Abstract
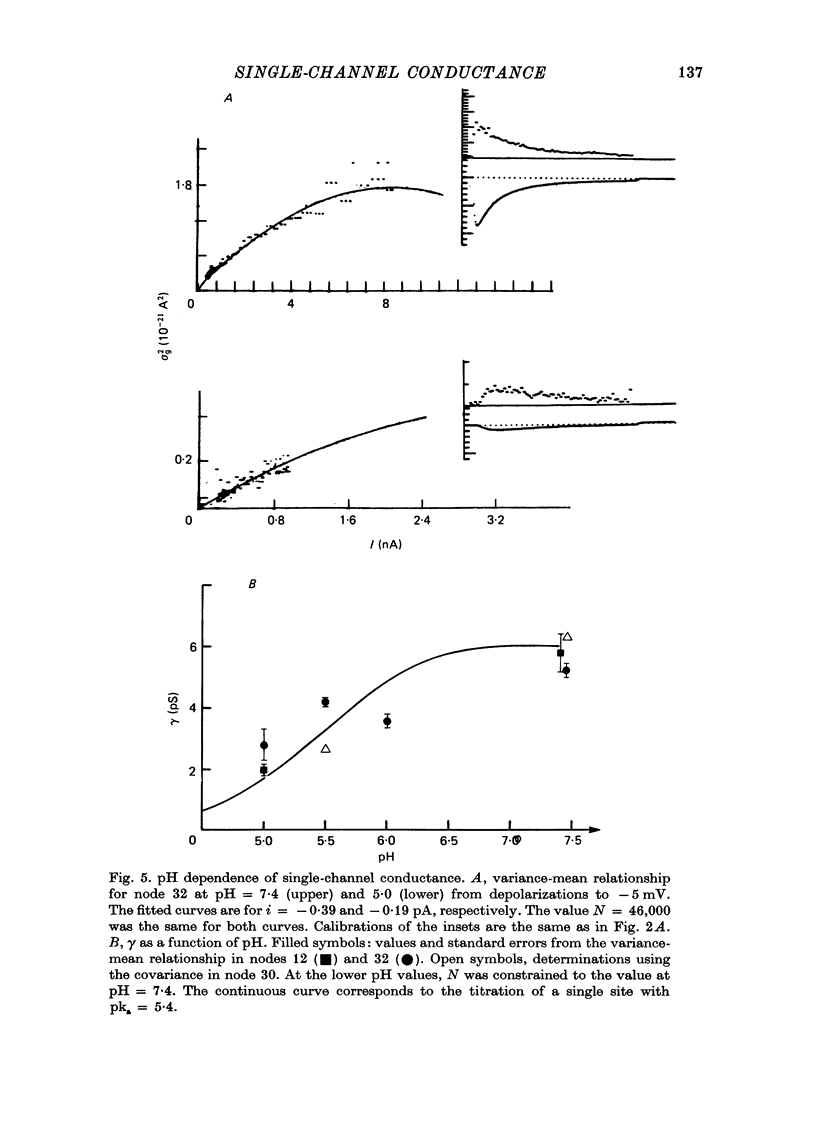
1. The single sodium channel conductance gamma and the number of channels N were estimated from fluctuation analysis in voltage-clamped nodes of Ranvier under conditions that decreased the size of the sodium current. 2. Reduction of the sodium current by depolarizing prepulses had no effect on gamma, and, in cases where it could be determined, had no significant effect on N. Partial block of the sodium conductance with tetrodotoxin and saxitoxin also did not affect gamma significantly, but reduced N. 3. gamma was reduced to about 40% of the control value at -5 mV when the pH of the external solution was reduced to 5.0. The pH dependence of gamma is consistent with the theories of Woodhull and of Drouin & Neumcke. 4. The differing effects of prepulses, toxins and pH are interpreted in view of the different time scales of channel inactivation or block under these conditions. 5. The nearly unchanged gamma with prepulses and partial toxin block provides further evidence for the absence of interactions among sodium channels.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Bezanilla F. Inactivation of the sodium channel. II. Gating current experiments. J Gen Physiol. 1977 Nov;70(5):567–590. doi: 10.1085/jgp.70.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLE K. S., MOORE J. W. Potassium ion current in the squid giant axon: dynamic characteristic. Biophys J. 1960 Sep;1:1–14. doi: 10.1016/s0006-3495(60)86871-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A., Morrow C. S. Binding to saxitoxin to electrically excitable neuroblastoma cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):218–222. doi: 10.1073/pnas.75.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P., Thiéry J., Tung Y., Kittel C. On the cooperativity of biological membranes. Proc Natl Acad Sci U S A. 1967 Feb;57(2):335–341. doi: 10.1073/pnas.57.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin H., Neumcke B. Specific and unspecific charges at the sodium channels of the nerve membrane. Pflugers Arch. 1974;351(3):207–229. doi: 10.1007/BF00586919. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L., Chen Y. D. On the theory of ion transport across the nerve membrane. II. Potassium ion kinetics and cooperativity (with x = 4). Proc Natl Acad Sci U S A. 1971 Aug;68(8):1711–1715. doi: 10.1073/pnas.68.8.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Common mode of action of three agents that decrease the transient change in sodium permeability in nerves. Nature. 1966 Jun 18;210(5042):1220–1222. doi: 10.1038/2101220a0. [DOI] [PubMed] [Google Scholar]
- Hille B. Pharmacological modifications of the sodium channels of frog nerve. J Gen Physiol. 1968 Feb;51(2):199–219. doi: 10.1085/jgp.51.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H. A., Bamberg E. Influence of membrane thickness and ion concentration on the properties of the gramicidin a channel. Autocorrelation, spectral power density, relaxation and single-channel studies. Biochim Biophys Acta. 1977 Jan 4;464(1):127–141. doi: 10.1016/0005-2736(77)90376-5. [DOI] [PubMed] [Google Scholar]
- Meves H., Vogel W. Inactivation of the asymmetrical displacement current in giant axons of Loligo forbesi. J Physiol. 1977 May;267(2):377–393. doi: 10.1113/jphysiol.1977.sp011818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J. The variance of sodium current fluctuations at the node of Ranvier. J Physiol. 1980 Oct;307:97–129. doi: 10.1113/jphysiol.1980.sp013426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TILLE J. A NEW INTERPRETATION OF THE DYNAMIC CHANGES OF THE POTASSIUM CONDUCTANCE IN THE SQUID GIANT AXON. Biophys J. 1965 Mar;5:163–171. doi: 10.1016/s0006-3495(65)86708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata M., Moore J. W., Kao C. Y., Fuhrman F. A. Blockage of sodium conductance increase in lobster giant axon by tarichatoxin (tetrodotoxin). J Gen Physiol. 1966 May;49(5):977–988. doi: 10.1085/jgp.49.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbricht W., Wagner H. H. The influence of pH on the rate of tetrodotoxin action on myelinated nerve fibres. J Physiol. 1975 Oct;252(1):185–202. doi: 10.1113/jphysiol.1975.sp011140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner H. H., Ulbricht W. The rates of saxitoxin action and of saxitoxin-tetrodotoxin interaction at the node of Ranvier. Pflugers Arch. 1975 Sep 29;359(4):297–315. doi: 10.1007/BF00581441. [DOI] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]


