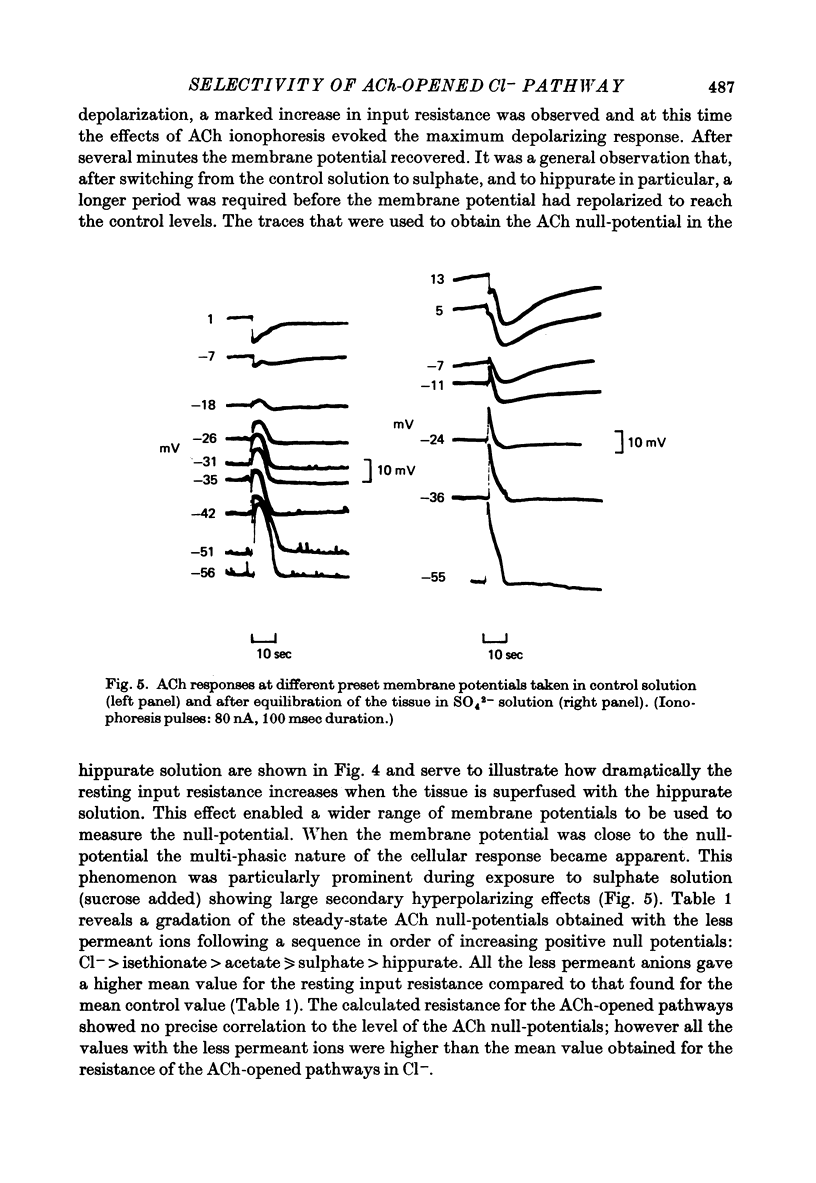
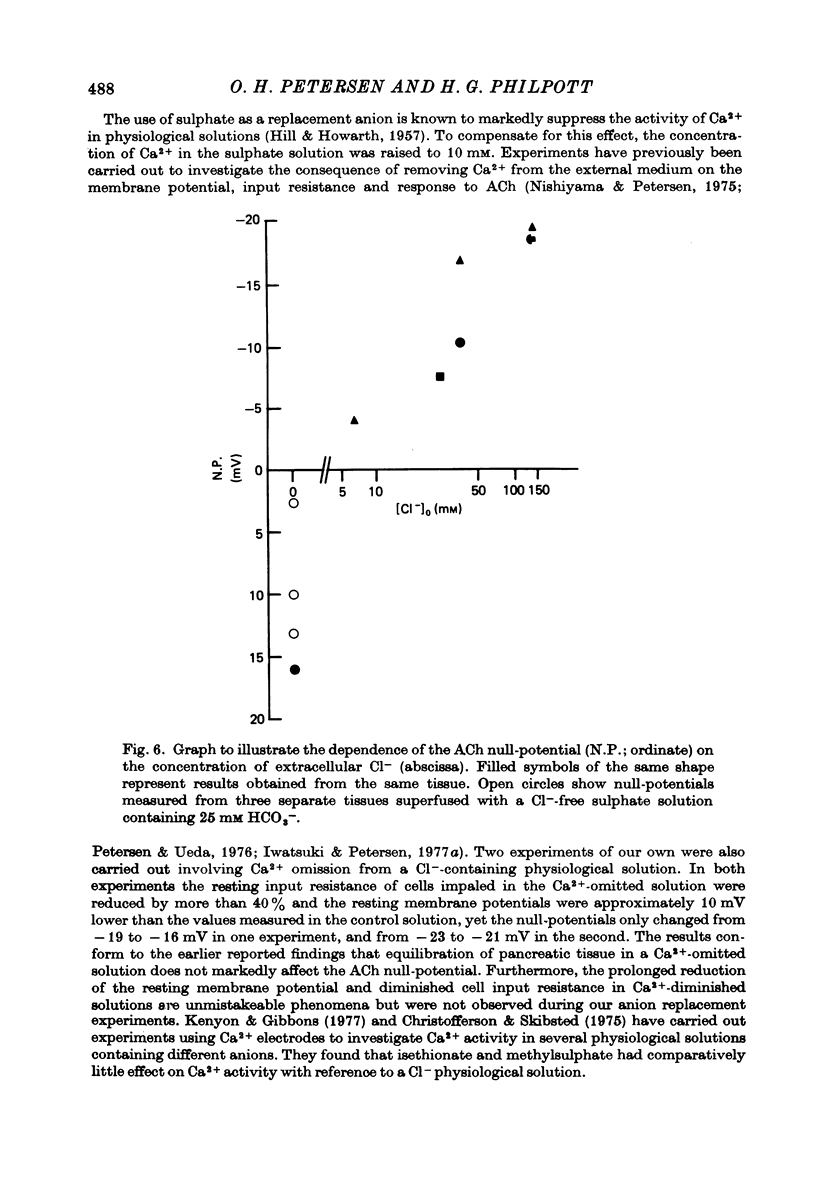
Abstract
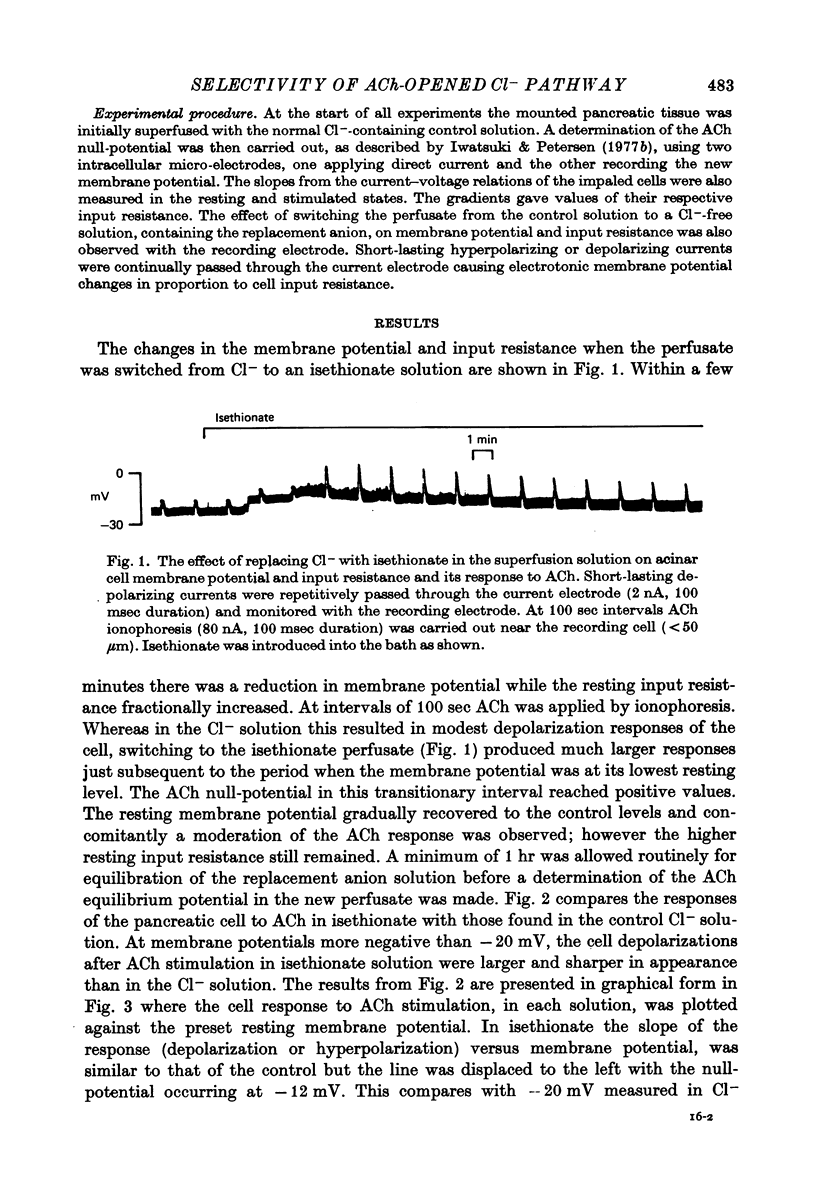
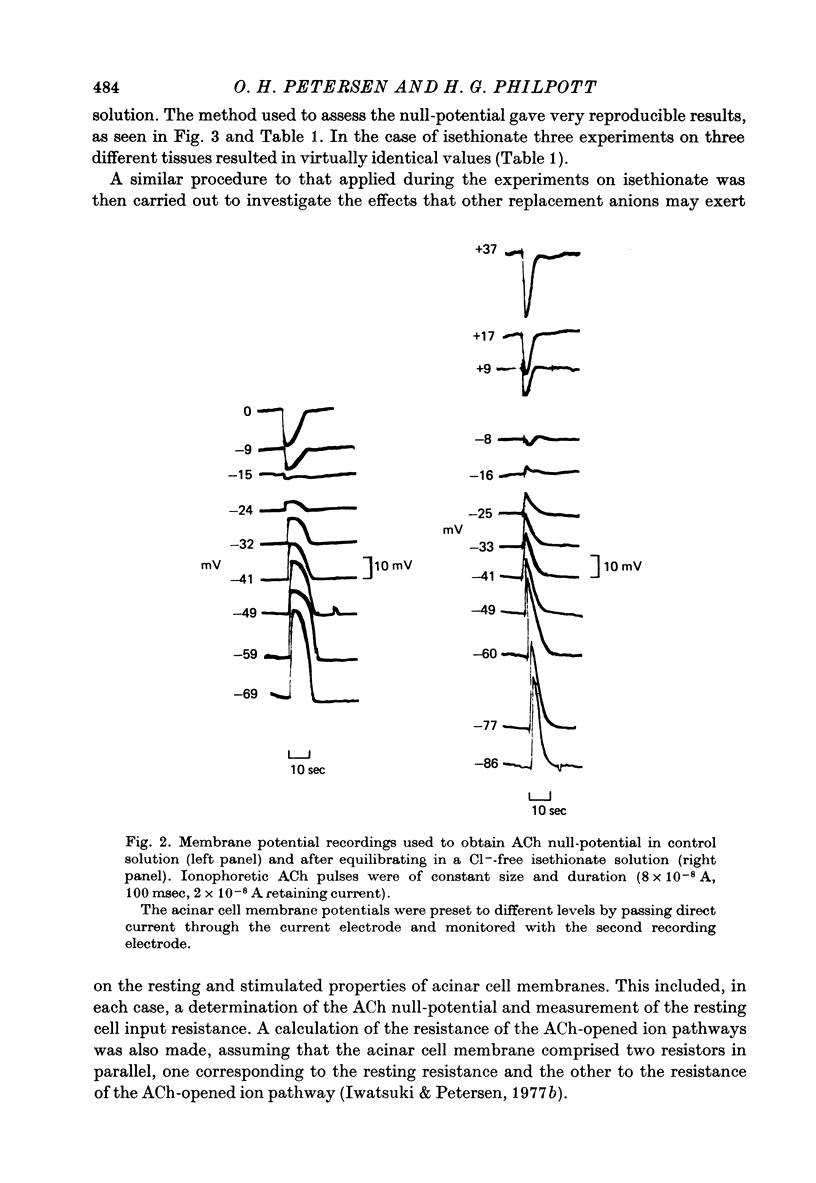
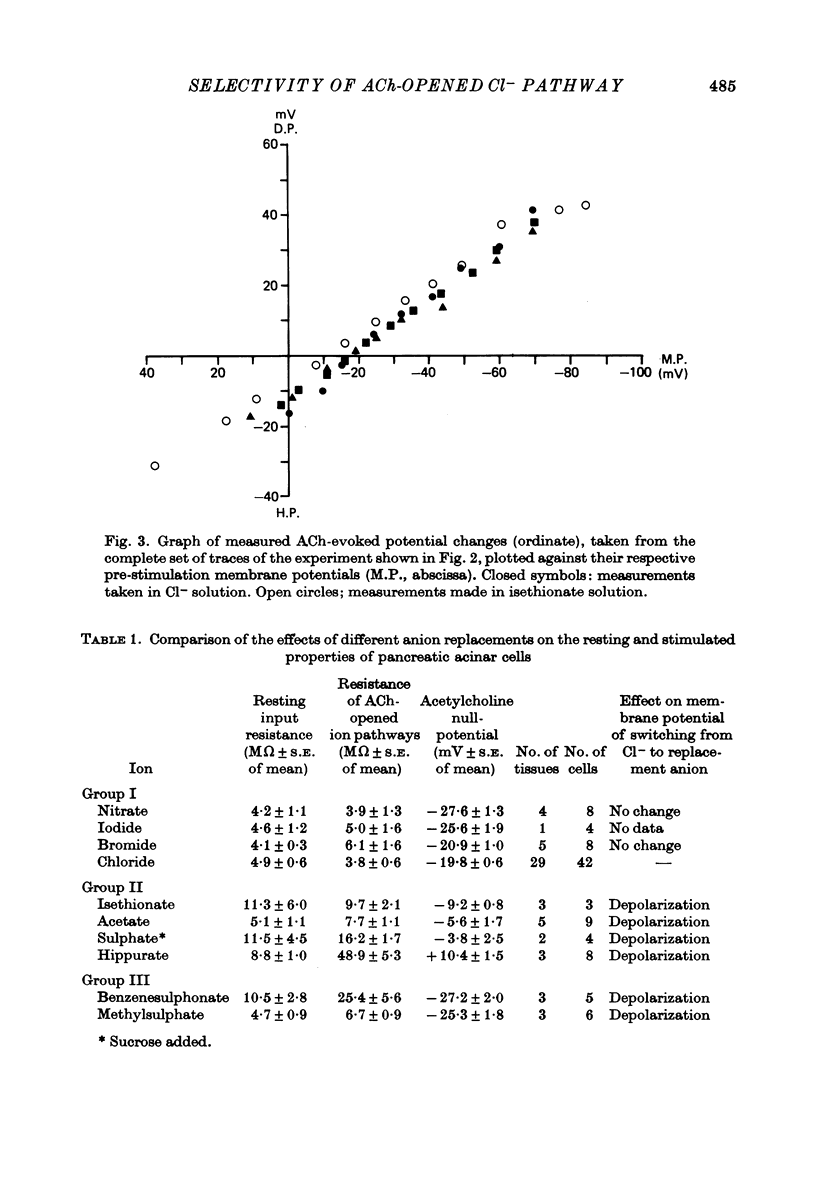
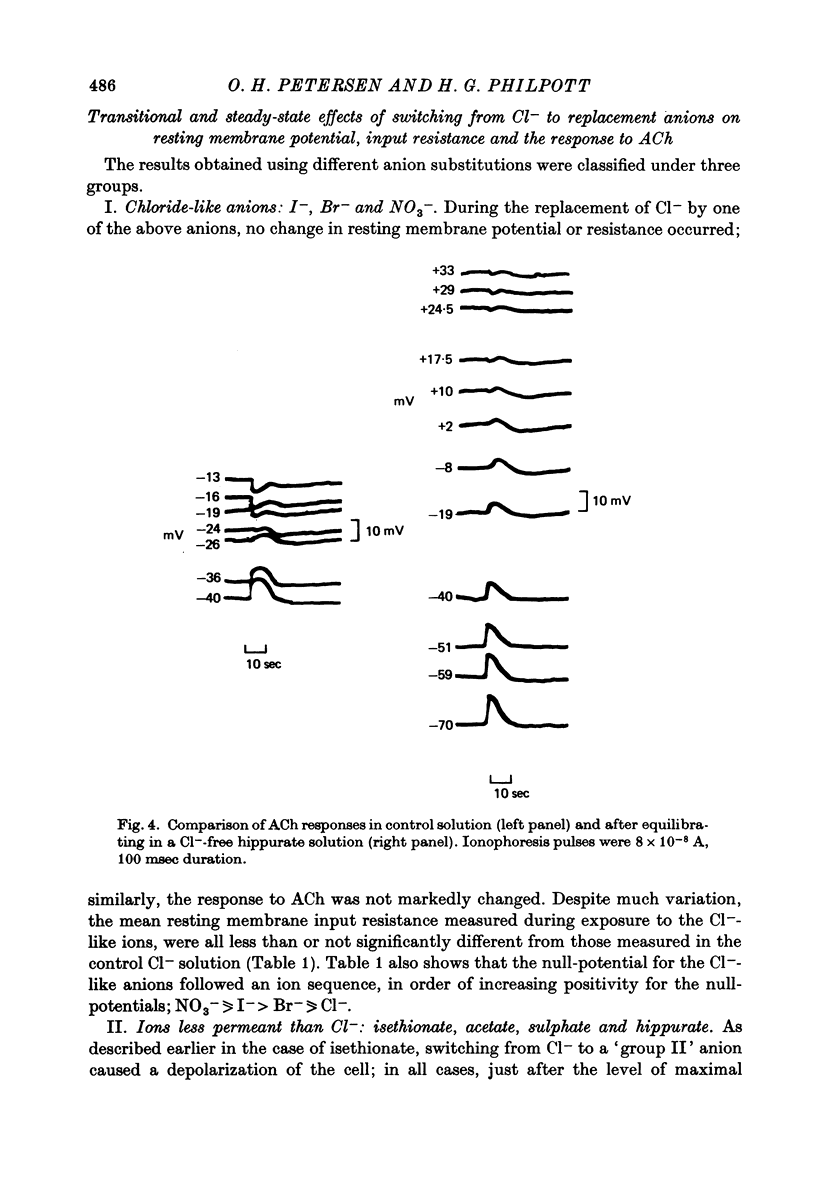
1. Anion replacement experiments were performed on superfused in vitro mouse pancreatic tissue and the effects on the electrical response of acinar cells to ACh investigated. 2. Electrical measurements were made with two micro-electrodes inserted into electrically coupled cells. ACh was applied by microionophoresis. Potential recordings were taken before, during and after changeover from the control superfusion fluid, containing Cl-, to one containing the substituted anion. 3. From the results obtained the tested anions were classified into three groups: I, Cl(-)-like anions: Br-, I- and NO3-, causing either no change or a negative displacement of the ACh null-potential, compared to that measured in the control Cl(-)-containing solution, and only small changes in the resting and stimulated electrical properties of the acinar cell, II, ions less permeable than Cl-: isethionate, acetate, sulphate and hippurate, showing a positive displacement of the ACh null-potential and a similar or increased resting cell input resistance, and III, methylsulphate and benzenesulphonate, causing a negatively displaced ACh null-potential but showing changes in the resting electrical properties of the acinar cells characteristic of anions in group II. 4. The ACh null-potential sequence, in order of decreasing negativity, was NO3- greater than or equal to benzenesulphonate greater than or equal to I- greater than or equal to methylsulphate greater than Br- greater than or equal to Cl- greater than isethionate greater than acetate greater than or equal to sulphate greater than hippurate. 5. Experiments involving the use of bicarbonate demonstrated that it does not contribute significantly to the value of the ACh null-potential. 6. The sequence of the anions in group I were compared to the Eisenman series I, suggesting that the ACh-opened Cl- pathway comprises a large hydrated ion channel bearing a lining of weak positive charges. 7. A quantitative relationship was sought between the ACh null-potential and extracellular Cl-. It was found that a tenfold reduction in the extracellular concentration resulted in a 15 mV positive shift of the null-potential.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casteels R., Meuwissen H. Interaction between potassium and chloride in smooth muscle cells of the guinea-pig taenia coli. J Physiol. 1968 Feb;194(2):76–7P. [PubMed] [Google Scholar]
- Christoffersen C. R., Skibsted L. H. Calcium ion activity in physiological salt solutions: influence of anions substituted for chloride. Comp Biochem Physiol A Comp Physiol. 1975 Oct 1;52(2):317–322. doi: 10.1016/s0300-9629(75)80094-6. [DOI] [PubMed] [Google Scholar]
- Eccles J., Nicoll R. A., Oshima T., Rubia F. J. The anionic permeability of the inhibitory postsynaptic membrane of hippocampal pyramidal cells. Proc R Soc Lond B Biol Sci. 1977 Sep 19;198(1133):345–361. doi: 10.1098/rspb.1977.0102. [DOI] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL A. V., HOWARTH J. V. The effect of potassium on the resting metabolism of the frog's sartorius. Proc R Soc Lond B Biol Sci. 1957 Aug 24;147(926):21–43. doi: 10.1098/rspb.1957.0034. [DOI] [PubMed] [Google Scholar]
- ITO M., KOSTYUK P. G., OSHIMA T. Further study on anion permeability of inhibitory post-synaptic membrane of cat motoneurones. J Physiol. 1962 Oct;164:150–156. doi: 10.1113/jphysiol.1962.sp007009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Pancreatic acinar cells: localization of acetylcholine receptors and the importance of chloride and calcium for acetylcholine-evoked depolarization. J Physiol. 1977 Aug;269(3):723–733. doi: 10.1113/jphysiol.1977.sp011925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Pancreatic acinar cells: the acetylcholine equilibrium potential and its ionic dependency. J Physiol. 1977 Aug;269(3):735–751. doi: 10.1113/jphysiol.1977.sp011926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon J. L., Gibbons W. R. Effects of low-chloride solutions on action potentials of sheep cardiac Purkinje fibers. J Gen Physiol. 1977 Nov;70(5):635–660. doi: 10.1085/jgp.70.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A., Petersen O. H. Pancreatic acinar cells: ionic dependence of acetylcholine-induced membrane potential and resistance change. J Physiol. 1975 Jan;244(2):431–465. doi: 10.1113/jphysiol.1975.sp010807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A., Petersen O. H. Pancreatic acinar cells: membrane potential and resistance change evoked by acetylcholine. J Physiol. 1974 Apr;238(1):145–158. doi: 10.1113/jphysiol.1974.sp010515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H., Philpott H. G. Pancreatic acinar cells: effects of micro-ionophoretic polypeptide application on membrane potential and resistance. J Physiol. 1979 May;290(2):305–315. doi: 10.1113/jphysiol.1979.sp012772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H., Ueda N. Pancreatic acinar cells: the role of calcium in stimulus-secretion coupling. J Physiol. 1976 Jan;254(3):583–606. doi: 10.1113/jphysiol.1976.sp011248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H., Ueda N. Secretion of fluid and amylase in the perfused rat pancreas. J Physiol. 1977 Jan;264(3):819–835. doi: 10.1113/jphysiol.1977.sp011696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson O. H., Iwatsuki N. The role of calcium in pancreatic acinar cell stimulus-secretion coupling: an electrophysiological approach. Ann N Y Acad Sci. 1978 Apr 28;307:599–617. doi: 10.1111/j.1749-6632.1978.tb41984.x. [DOI] [PubMed] [Google Scholar]
- Philpott H. G., Petersen O. H. Extracellular but not intracellular application of peptide hormones activates pancreatic acinar cells. Nature. 1979 Oct 25;281(5733):684–686. doi: 10.1038/281684a0. [DOI] [PubMed] [Google Scholar]
- Philpott H. G., Petersen O. H. Separate activation sites for cholecystokinin and bombesin on pancreatic acini: an electrophysiological study employing a competitive antagonist for the action of CCK. Pflugers Arch. 1979 Nov;382(3):263–267. doi: 10.1007/BF00583711. [DOI] [PubMed] [Google Scholar]
- Woodbury J. W., Miles P. R. Anion conductance of frog muscle membranes: one channel, two kinds of pH dependence. J Gen Physiol. 1973 Sep;62(3):324–353. doi: 10.1085/jgp.62.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright E. M., Diamond J. M. Anion selectivity in biological systems. Physiol Rev. 1977 Jan;57(1):109–156. doi: 10.1152/physrev.1977.57.1.109. [DOI] [PubMed] [Google Scholar]


