient to evoke maximal inward current was preceded by a conditioning pulse of variable amplitude (0-120 mV).
2. The amplitude of the current recorded during the test pulse was related to the potential of the conditioning pulse. Reduction of test pulse current was taken as an index of Ca current inactivation. The current recorded during a test pulse showed a progressive decrease to a minimum as the potential of the conditioning pulse approached +10 to +30 mV. Further increase in conditioning pulse amplitude was accompanied by a progressive restoration of the test pulse current. Conditioning pulses near the calcium equilibrium potential had only a slight inactivating effect on the test pulse current.
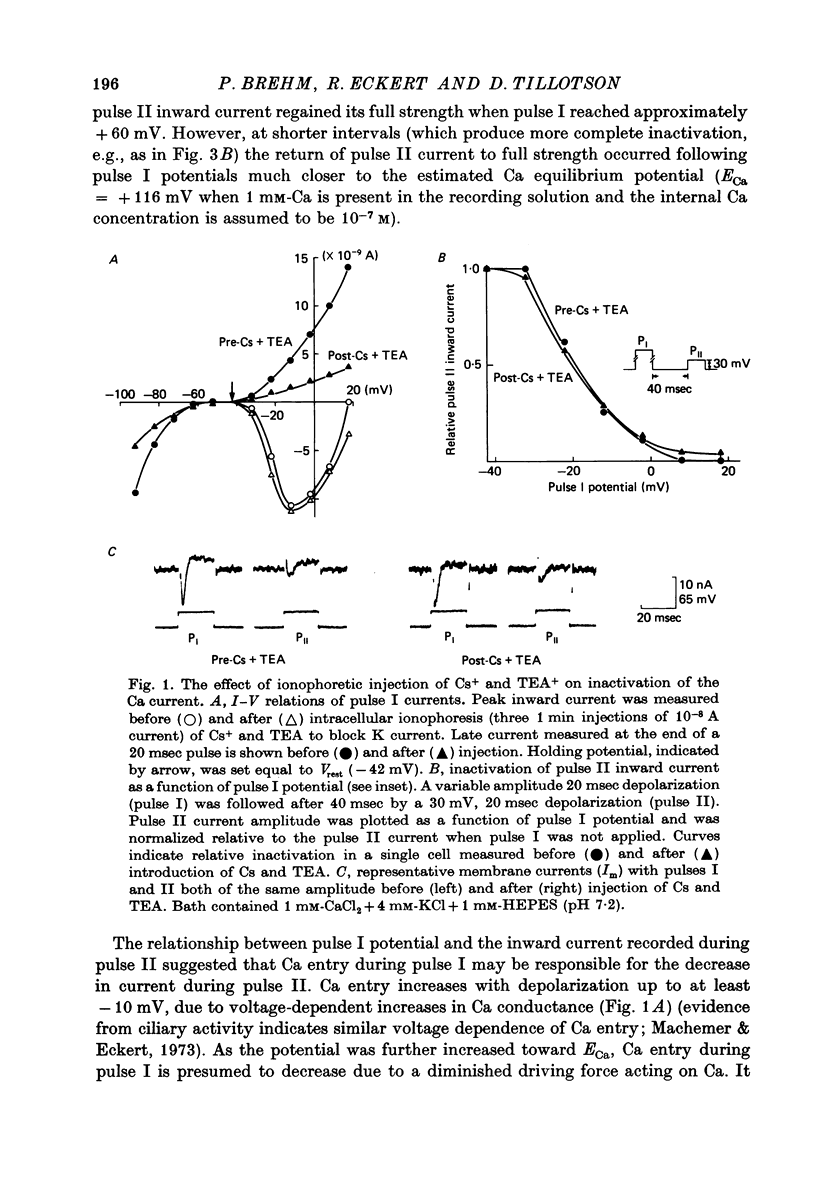
3. Injection of a mixture of Cs and TEA which blocked late outward current had essentially no effect on the inward current or its inactivation.
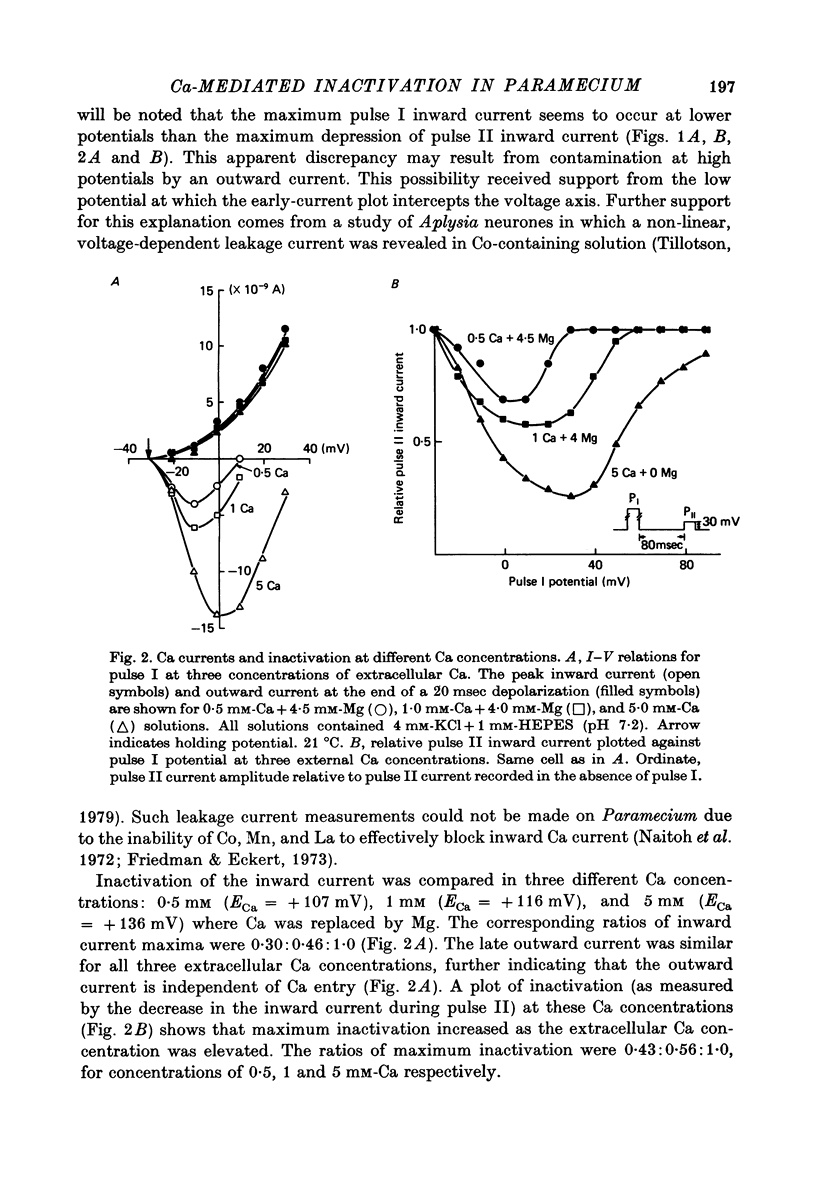
4. Elevation of external Ca from 0·5 to 5 mM was accompanied by increased inactivation of the test pulse current. The enhanced inactivation of the test pulse current was approximately proportional to the increase in current recorded during the conditioning pulse.
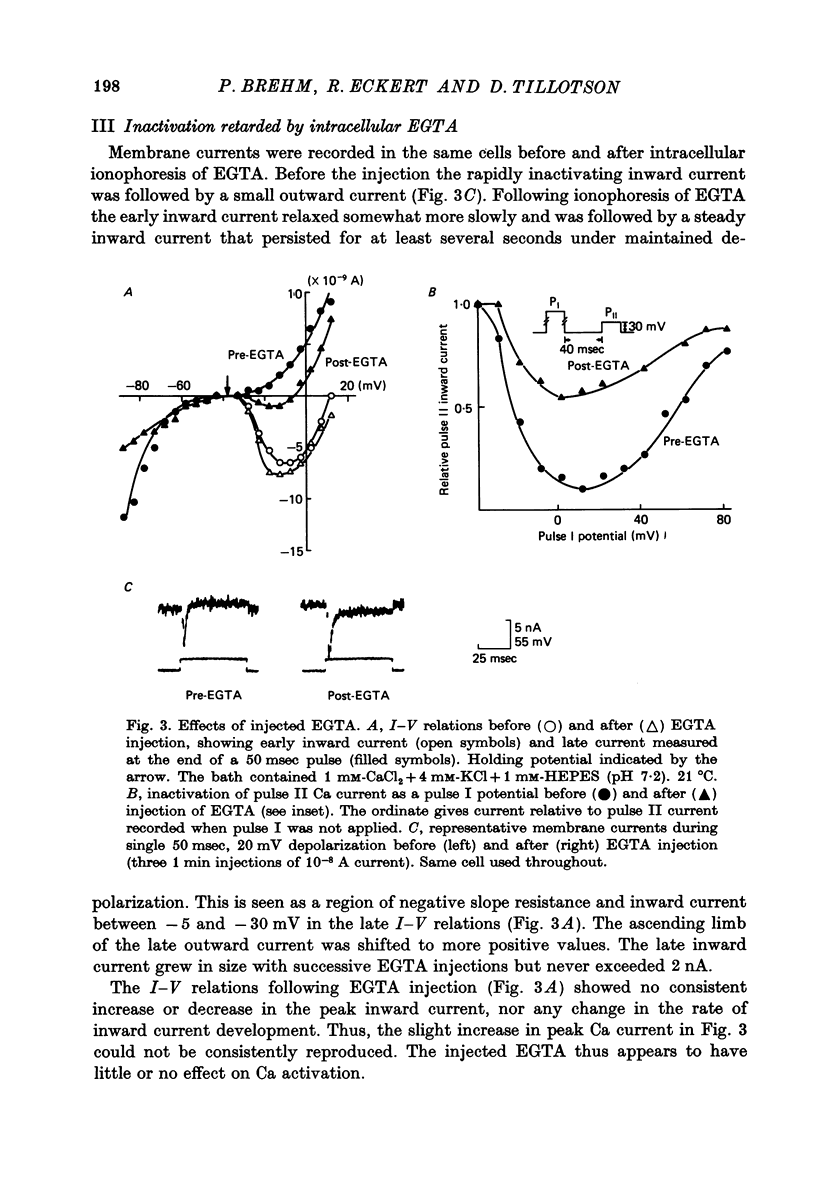
5. Following injection of the Ca chelating agent, EGTA, the inactivation of the test pulse current was diminished; in addition, the transient inward current relaxed slightly more slowly, and the transient was followed by a steady net inward current.
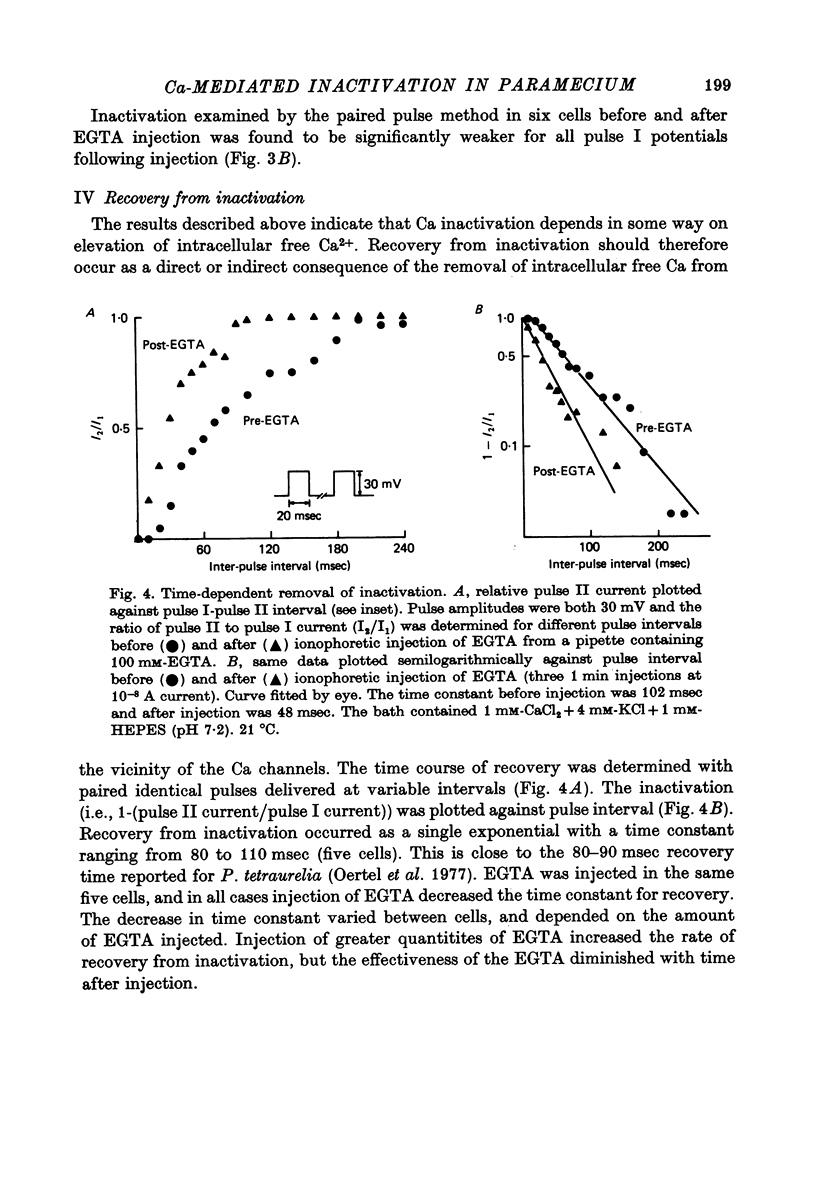
6. The time course of recovery from inactivation in the double pulse experiment approximated a single exponential having a time constant of 80-110 msec. Injection of EGTA shortened the time constant by as much as 50%.
7. It is concluded that interference with the entry of Ca or enhanced removal of intracellular free Ca2+ interferes with the process of Ca current inactivation, while enhanced entry of Ca promotes the process of inactivation. While the mechanism of inactivation is unknown, arguments are presented that the accumulation of intracellular Ca influences the Ca channel conductance.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Lee K. S., Brown A. M. The calcium current of Helix neuron. J Gen Physiol. 1978 May;71(5):509–531. doi: 10.1085/jgp.71.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Inactivation of the sodium channel. I. Sodium current experiments. J Gen Physiol. 1977 Nov;70(5):549–566. doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm P., Dunlap K., Eckert R. Calcium-dependent repolarization in Paramecium. J Physiol. 1978 Jan;274:639–654. doi: 10.1113/jphysiol.1978.sp012171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm P., Eckert R. Calcium entry leads to inactivation of calcium channel in Paramecium. Science. 1978 Dec 15;202(4373):1203–1206. doi: 10.1126/science.103199. [DOI] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Rate constants associated with changes in sodium conductance in axons perfused with sodium fluoride. J Physiol. 1970 Dec;211(3):679–705. doi: 10.1113/jphysiol.1970.sp009299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clusin W., Spray D. C., Bennett M. V. Activation of a voltage-insensitive conductance by inward calcium current. Nature. 1975 Jul 31;256(5516):425–427. doi: 10.1038/256425a0. [DOI] [PubMed] [Google Scholar]
- Dunlap K. Localization of calcium channels in Paramecium caudatum. J Physiol. 1977 Sep;271(1):119–133. doi: 10.1113/jphysiol.1977.sp011993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R. Bioelectric control of ciliary activity. Science. 1972 May 5;176(4034):473–481. doi: 10.1126/science.176.4034.473. [DOI] [PubMed] [Google Scholar]
- Eckert R., Brehm P. Ionic mechanisms of excitation in Paramecium. Annu Rev Biophys Bioeng. 1979;8:353–383. doi: 10.1146/annurev.bb.08.060179.002033. [DOI] [PubMed] [Google Scholar]
- Eckert R., Naitoh Y. Passive electrical properties of Paramecium and problems of ciliary coordination. J Gen Physiol. 1970 Apr;55(4):467–483. doi: 10.1085/jgp.55.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S. Ca-dependent action potential. Membranes. 1975;3:359–381. [PubMed] [Google Scholar]
- Hagiwara S., Nakajima S. Effects of the intracellular Ca ion concentration upon the excitability of the muscle fiber membrane of a barnacle. J Gen Physiol. 1966 Mar;49(4):807–818. doi: 10.1085/jgp.49.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A. Effects of calcium and calcium-chelating agents on the inward and outward current in the membrane of mollusc neurones. J Physiol. 1977 Sep;270(3):569–580. doi: 10.1113/jphysiol.1977.sp011969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machemer H., Eckert R. Electrophysiological control of reversed ciliary beating in Paramecium. J Gen Physiol. 1973 May;61(5):572–587. doi: 10.1085/jgp.61.5.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Meves H. Inactivation of the sodium permeability in squid giant nerve fibres. Prog Biophys Mol Biol. 1978;33(2):207–230. doi: 10.1016/0079-6107(79)90029-4. [DOI] [PubMed] [Google Scholar]
- Naito Y., Kaneko H. Control of ciliary activities by adenosinetriphosphate and divalent cations in triton-extracted models of Paramecium caudatum. J Exp Biol. 1973 Jun;58(3):657–676. doi: 10.1242/jeb.58.3.657. [DOI] [PubMed] [Google Scholar]
- Naitoh Y., Eckert R., Friedman K. A regenerative calcium response in Paramecium. J Exp Biol. 1972 Jun;56(3):667–681. doi: 10.1242/jeb.56.3.667. [DOI] [PubMed] [Google Scholar]
- Oertel D., Schein S. J., Kung C. Separation of membrane currents using a Paramecium mutant. Nature. 1977 Jul 14;268(5616):120–124. doi: 10.1038/268120a0. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Takahashi K., Yoshii M. Two components of the calcium current in the egg cell membrane of the tunicate. J Physiol. 1976 Feb;255(2):527–561. doi: 10.1113/jphysiol.1976.sp011294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Satow Y. Internal calcium concentration and potassium permeability in Paramecium. J Neurobiol. 1978 Jan;9(1):81–91. doi: 10.1002/neu.480090107. [DOI] [PubMed] [Google Scholar]
- Standen N. B. Properties of a calcium channel in snail neurones. Nature. 1974 Jul 26;250(464):340–342. doi: 10.1038/250340a0. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yoshii M. Effects of internal free calcium upon the sodium and calcium channels in the tunicate egg analysed by the internal perfusion technique. J Physiol. 1978 Jun;279:519–549. doi: 10.1113/jphysiol.1978.sp012360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillotson D. Inactivation of Ca conductance dependent on entry of Ca ions in molluscan neurons. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1497–1500. doi: 10.1073/pnas.76.3.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]



