Abstract
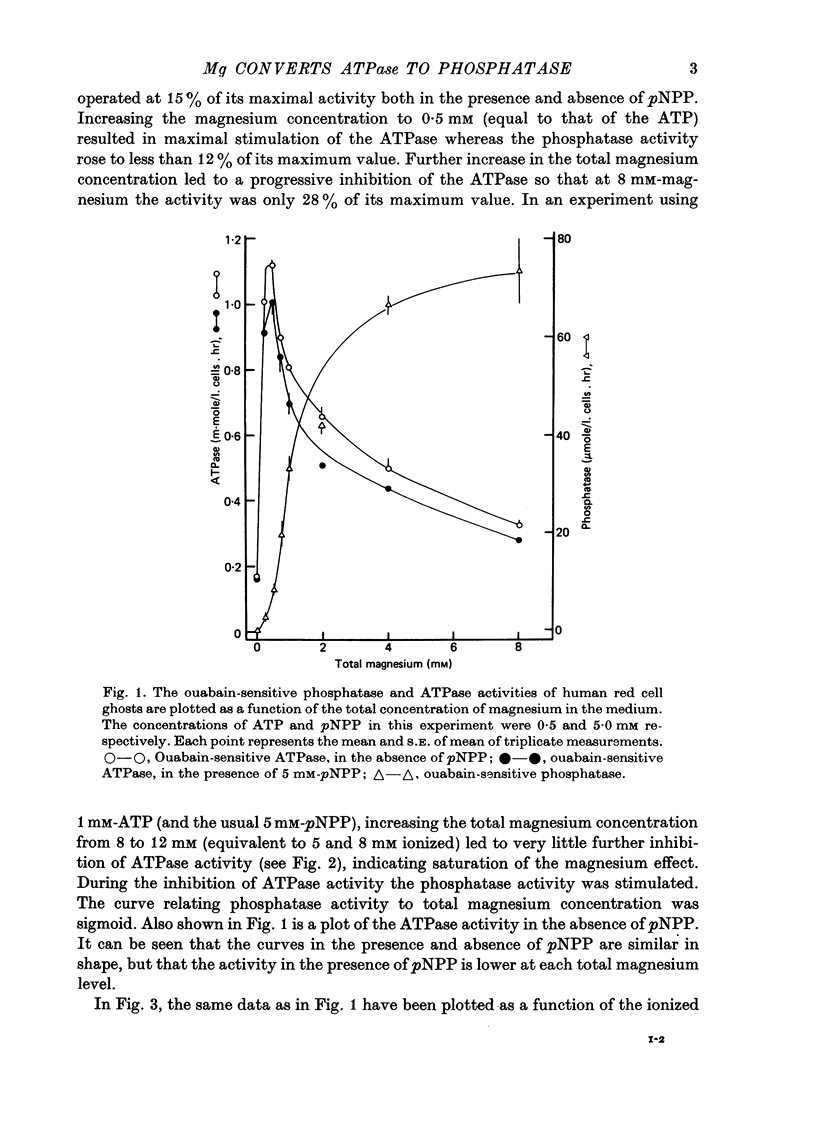
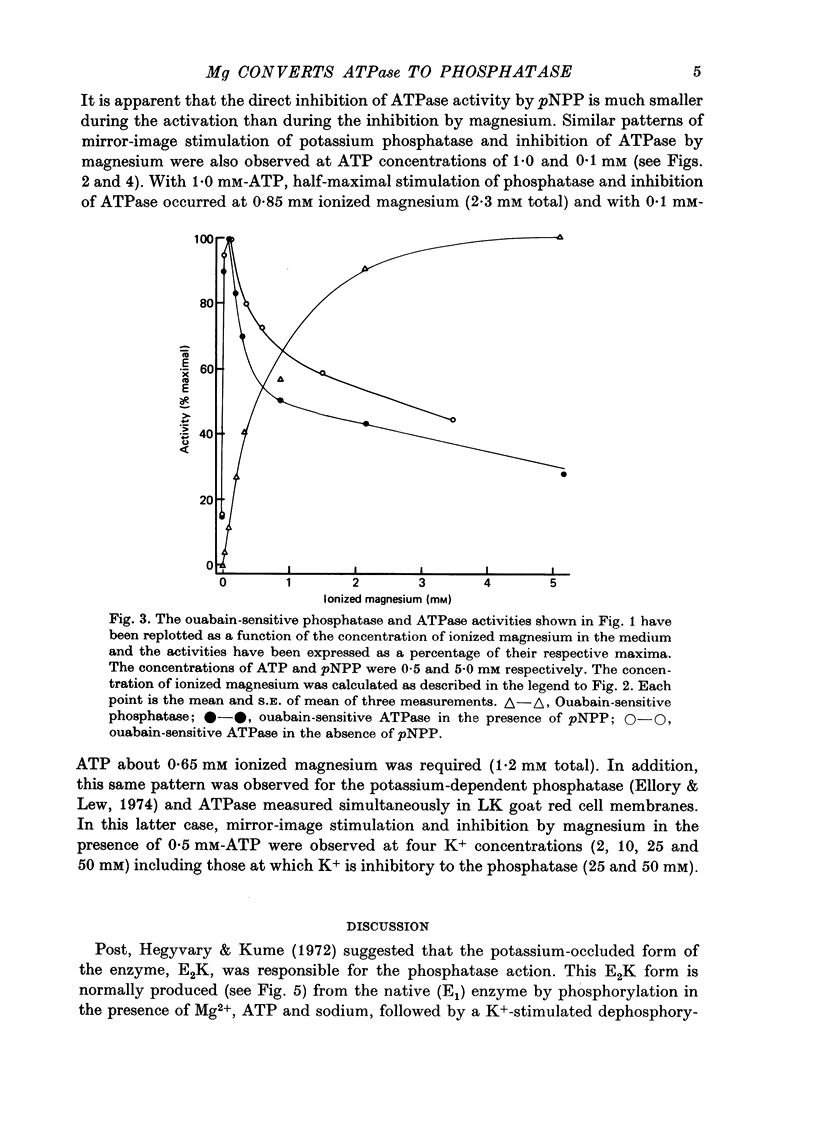
1. The ATPase and phosphatase activities of red cell membranes were measured simultaneously as a function of the magnesium content of the medium. 2. It was found that when the magnesium concentration was greater than that of ATP, magnesium inhibited the ATPase and simultaneously stimulated the phosphatase. The concentrations of magnesium needed for half-maximal stimulation of the phosphatase and half-maximal inhibition of the ATPase were similar. 3. It is suggested that increasing the concentration of magnesium directly causes a change in the conformation of the enzyme from one which favours ATPase activity to one which favours phosphatase activity.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaugé L. A., Glynn I. M. The equilibrium between different conformations of the unphosphorylated sodium pump: effects of ATP and of potassium ions, and their relevance to potassium transport. J Physiol. 1980 Feb;299:367–383. doi: 10.1113/jphysiol.1980.sp013130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H., Jänig G. R., Gerber G., Ruckpaul K., Rapoport S. M. Interaction of haemoglobin with ions. Interactions among magnesium, adenosine 5'-triphosphate, 2,3-bisphosphoglycerate, and oxygenated and deoxygenated human haemoglobin under simulated intracellular conditions. Eur J Biochem. 1973 Oct 18;38(3):553–562. doi: 10.1111/j.1432-1033.1973.tb03090.x. [DOI] [PubMed] [Google Scholar]
- DUNHAM E. T., GLYNN I. M. Adenosinetriphosphatase activity and the active movements of alkali metal ions. J Physiol. 1961 Apr;156:274–293. doi: 10.1113/jphysiol.1961.sp006675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Pouchan M. I., Rega A. F. Potassium activated phosphatase from human red blood cells. The mechanism of potassium activation. J Physiol. 1969 Jun;202(2):305–327. doi: 10.1113/jphysiol.1969.sp008813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hexum T., Samson F. E., Jr, Himes R. H. Kinetic studies of membrane (Na+-K+-Mg2+)-ATPase. Biochim Biophys Acta. 1970 Aug 15;212(2):322–331. doi: 10.1016/0005-2744(70)90213-5. [DOI] [PubMed] [Google Scholar]
- Jorgensen P. L. Purification and characterization of (Na+ + K+)-ATPase. VI. Differential tryptic modification of catalytic functions of the purified enzyme in presence of NaCl and KCl. Biochim Biophys Acta. 1977 Apr 1;466(1):97–108. doi: 10.1016/0005-2736(77)90211-5. [DOI] [PubMed] [Google Scholar]
- Jorgensen P. L., Skou J. C., Solomonson L. P. Purification and characterization of (Na+ + K+)-ATPase. II. Preparation by zonal centrifugation of highly active (Na+ + K+)-ATPase from the outer medulla of rabbit kidneys. Biochim Biophys Acta. 1971 Apr 13;233(2):381–394. doi: 10.1016/0005-2736(71)90335-x. [DOI] [PubMed] [Google Scholar]
- Karlish S. J., Yates D. W., Glynn I. M. Conformational transitions between Na+-bound and K+-bound forms of (Na+ + K+)-ATPase, studied with formycin nucleotides. Biochim Biophys Acta. 1978 Jul 7;525(1):252–264. doi: 10.1016/0005-2744(78)90219-x. [DOI] [PubMed] [Google Scholar]
- Ottolenghi P. The relipidation of delipidated Na,K-ATPase. An analysis of complex formation with dioleoylphosphatidylcholine and with dioleoylphosphatidylethanolamine. Eur J Biochem. 1979 Aug 15;99(1):113–131. doi: 10.1111/j.1432-1033.1979.tb13238.x. [DOI] [PubMed] [Google Scholar]
- Post R. L., Hegyvary C., Kume S. Activation by adenosine triphosphate in the phosphorylation kinetics of sodium and potassium ion transport adenosine triphosphatase. J Biol Chem. 1972 Oct 25;247(20):6530–6540. [PubMed] [Google Scholar]
- Post R. L., Toda G., Kume S., Taniguchi K. Synthesis of adenosine triphosphate by way of potassium-sensitive phosphoenzyme of sodium, potassium adenosine triphosphatase. J Supramol Struct. 1975;3(5-6):479–497. doi: 10.1002/jss.400030508. [DOI] [PubMed] [Google Scholar]
- Robinson J. D. Kinetic studies on a brain microsomal adenosine triphosphatase. II. Potassium-dependent phosphatase activity. Biochemistry. 1969 Aug;8(8):3348–3355. doi: 10.1021/bi00836a032. [DOI] [PubMed] [Google Scholar]
- Robinson J. D. Nucleotide and divalent cation interactions with the (Na+ plus K+)-dependent ATPase. Biochim Biophys Acta. 1974 Mar 21;341(1):232–247. doi: 10.1016/0005-2744(74)90084-9. [DOI] [PubMed] [Google Scholar]
- Sen A. K., Tobin T. A cycle for ouabain inhibition of sodium- and potassium-dependent adenosine triphosphatase. J Biol Chem. 1969 Dec 25;244(24):6596–6604. [PubMed] [Google Scholar]
- Skou J. C. Effect of ATP on the intermediary steps of the reaction of the (Na+ plus K+)-dependent enzyme system. 3. Effect on the p-nitrophenylphosphatase activity of the system. Biochim Biophys Acta. 1974 Mar 15;339(2):258–273. [PubMed] [Google Scholar]


