Abstract
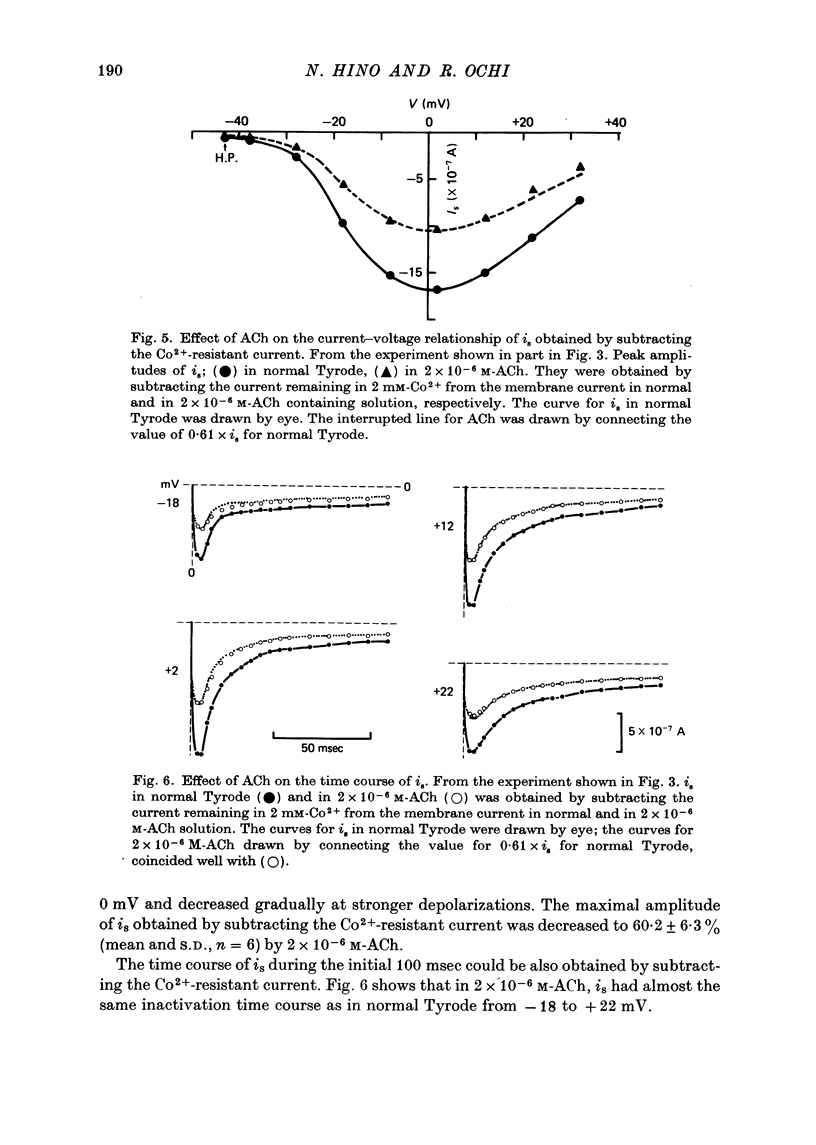
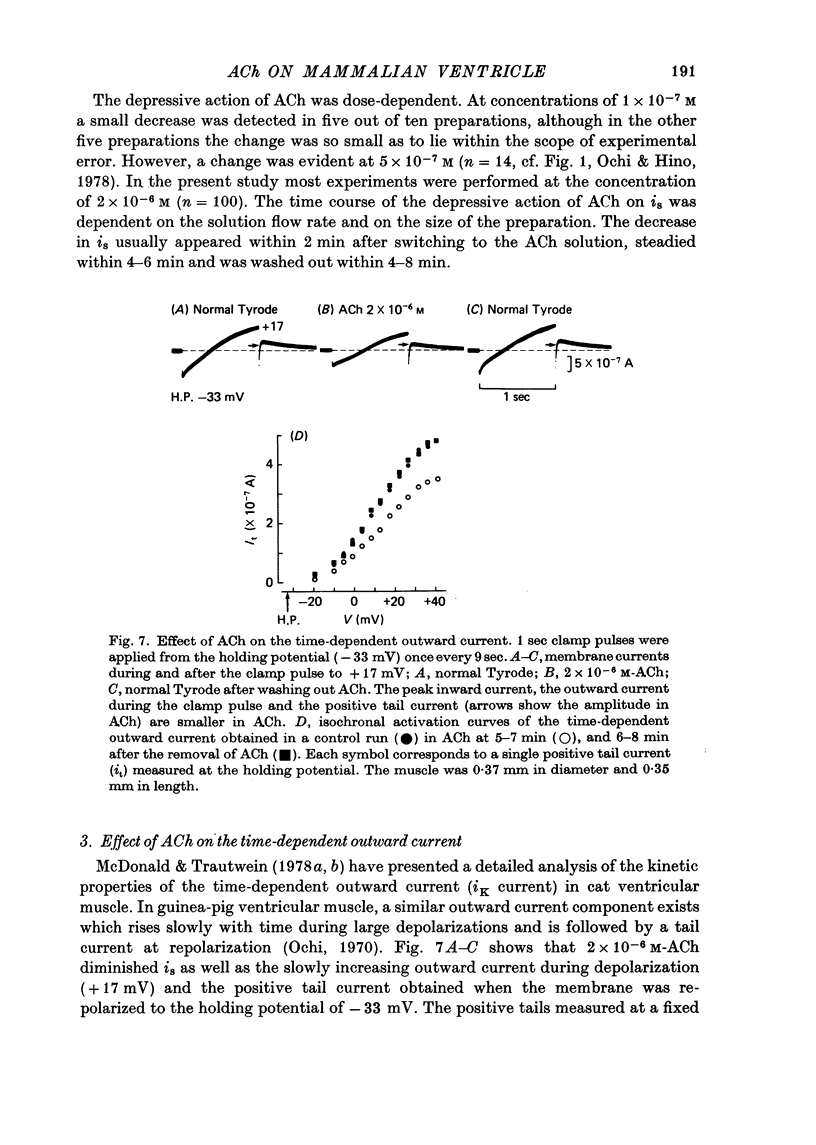
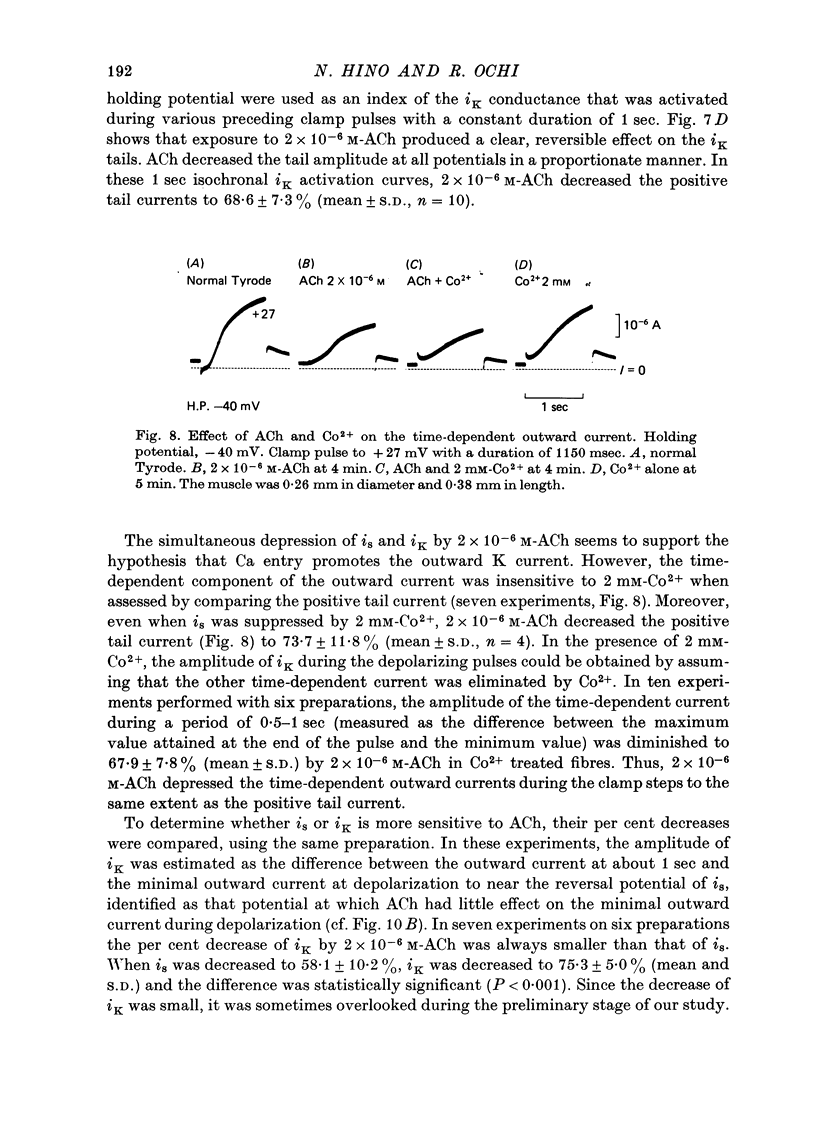
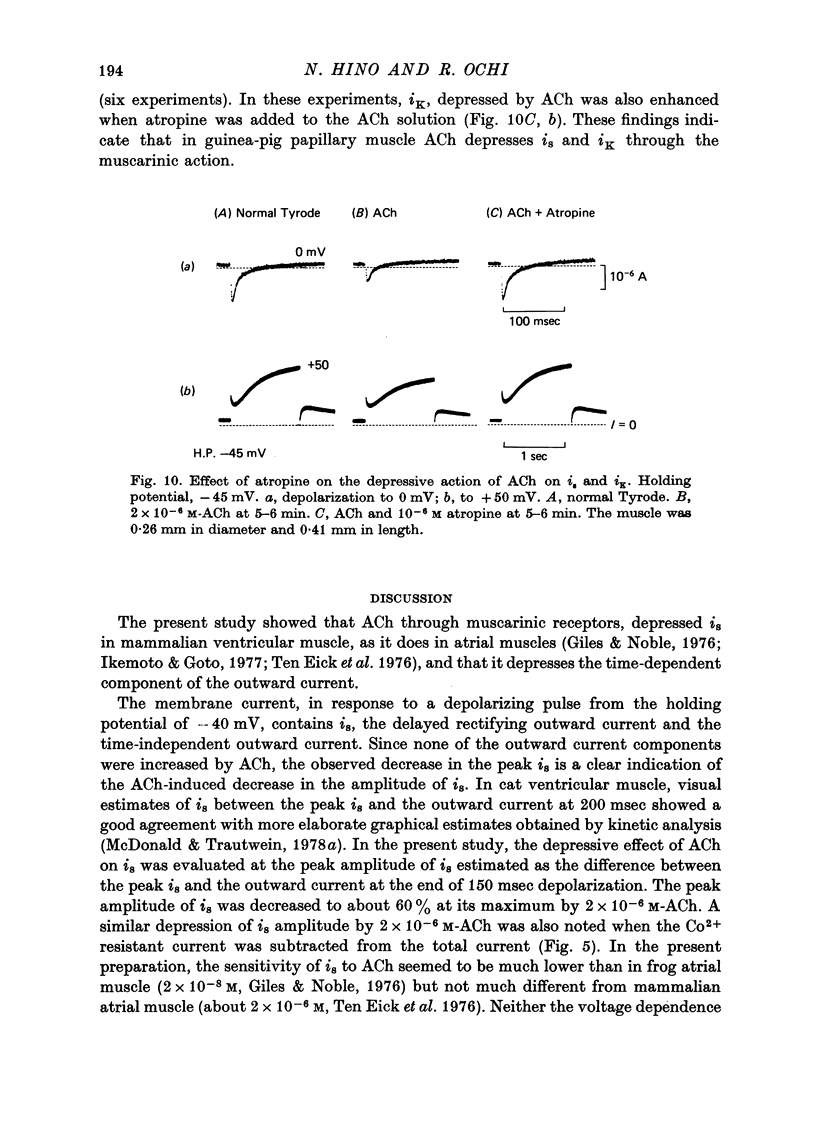
1. The effect of acetylcholine (ACh) on the membrane current components in guinea-pig papillary muscle was studied by the single sucrose gap voltage clamp method. 2. The slow inward current (is) elicited by various depolarizations from the holding potential of -40 mV was evidently diminished by ACh greater than 5 x 10(-7) M. The amplitude of is, estimated as the difference between the peak of is and the current at 150 msec, was reduced to 56.8 +/- 12.9% (mean +/- S.D., n = 34) by 2 x 10(-8) M-ACh when estimated at the membrane potential where the amplitude was maximal. 3. is was suppressed almost completely by 2 mM-Co2+, is obtained by subtracting the current remaining in 2 mM-Co2+ was also diminished to the same extent by 2 x 10(-6) M-Ach with little change in its time course; the degree of decrease was not dependent on the membrane potential. 4. The time-dependent outward current during the depolarizing pulse was also diminished by 2 x 10(-6) M-Ach. Correspondingly, the positive tail current obtained when the membrane was repolarized to the holding potential of -40 mV after various 1 sec depolarizations was reduced in a proportionate manner to 68.6 +/- 7.3% (n = 10). 5. The time-independent inward rectifying outward current was not affected by 2 x 10(-6) M-Ach. 6. Atropine (10(-6) M) restored is and the time-dependent outward current which had been previously depressed by 2 x 10(-6) M-ACh. 7. ACh may decrease tension development by depressing is, thereby preserving the duration of the action potential with relatively little change, since, different from atrial muscle, ACh does not increase the outward current in mammalian ventricular muscle.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey J. C., Watanabe A. M., Besch H. R., Jr, Lathrop D. A. Acetylcholine antagonism of the electrophysiological effects of isoproterenol on canine cardiac Purkinje fibers. Circ Res. 1979 Mar;44(3):378–383. doi: 10.1161/01.res.44.3.378. [DOI] [PubMed] [Google Scholar]
- Gadsby D. C., Wit A. L., Cranefield P. F. The effects of acetylcholine on the electrical activity of canine cardiac Purkinje fibers. Circ Res. 1978 Jul;43(1):29–35. doi: 10.1161/01.res.43.1.29. [DOI] [PubMed] [Google Scholar]
- Garnier D., Nargeot J., Ojeda C., Rougier O. The action of acetylcholine on background conductance in frog atrial trabeculae. J Physiol. 1978 Jan;274:381–396. doi: 10.1113/jphysiol.1978.sp012154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles W., Noble S. J. Changes in membrane currents in bullfrog atrium produced by acetylcholine. J Physiol. 1976 Sep;261(1):103–123. doi: 10.1113/jphysiol.1976.sp011550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFMAN B. F., SUCKLING E. E. Cardiac cellular potentials; effect of vagal stimulation and acetylcholine. Am J Physiol. 1953 May;173(2):312–320. doi: 10.1152/ajplegacy.1953.173.2.312. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. Surface density of calcium ions and calcium spikes in the barnacle muscle fiber membrane. J Gen Physiol. 1967 Jan;50(3):583–601. doi: 10.1085/jgp.50.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto Y., Goto M. Effects of ACh on slow inward current and tension components of the bullfrog atrium. J Mol Cell Cardiol. 1977 Apr;9(4):313–326. doi: 10.1016/s0022-2828(77)80037-0. [DOI] [PubMed] [Google Scholar]
- Inui J., Imamura H. Effects of acetylcholine on calcium-dependent electrical and mechanical responses in the guinea-pig papillary muscle partially depolarized by potassium. Naunyn Schmiedebergs Arch Pharmacol. 1977 Aug;299(1):1–7. doi: 10.1007/BF00508630. [DOI] [PubMed] [Google Scholar]
- Keely S. L., Jr, Lincoln T. M., Corbin J. D. Interaction of acetylcholine and epinephrine on heart cyclic AMP-dependent protein kinase. Am J Physiol. 1978 Apr;234(4):H432–H438. doi: 10.1152/ajpheart.1978.234.4.H432. [DOI] [PubMed] [Google Scholar]
- Kléber A. G. Effects of sucrose solution on the longitudinal tissue resistivity of trabecular muscle from mammalian heart. Pflugers Arch. 1973 Dec 18;345(3):195–205. doi: 10.1007/BF00586334. [DOI] [PubMed] [Google Scholar]
- Maughan D. W. Potassium movement during hyperpolarization of cardiac muscle. J Membr Biol. 1976 Aug 26;28(2-3):241–262. doi: 10.1007/BF01869699. [DOI] [PubMed] [Google Scholar]
- McAllister R. E., Noble D. The time and voltage dependence of the slow outward current in cardiac Purkinje fibres. J Physiol. 1966 Oct;186(3):632–662. doi: 10.1113/jphysiol.1966.sp008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. F., Trautwein W. Membrane currents in cat myocardium: separation of inward and outward components. J Physiol. 1978 Jan;274:193–216. doi: 10.1113/jphysiol.1978.sp012143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. F., Trautwein W. The potassium current underlying delayed rectification in cat ventricular muscle. J Physiol. 1978 Jan;274:217–246. doi: 10.1113/jphysiol.1978.sp012144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New W., Trautwein W. Inward membrane currents in mammalian myocardium. Pflugers Arch. 1972;334(1):1–23. doi: 10.1007/BF00585997. [DOI] [PubMed] [Google Scholar]
- Noma A., Trautwein W. Relaxation of the ACh-induced potassium current in the rabbit sinoatrial node cell. Pflugers Arch. 1978 Nov 30;377(3):193–200. doi: 10.1007/BF00584272. [DOI] [PubMed] [Google Scholar]
- Ochi R., Hashimoto K. The effect of procaine on the passive electrical properties of guinea-pig ventricular muscle. Pflugers Arch. 1978 Dec 15;378(1):1–7. doi: 10.1007/BF00581951. [DOI] [PubMed] [Google Scholar]
- Ochi R. The slow inward current and the action of manganese ions in guinea-pig's myocardium. Pflugers Arch. 1970;316(1):81–94. doi: 10.1007/BF00587898. [DOI] [PubMed] [Google Scholar]
- Reuter H., Scholz H. A study of the ion selectivity and the kinetic properties of the calcium dependent slow inward current in mammalian cardiac muscle. J Physiol. 1977 Jan;264(1):17–47. doi: 10.1113/jphysiol.1977.sp011656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMIDT R. F. Uber die Acetylcholin-Empfindlichkeit verschiedener Herzabschnitte. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1958;233(6):531–540. [PubMed] [Google Scholar]
- Schmid P. G., Greif B. J., Lund D. D., Roskoski R., Jr Regional choline acetyltransferase activity in the guinea pig heart. Circ Res. 1978 May;42(5):657–660. doi: 10.1161/01.res.42.5.657. [DOI] [PubMed] [Google Scholar]
- Simurda J., Simurdova M., Braveny P., Sumbera J. Slow inward current and action potentials of papillary muscles under non-steady state conditions. Pflugers Arch. 1976 Apr 6;362(3):209–218. doi: 10.1007/BF00581172. [DOI] [PubMed] [Google Scholar]
- Ten Eick R., Nawrath H., McDonald T. F., Trautwein W. On the mechanism of the negative inotropic effect of acetylcholine. Pflugers Arch. 1976 Feb 24;361(3):207–213. doi: 10.1007/BF00587284. [DOI] [PubMed] [Google Scholar]
- Trautwein W., McDonald T. F., Tripathi O. Calcium conductance and tension in mammalian ventricular muscle. Pflugers Arch. 1975;354(1):55–74. doi: 10.1007/BF00584503. [DOI] [PubMed] [Google Scholar]
- Watanabe A. M., Besch H. R., Jr Interaction between cyclic adenosine monophosphate and cyclic gunaosine monophosphate in guinea pig ventricular myocardium. Circ Res. 1975 Sep;37(3):309–317. doi: 10.1161/01.res.37.3.309. [DOI] [PubMed] [Google Scholar]


