Abstract
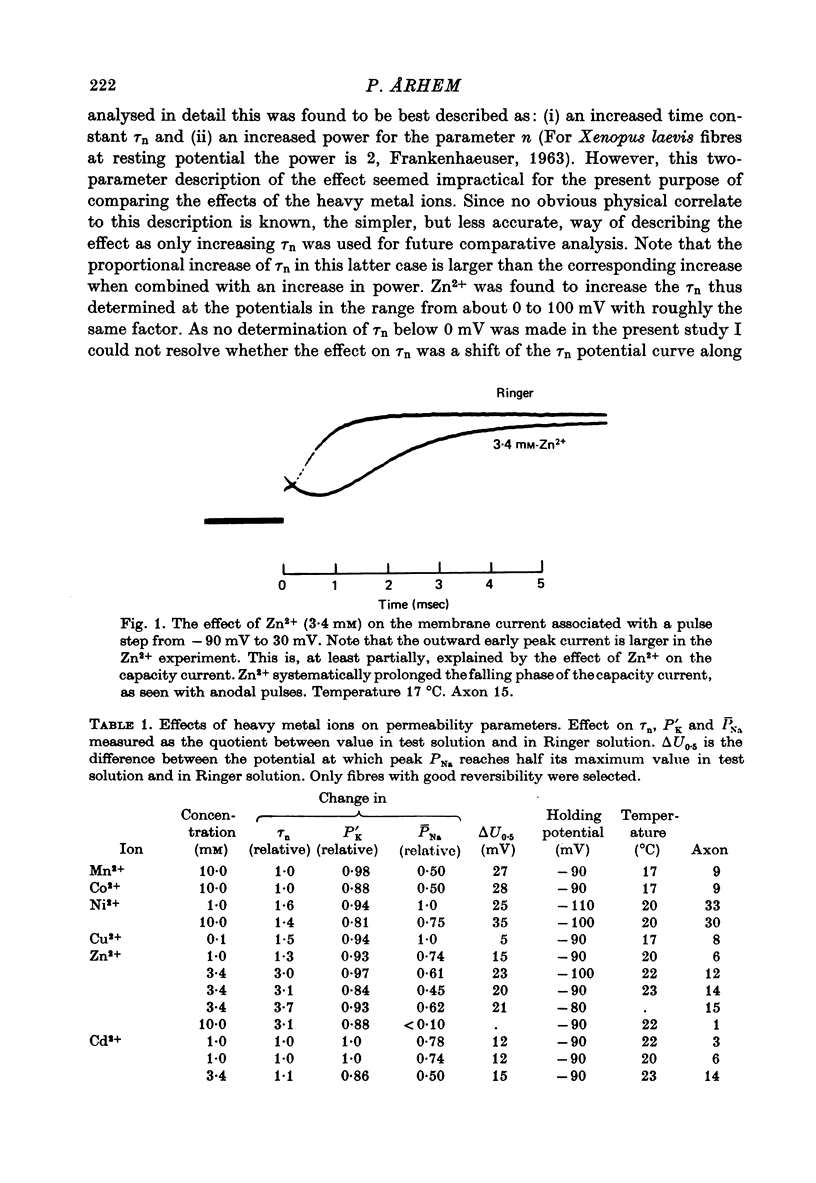
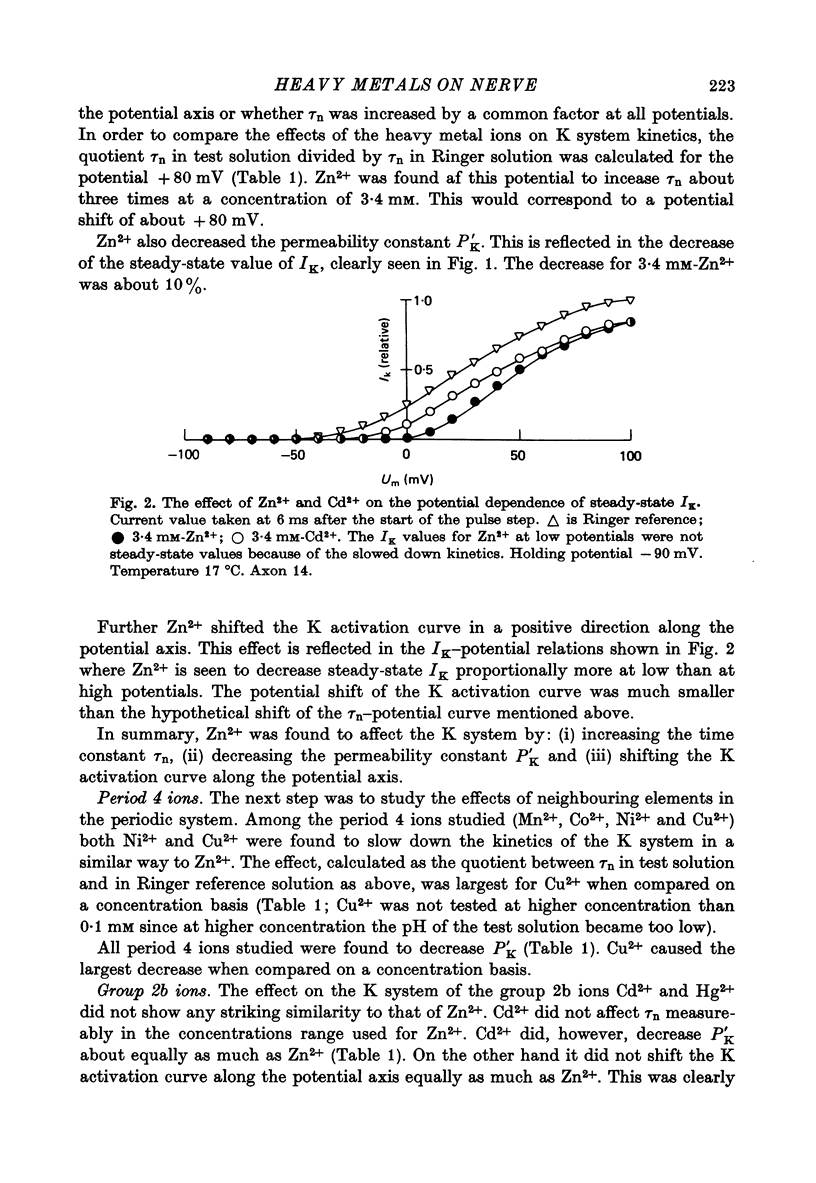
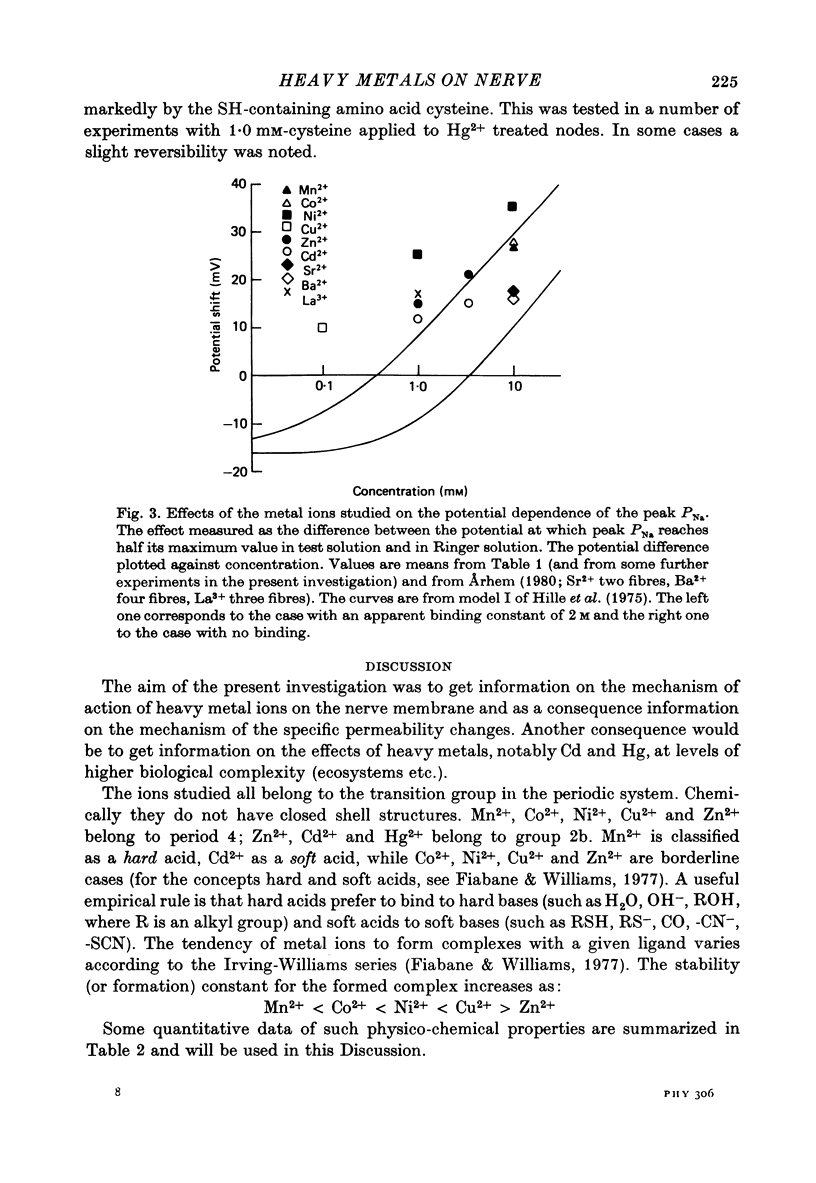
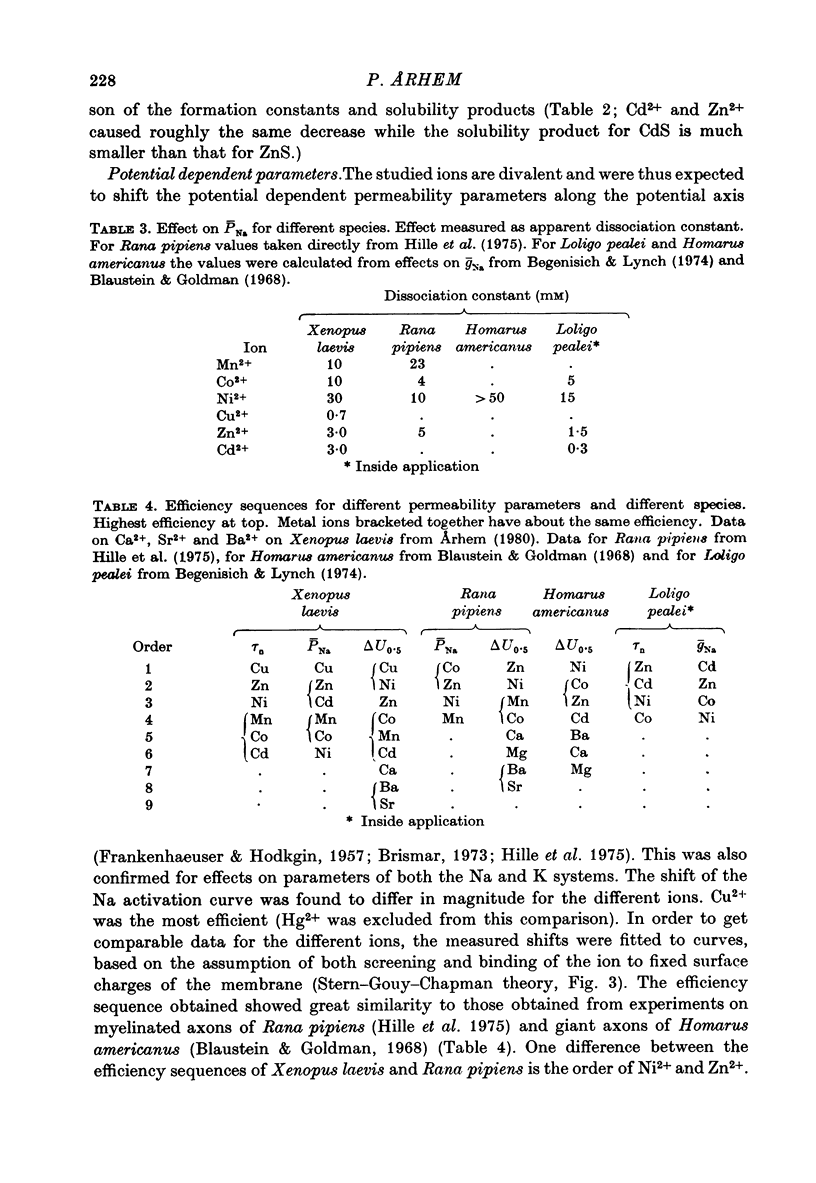
1. The effect of the heavy metal ions Mn2+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+ and Hg2+ on permeability parameters of the nerve membrane was investigated. The ions were applied externally to single myelinated fibres of Xenopus laevis. The ionic currents, associated with potential steps were measured and analysed. 2. Zn2+ reversibly slowed down the kinetics of the K system. The effect at large potential steps was described as an increase of tau n. 3.4 mM-Zn2+ increased tau n about three times. 3. Ni2+ and Cu2+ also reversibly increased tau n in a similar way. The effect was larger than that of Zn2+. The other period 4 ions tested did not affect tau n markedly. Nor did the group 2b ion Cd2+. 4. The relative efficiency of the different ions agreed well with their tendency to form complexes with certain ligands. The effect was not a simple function of affinity for sulphydryl groups. 5. All the studied ions decreased PNa. The decrease was reversible except for that caused by Hg2+. Cu2+ caused the largest reversible effect. Hg2+ irreversibly decreased PNa at low concentrations (1-10 microM). 6. All ions studied shifted the Na activation curve in a positive direction along the potential axis. The effect was reversible except for that caused by Hg2+. The largest reversible shift was caused by Cu2+. 7. The relative efficiency of the different ions on the parameters studied in Xenopus axons showed great similarity to the corresponding efficiency on axons from other species, although some differences were noted.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arhem P. Effects of rubidium, caesium, strontium, barium and lanthanum on ionic currents in myelinated nerve fibres from Xenopus laevis. Acta Physiol Scand. 1980 Jan;108(1):7–16. doi: 10.1111/j.1748-1716.1980.tb06494.x. [DOI] [PubMed] [Google Scholar]
- Arhem P., Frankenhaeuser B., Moore L. E. Ionic currents at resting potential in nerve fibres from Xenopus laevis. Potential clamp experiments. Acta Physiol Scand. 1973 Aug;88(4):446–454. doi: 10.1111/j.1748-1716.1973.tb05474.x. [DOI] [PubMed] [Google Scholar]
- Armstrong C. M. Ionic pores, gates, and gating currents. Q Rev Biophys. 1974 May;7(2):179–210. doi: 10.1017/s0033583500001402. [DOI] [PubMed] [Google Scholar]
- BENESCH R. E., BENESCH R. Relation between erythrocyte integrity and sulfhydryl groups. Arch Biochem Biophys. 1954 Jan;48(1):38–42. doi: 10.1016/0003-9861(54)90302-1. [DOI] [PubMed] [Google Scholar]
- Begenisich T., Lynch C. Effects of internal divalent cations on voltage-clamped squid axons. J Gen Physiol. 1974 Jun;63(6):675–689. doi: 10.1085/jgp.63.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Goldman D. E. The action of certain polyvalent cations on the voltage-clamped lobster axon. J Gen Physiol. 1968 Mar;51(3):279–291. doi: 10.1085/jgp.51.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brismar T. Effects of ionic concentration on permeability properties of nodal membrane in myelinated nerve fibres of Xenopus laevis. Potential clamp experiments. Acta Physiol Scand. 1973 Apr;87(4):474–484. doi: 10.1111/j.1748-1716.1973.tb05414.x. [DOI] [PubMed] [Google Scholar]
- Conti F., Hille B., Neumcke B., Nonner W., Stämpfli R. Measurement of the conductance of the sodium channel from current fluctuations at the node of Ranvier. J Physiol. 1976 Nov;262(3):699–727. doi: 10.1113/jphysiol.1976.sp011616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE F. A., FRANKENHAEUSER B. Membrane currents in isolated frog nerve fibre under voltage clamp conditions. J Physiol. 1958 Aug 29;143(1):76–90. doi: 10.1113/jphysiol.1958.sp006045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE F. A., FRANKENHAEUSER B. Sodium currents in the myelinated nerve fibre of Xenopus laevis investigated with the voltage clamp technique. J Physiol. 1959 Oct;148:188–200. doi: 10.1113/jphysiol.1959.sp006281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. A QUANTITATIVE DESCRIPTION OF POTASSIUM CURRENTS IN MYELINATED NERVE FIBRES OF XENOPUS LAEVIS. J Physiol. 1963 Nov;169:424–430. doi: 10.1113/jphysiol.1963.sp007268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Woodhull A. M., Shapiro B. I. Negative surface charge near sodium channels of nerve: divalent ions, monovalent ions, and pH. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):301–318. doi: 10.1098/rstb.1975.0011. [DOI] [PubMed] [Google Scholar]
- Robinson J. D. Interaction between protein sulphydryl groups and lipid double bonds in biological membranes. Nature. 1966 Oct 8;212(5058):199–200. doi: 10.1038/212199a0. [DOI] [PubMed] [Google Scholar]
- Segall H. J., Wood J. M. Reaction of methyl mercury with plasmalogens suggests a mechanism for neurotoxicity of metal-alkyls. Nature. 1974 Mar 29;248(447):456–458. doi: 10.1038/248456a0. [DOI] [PubMed] [Google Scholar]
- Shrager P. Slow sodium inactivation in nerve after exposure to sulhydryl blocking reagents. J Gen Physiol. 1977 Feb;69(2):183–202. doi: 10.1085/jgp.69.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield P. R. The effect of zinc ions on the gating of the delayed potassium conductance of frog sartorius muscle. J Physiol. 1975 Oct;251(3):711–735. doi: 10.1113/jphysiol.1975.sp011118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHASHI H., MURAI T., SASAKI T. Plateau formation and sulphydryl groups in the plasma membrane. Nature. 1958 Dec 13;182(4650):1675–1677. doi: 10.1038/1821675a0. [DOI] [PubMed] [Google Scholar]



