Abstract
Macrophages (Mφ) play a key role in the pathogenesis of invasive meningococcal infections. The roles of two pattern recognition molecules, the Mφ scavenger receptor (SR-A) and Toll-like receptor 4 (TLR-4), have been investigated using bone marrow culture-derived Mφ (BMMφ). Surprisingly, a comparison of BMMφ from wild-type and SR-A knockout (SR-A−/−) mice showed that nonopsonic phagocytosis of meningococci was mediated almost exclusively via SR-A. Previous studies have demonstrated only a partial involvement of the receptor in the uptake of other bacteria, such as Escherichia coli. Interestingly, we also show that lipopolysaccharide (LPS) was not the ligand for the receptor on these organisms. Further study of the downstream events of SR-A-mediated ingestion of Neisseria meningitidis demonstrated that SR-A was not required for cytokine production. To determine the bacterial and host factors required to stimulate Mφ activation, we examined TLR-4-deficient Mφ from C3H/HeJ mice and LPS-deficient meningococci. TLR-4-deficient cells elaborated reduced amounts of tumor necrosis factor alpha, interleukin-12 (IL-12), and IL-10, even though ingestion via SR-A was unaffected in these cells. Similarly, although there was no change in SR-A-mediated ingestion of LPS-deficient meningococci, the mutant failed to stimulate a Mφ-dependent cytokine response. Thus, we show that Mφ SR-A mediates opsonin-independent uptake of N. meningitidis independently of lipid A and that this activity is uncoupled from the Mφ secretion of proinflammatory cytokines, which provides a basis for further investigation of the role of this receptor in meningococcal disease in humans.
Meningococcal meningitis and septicemia are life-threatening infections, especially in infants. Neisseria meningitidis is a commensal of the nasopharynx but occasionally crosses the epithelial barrier of the respiratory tract and enters the bloodstream, resulting in potentially fatal septicemia and meningitis (1, 39).
Many aspects of the host response to meningococcal infection remain obscure. Most studies have focused on the protective (bactericidal) activity mediated by antibody and complement (26, 45) and the inflammatory responses that result from circulating endotoxin, a correlate of disease severity (1). Large numbers of neutrophils are recruited to sites of acute infection, and epithelial cells in the nasopharynx and choroid plexus may provide barriers to invasion. Bacterial pili and Opa/Opc proteins are required for attachment to these cells (40, 42), and meningococci, sensitive to uptake by neutrophils, are potent activators of leukocytes in vitro (14, 32). However, we know little of factors governing the initial host-microbial interactions which result in clearance, colonization, or spread from the nasopharynx and why some individuals progress to fulminant septicemia.
The potential role of macrophages (Mφ) has hitherto received little attention aside from their proinflammatory response following exposure to meningococcal endotoxin (2, 21, 28). Monocytes are readily mobilized to sites of infection for local activation (8), and resident Mφ are present in the nasopharynx and systemically, including at the blood-cerebrospinal fluid (CSF) interface (9). Meningococci were first observed within Mφ in CSF from patients (3, 18) and have been detected in Mφ-like cells following experimental infection of explanted human rhinopharyngeal mucosa (30). Thus, Mφ may be important during the initial and subsequent interactions between host and meningococci and during dissemination, as well as in the generation of acquired immunity.
Mφ express several surface molecules that aid in recognition of and response to microorganisms, including the pattern recognition molecules (PRM), such as CD14, the toll-like receptors (TLR), and the scavenger receptors (SR), which are able to recognize conserved motifs on pathogen surfaces directly (5, 20, 24). N. meningitidis binds to Mφ in the absence of serum, but the receptors have not been identified (31). CD14 and the class A Mφ SR (SR-A) (24), a trimeric transmembrane glycoprotein, are candidate receptors, as they bind lipopolysaccharide (LPS), either directly or through LPS-binding protein. CD14 is highly expressed on monocytes and down-regulated on tissue Mφ (20, 47), whereas SR-A, present on resident Mφ, is absent on monocytes (10, 16).
In vivo studies have shown that both TLR-4 and SR-A function in defense against pathogens and endotoxic shock, though their roles are contrasting. CD14- and TLR-4-deficient animals are resistant to endotoxic shock and infection by gram-negative organisms (13), supporting their roles in the generation of inflammation. Conversely, SR-A−/− animals have increased susceptibility to gram-positive infection in vivo (34, 37), and Mycobacterium bovis BCG-primed SR-A−/− mice are more sensitive to LPS (12), suggesting a role for SR-A in limiting endotoxemia. Using SR-A−/− mice, it was shown that the direct binding and uptake of various gram-positive and gram-negative organisms are mediated in part by SR-A+/+ Mφ in vitro (25). Therefore, we examined the role of SR-A in nonopsonic phagocytosis of N. meningitidis and cytokine secretion following bacterial contact with Mφ.
We show that N. meningitidis binds to bone marrow culture-derived Mφ (BMMφ) almost exclusively through SR-A. Although meningococcal entry into Mφ resulted in bacterial killing and cytokine production, we were unable to demonstrate any SR-A-mediated contribution to the induction of proinflammatory cytokines. Conversely, TLR-4 mutant Mφ were unable to signal cytokine release, but phagocytosis was unaltered in these cells. Examination of bacterial components required for Mφ cytokine secretion showed that the ligand for SR-A-mediated phagocytosis was not lipid A, although it was required for Mφ activation. Thus, we demonstrate in vitro that ingestion of meningococci by Mφ, mediated by SR-A, can be dissociated from the production of inflammatory cytokines. These findings provide a critical insight upon which to pursue subsequent in vivo studies of the complex interplay of meningococci and host cells in the pathogenesis of invasive N. meningitidis disease.
MATERIALS AND METHODS
Reagents.
Poly(I), poly(C), and Ficoll-Hypaque were obtained from Pharmacia Biotech (St. Albans, United Kingdom). X-VIVO 10 culture medium was from Bio-Whittaker (Reading, United Kingdom), while all other culture media were from Gibco (Paisley, United Kingdom). Rhodamine green X (RdGnX) was obtained from Molecular Probes (Eugene, Oreg.), and Escherichia coli LPS and Luria broth were obtained from Difco Laboratories (West Molesey, United Kingdom). Cytokine detection using flow cytometry kits, brefeldin A, and enzyme-linked immunosorbent assay (ELISA) kits were obtained from PharMingen (San Diego, Calif.), and brain heart infusion broth was from Merck (Poole, United Kingdom). Purified 2F8, rat anti-mouse SR-A monoclonal antibody, has been described previously (6) and was prepared using affinity chromatography. CAMPATH IG, the isotype-matched control of 2F8, was a kind gift from H. Waldmann, Sir William Dunn School of Pathology, Oxford University. Unless stated otherwise, all other chemical reagents were from Sigma (Poole, United Kingdom), and plastic products were from Becton Dickinson Labware (Oxford, United Kingdom).
Animals.
SR-A−/− mice were produced as described previously (35). These cells do not express any of the SR-A isforms. C3H/HeN and C3H/HeJ mice were purchased from Harlan-Olac. SR-A−/−, 129/ICR (wild-type [WT] controls), and C3H/HeN and C3H/HeJ animals of the same sex were used at 8 to 12 weeks of age. All animals used were handled in accordance with guidelines issued by the Home Office, United Kingdom.
Cell isolation and culture.
Mφ were obtained as described previously (25). BMMφ were cultured in RPMI supplemented with 10% fetal calf serum (FCS) (Sigma, Reading, United Kingdom) and 15% L-cell conditioned medium; human cells were cultured in X-VIVO with 2% autologous human serum. CHO K1 cells (a nonphagocytic hamster ovary cell line) were routinely cultured in Ham's F-12 medium supplemented with 10% FCS. CHO cells stably transfected with SR-AII were produced as described previously (10) and maintained in Ham's F-12 medium containing 3% lipoprotein-deficient FCS, 250 μmol of mevalonate/liter, 40 μmol of mevastatin/liter, and 3 μg of acetylated low-density lipoprotein/ml (7). OPTIMEM, a serumless medium, was used for all assays.
Bacterial culture and labeling.
N. meningitidis MC58 (40), 44/76 (15), capsule mutants (MC58 ⊄ 3) (44), 44/76lpxA (38), and the Centers for Disease Control and Prevention reference strains, F8238 and C11, were treated as described previously (40). For fluorescent labeling, N. meningitidis cells were fixed in 70% ethanol and labeled with RdGnX according to the manufacturer's protocol.
Association of ethanol-fixed bacteria with Mφ.
For flow cytometry, Mφ were incubated for 2 h at 37°C with RdGnX-labeled bacteria as described previously (25). As required, cells were preincubated for 30 min with inhibitor, which was retained throughout the assay. Poly(I) and poly(C) were used at 50 μg/ml, cytochalasin D was used at 2 μM, and 2F8 and CAMPATH IG were used at 15 μg/ml. Cells for flow cytometry were washed and harvested with phosphate-buffered saline (PBS) containing 10 mM EDTA and 4 mg of lidocaine-HCl/ml before fixation with 4% formaldehyde. Fluorescence was analyzed on a FACScan (Becton Dickinson, Mountain View, Calif.) using the FL-1 or FL-2 photomultiplier where appropriate, and the results were analyzed with CellQuest software. The mean fluorescence of unloaded control cells was subtracted from the mean fluorescence of each assay condition, and the average was determined. The results are representative of at least three independent experiments. The statistical significance of the results was determined using the paired Student t test, and significance was tested at the 95% confidence level (P ≤ 0.05). The abovementioned assay measures the total association or uptake of bacteria to cells and does not distinguish between binding and ingestion. Attempts to modify the flow cytometric assay to distinguish between binding and internalization have been described elsewhere (25). Cells for fluorescence microscopy were not harvested, but PBS was added to the Mφ and the cells were observed using a Zeiss Axiovert 25 CTL microscope equipped with a 50-W mercury vapor lamp fitted with standard filter sets for fluorescein isothiocyanate and rhodamine fluorescence. The fields chosen for photography were representative of the whole well.
ELISAs and intracellular cytokine assays.
Mφ were incubated with N. meningitidis, and supernatants or cells were tested using cytokine ELISAs or intracellular tumor necrosis factor alpha (TNF-α) assays according to the manufacturer's protocol. One microgram of LPS per milliliter was used as a positive control. Each condition was examined in quadruplicate for cytokine ELISA.
Uptake of live N. meningitidis by Mφ.
Assays were performed as described previously (40, 41). Briefly, bacteria were harvested after 12 to 18 h of growth on brain heart infusion agar plates and resuspended in PBS. To remove large aggregates, the bacteria were centrifuged at 85 × g for 1 min and then counted before addition to Mφ cultures at the required concentration for 2 h at 37°C. Mφ were washed to remove unbound organisms, and the total number of CFU was estimated after addition of saponin (1% final concentration). The number of cell-associated bacteria was determined after correction for attachment to exposed bacteriological plastic, as described previously (40, 41). All bacteria were equally resistant to treatment with 1% saponin. To determine the number of internalized bacteria and to examine their killing by Mφ over time, Mφ were incubated with bacteria as described above and then washed, and fresh culture medium containing 200 μg of gentamicin/ml was added to each well for 1.5 h to eliminate extracellular bacteria. A control for gentamicin killing was included in each assay by incubating bacteria alone with the antibiotic, which killed all the organisms. Following gentamicin treatment, the Mφ were washed and lysed with saponin and the number of CFU was estimated as described above when examining intracellular bacteria only, or the cells were washed and placed in culture medium, and at each time point of a time course they were lysed and the number of bacteria was determined. Each variable was assayed in triplicate, and each experiment was repeated at least three times. The statistical significance of the results was determined using the paired Student t test, and significance was tested at the 95% confidence level (P ≤ 0.05).
EM.
Mφ were plated on 35-mm-diameter bacteriological-plastic petri dishes and incubated with live MC58 bacteria. At various times, cells were fixed for electron microscopy (EM) by standard protocols. Sections were examined on a Zeiss Omega 912 electron microscope (LEO Electron Microscope Ltd., Oberkochen, Germany), and digital images were captured with the integrated Soft Imaging Software (Munster, Germany) image analysis package.
RESULTS
Mφ deficient in SR-A display markedly reduced ingestion of ethanol-fixed N. meningitidis.
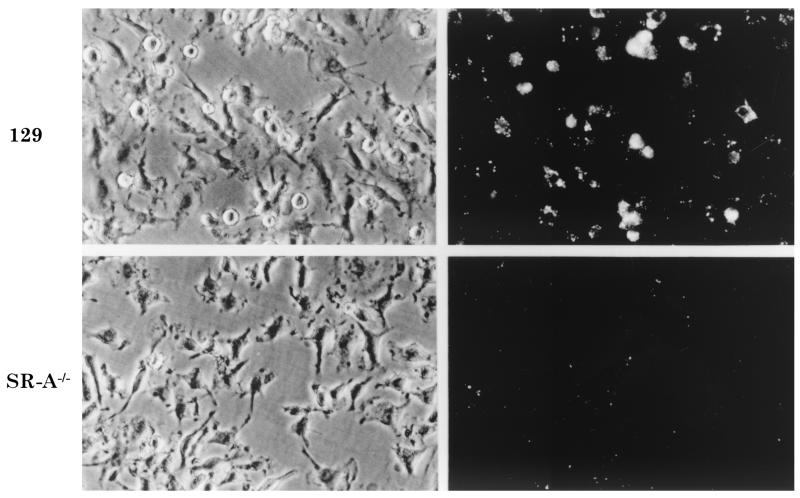
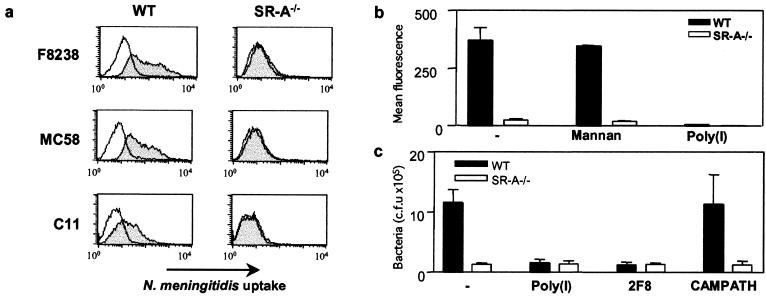
To assess the roles of Mφ and SR-A in interaction with meningococci, we compared bacterial phagocytosis by WT and SR-A−/− BMMφ. Using this method, SR-A-deficient Mφ had an approximately 30 to 55% reduction in the ingestion of E. coli (25) but were still able to internalize organisms through other receptors. WT and SR-A−/− Mφ were incubated with 20 ethanol-fixed RdGnX-labeled N. meningitidis serogroup A (strain F8238) bacteria per cell for 2 h in the absence of serum. Fluorescence microscopy (Fig. 1) and flow cytometry (Fig. 2a) demonstrated that uptake of F8238 by WT Mφ was heterogeneous, but most cells ingested bacteria. In contrast, surprisingly, the binding of meningococci to SR-A−/− BMMφ was minimal. Comparison of fluorescence showed that SR-A−/− Mφ associated with 88 to 94% fewer N. meningitidis bacteria than WT cells (Fig. 2b), even over a range of bacterial doses (not shown). Poly(I) (Fig. 2b), a general SR inhibitor, and 2F8 (not shown), an anti-SR-A monoclonal antibody, blocked N. meningitidis association to WT Mφ, confirming specific binding through SR-A, whereas mannan, an inhibitor of another PRM, the mannose receptor (Fig. 2b), had no effect. The presence of opsonizing serum increased uptake via SR-A−/− Mφ but not to WT levels, and uptake by WT cells was still partially blocked by 2F8 (not shown). EM confirmed the presence of intracellular bacteria within phagosomal membranes (Fig. 3).
FIG. 1.
SR-A−/− Mφ show greatly reduced ability to ingest N. meningitidis. BMMφ from WT or SR-A−/− mice were incubated with ethanol-fixed RdGnX-F8238 (20 bacteria per Mφ) for 2 h at 37°C and analyzed by fluorescence microscopy. Each field depicted is representative of the whole population, and representative fields derived by fluorescence and phase microscopy are shown. Magnification, ×600.
FIG. 2.
Characterization of SR-A-dependent uptake of N. meningitidis by Mφ. (a) Flow cytometry of WT or SR-A−/− Mφ ingestion of ethanol-fixed, RdGnX-F8238, -MC58, or -C11. The shaded line represents Mφ incubated with bacteria, and the open line represents control cells. (b) BMMφ were incubated with 20 ethanol-fixed RdGnX-MC58 bacteria per cell in the presence or absence of inhibitors at 37°C, and the mean fluorescence for each population was determined by flow cytometry. The average mean fluorescence for each condition is shown. (c) BMMφ were incubated with 50 live MC58 bacteria per cell in the presence or absence of SR-A inhibitors at 37°C. The number of bacteria associated with the Mφ was determined by colony assay. The average number of colonies obtained for each condition is shown. The error bars indicate standard deviations.
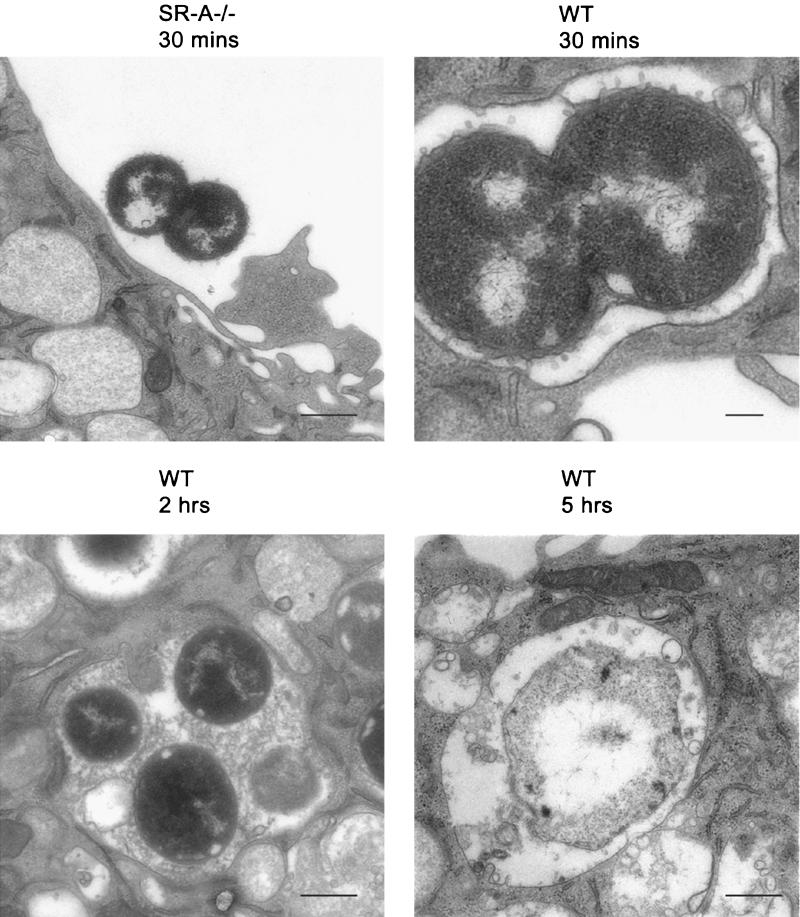
FIG. 3.
EM of N. meningitidis uptake. WT and SR-A−/− BMMφ were incubated for various times with 150 live MC58 bacteria per cell at 37°C. At various intervals, the cells were washed to remove extracellular bacteria before being processed and analyzed by EM. The fields chosen are representative of the whole Mφ population. Bar = 400 nm; upper right panel, bar = 200 nm.
SR-A-mediated uptake was independent of the capsular polysaccharides; serogroup B (MC58), C (C11) (Fig. 2a), and W135 (not shown) strains produced similar results. Further characterization of Mφ recognition of N. meningitidis via SR-A using laboratory-derived or natural mutants showed that the presence or absence of pili, capsule, or Opa or Opc protein did not alter SR-A-mediated binding (not shown). Also, binding was conserved over the genus Neisseria, as Neisseria gonorrhoeae, Neisseria lactamica, Neisseria mucosa, and Neisseria sicca all showed dramatic reductions (85 to 90%) in their associations with SR-A−/− Mφ (not shown) compared with WT cells. We used N. meningitidis strain MC58 in further studies because of the public health importance of serogroup B infections.
We were unable to test the binding of N. meningitidis to human SR-A expressed on Mφ, as specific inhibitors for human SR-A are not available. However, when we examined MC58 binding by all polyanion-sensitive SR in human Mφ, the uptake of MC58 by primary human monocyte-derived Mφ in the presence of poly(I) was blocked 69% ± 9% (not shown) whereas poly(C), a cognate non-SR ligand, had no effect. Also, CHO cells stably transfected with human SR-A associated with significantly more ethanol-fixed RdGnX-labeled MC58 (RdGnX-MC58) bacteria than control untransfected CHO K1 cells. Therefore, SR are also dominant receptors in nonopsonic meningococcal uptake by human Mφ, although they do express other molecules that may bind N. meningitidis, and human SR-A is able to bind to N. meningitidis.
Mφ ingest live N. meningitidis bacteria through SR-A.
To confirm that this specific interaction was not an artifact of bacterial fixation, BMMφ were incubated with 50 live MC58 bacteria per cell for 2 h, and bacterial association was determined by colony counting (Fig. 2c). SR-A−/− Mφ consistently associated with 90% fewer bacteria, including F8238 and C11 (not shown), than did WT Mφ. Poly(I) and 2F8 inhibited binding to WT Mφ, but an isotype-matched control, CAMPATH IG, had no effect (Fig. 2c). Gentamicin was used in assays to measure bacterial internalization (36), and the difference in uptake between WT and SR-A Mφ was maintained (not shown).
N. meningitidis inside phagosomes is killed by Mφ.
Our system provides a good model for the examination of the downstream cellular events following SR-A ligation. It also can provide further insight into the role of Mφ in meningococcal disease, and for this reason, the uptake of live N. meningitidis bacteria by BMMφ from SR-A−/− and WT mice was followed over time using EM. BMMφ were incubated with 150 live MC58 bacteria per cell for various times before fixation. As observed by flow cytometry and fluorescence microscopy, most SR-A−/− Mφ contained no bacteria, although some organisms were detected close to the cell surface as classical diplococci with numerous membrane blebs (Fig. 3, upper left). By 30 min, WT Mφ contained many organisms closely apposed to the phagosome membrane (Fig. 3, upper right), confirming that the organisms were ingested by the cells. Following the ingested bacteria over time, EM revealed that the phagosomes became more spacious with time (Fig. 3, lower left) and by 2 h were filled with amorphous material likely of bacterial origin. Intracellular organisms lacked membrane blebs and now appeared as single bacteria. After 5 h, only bacterial remnants remained in the phagosomes (Fig. 3, lower right), showing that the Mφ are able to kill the ingested MC58. Colony assays confirmed that SR-A−/− Mφ took up fewer living organisms (Table 1).
TABLE 1.
Killing of N. meningitidis by BMMφ
| Timea (h) | No. of CFU (%)b
|
|
|---|---|---|
| WT | SR-A−/− | |
| 2.5 | 431 ± 169 (100) | 76 ± 10* (100) |
| 4.5 | 44 ± 17 (10) | 10 ± 18 (13) |
| 6 | 125 ± 21 (29) | 40 ± 39 (52*) |
Time elapsed from addition of bacteria to wells.
Results show the mean number of bacteria (CFU) per well when 105 Mφ were infected with MC58 bacteria for 1 h, extracellular bacteria were removed by gentamicin and washing, and then the Mφ were lysed over time to determine the number of associated bacteria. The numbers in parentheses are the percentages of bacteria for those times compared with the number of meningococci obtained at the first time point that could be assessed, 2.5 h. Triplicate conditions from one experiment representative of at least three similar assays are shown.
No significant difference (P > 0.05) between WT and SR-A−/− Mφ.
Mφ cytokine production is independent of SR-A.
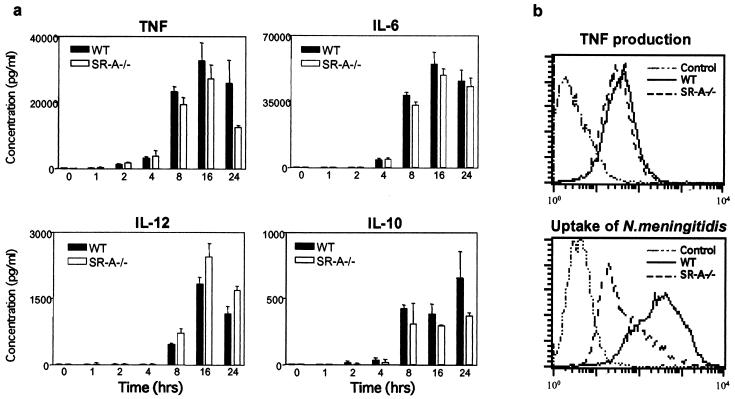
Cytokines commonly produced by Mφ and previously detected in the circulation or CSF of patients infected with N. meningitidis (1) were assayed. TNF-α was detected within 2 h of infection in vitro, peaking at 8 to 16 h (Fig. 4a). Secretion of interleukin-6 (IL-6), IL-12, and IL-10 coincided, or closely followed, that of TNF-α (Fig. 4a). We tested whether one or more factors present in the bacterial culture supernatant were responsible for the induction of cytokines. The bacterial suspension was filtered and centrifuged to remove all particulate matter before incubation with the Mφ for 24 h. Some secretion was observed; however, the amount of cytokines secreted was less than that of cells treated with whole bacteria (not shown).
FIG. 4.
Mφ proinflammatory cytokine secretion following challenge with N. meningitidis. (a) WT and SR-A−/− BMMφ were incubated with ethanol-fixed MC58 bacteria at 37°C. At different times, the culture supernatant was harvested and analyzed by cytokine ELISA for production of TNF-α, IL-6, IL-10, and IL-12. The results are representative of at least two similar experiments, and the average of triplicate conditions is plotted. (b) BMMφ were incubated with ethanol-fixed RdGnX-MC58 (40 bacteria per cell) in the presence of brefeldin A. After incubation, the Mφ were fixed with 4% paraformaldehyde, stained with a phycoerythrin (PE)-labeled anti-TNF-α antibody, and analyzed by flow cytometry. The results are representative of at least two similar experiments. Note that preincubation of the anti-TNF-α antibody with recombinant mouse TNF-α or of the cells with an unlabeled anti-TNF-α antibody blocked the binding of the PE-labeled anti-TNF-α (not shown), confirming specificity. The error bars indicate standard deviations.
Interestingly, comparison of cytokine production by WT and SR-A−/− cells showed that the kinetics of cytokine output were comparable, and cytokine levels were not consistently reduced in SR-A−/− Mφ (Fig. 4a), nor did they correlate with uptake. We therefore examined cytokine production by individual cells using TNF-α as a model cytokine. Mφ were incubated with MC58 in the presence of brefeldin A for 5 h (Fig. 4b). SR-A−/− Mφ did not ingest many bacteria, but most of the cell population produced levels of TNF-α comparable to that of WT cells (Fig. 4b). Similarly, when the BMMφ were incubated with live MC58 bacteria for 5 h, only a small decrease in TNF-α expression by SR-A−/− Mφ was detected after exposure to the live bacteria (not shown). Taken together, these data confirm that to a considerable extent, ingestion via SR-A can be dissociated from the Mφ response to N. meningitidis.
Lipid A is not the ligand for SR-A on N. meningitidis but is required for cytokine induction.
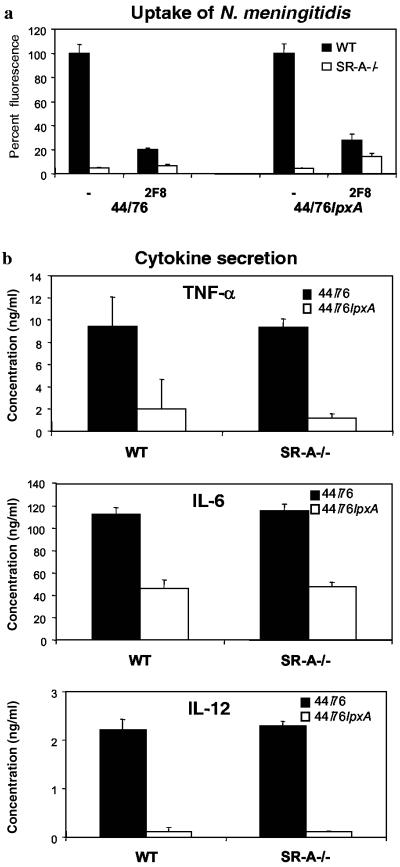
The data described above suggest that ingestion of meningococci via SR-A is not necessary for Mφ activation and that it requires other receptors. We wanted to determine the components required for the induction of the Mφ response to N. meningitidis, both on the cell and on the bacteria. Lipid A is a known activator of Mφ, and we therefore examined a well-characterized mutant strain of N. meningitidis totally lacking LPS (44/76lpxA) (33). Also, the lipid A moiety has been shown to bind SR-A (11) and was the best candidate ligand for the specific binding we observed, especially as the presence or absence of capsule, pili, or Opa and Opc proteins did not alter SR-A-mediated binding of meningococci (see above). Surprisingly, as with the WT strain of N. meningitidis (44/76), SR-A−/− Mφ incubated with 44/76lpxA associated with 96% fewer bacteria than WT Mφ (Fig. 5a), showing that LPS was not responsible for specific SR-A binding in this system. Compared with the control strain of N. meningitidis, more 44/76lpxA organisms were taken up by both Mφ populations and 2F8 blocked most bacterial binding by WT Mφ, while CAMPATH IG had no effect (not shown). Thus, these data show that another ligand(s) exists for SR-A on the bacterial cell surface.
FIG. 5.
Lipid A mutants of N. meningitidis do not induce TNF-α, IL-12, and IL-6 production by WT and SR-A−/− Mφ. (a) WT and SR-A−/− Mφ were incubated with 44/76 or the lipid A mutant (44/76lpxA) in the presence or absence of 2F8 (15 μg/ml). SR-A-mediated ingestion is expressed as a percentage of WT fluorescence. The average fluorescence values for WT and SR-A−/− Mφ incubated with 44/76, 44/76 plus 2F8, lpxA, and lpxA plus 2F8 were (459, 22); (93, 30); (1,050, 44); and (293, 155), respectively. (b) WT and SR-A−/− Mφ were incubated with ethanol-fixed 44/76 or 44/76lpxA bacteria at 37°C. After 24 h, the culture supernatant was removed and centrifuged to remove particulate matter before cytokine ELISA. The average concentration of triplicate conditions is plotted; the results are from a single experiment but are representative of at least three similar assays. Note that cells incubated with culture medium containing PBS or culture medium alone did not stimulate the Mφ to secrete cytokines (not shown). The error bars indicate standard deviations.
To examine the role of lipid A in cytokine secretion, WT and SR-A−/− Mφ were incubated with ethanol-fixed 44/76 or 44/76lpxA bacteria for 24 h before the culture medium was analyzed by ELISA (Fig. 5b). Similar to MC58, 44/76 bacteria induced TNF-α, IL-6, and IL-12 production by WT and SR-A−/− cells. However, the induction of proinflammatory cytokines by the lipid A-deficient mutant, 44/76lpxA, was reduced (Fig. 5b) even over a range of bacterial doses (not shown). TNF-α was decreased by 76 to 87%, and IL-12 was decreased by 89 to 95%. The IL-6 concentration in the culture supernatant was also reduced, although only by 41 to 59%, implying a greater contribution from other surface molecules in IL-6 release.
Mφ require TLR-4 for activation following exposure to N. meningitidis.
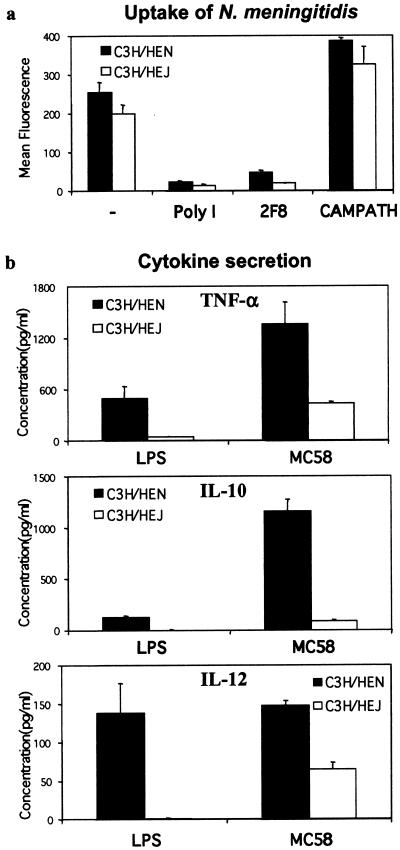
To determine what cellular factors were required for cytokine production following uptake via SR-A, we examined TLR-4-deficient Mφ. It has been shown that TLR-4 is required for proinflammatory cytokine release in response to LPS (23). We followed cytokine production after uptake of N. meningitidis through SR-A by Mφ isolated from C3H/HeN and C3H/HeJ animals (Fig. 6). Analysis of N. meningitidis uptake by C3H/HeN and C3H/HeJ BMMφ using flow cytometry showed that C3H/HeN Mφ associated with more N. meningitidis bacteria than C3H/HeJ cells, but the difference was not significant (Fig. 6a) (P > 0.05). Both 2F8 and poly(I) blocked 80 to 90% of bacterial uptake by both types of Mφ, confirming that the contribution of SR-A to N. meningitidis uptake was maintained in these strains.
FIG. 6.
CH3/HeJ BMMφ produce less cytokine than C3H/HeJ cells following N. meningitidis uptake. (a) BMMφ were incubated with 20 ethanol-fixed RdGnX-MC58 bacteria per Mφ in the presence or absence of poly(I) (50 μg/ml), 2F8 (15 mg/ml), or CAMPATH IG (15 μg/ml) at 37°C for 2 h. The Mφ were then analyzed by flow cytometry, and the average fluorescence for each condition is shown. (b) C3H/HeN and C3H/HeJ Mφ were incubated with E. coli LPS (1 μg/ml) or ethanol-fixed MC58 bacteria at 37°C. After 24 h, the culture supernatant was removed and centrifuged to remove particulate matter before cytokine ELISA. The average concentration of triplicate conditions is plotted; the results are from a single experiment but are representative of at least three similar assays. Note that cells incubated with culture medium alone did not stimulate the Mφ to secrete cytokines (not shown). The error bars indicate standard deviations.
Cytokine production by C3H/HeN and C3H/HeJ BMMφ was examined when the cells were challenged for 24 h with E. coli LPS, as a positive control, or ethanol-fixed MC58. The secretion of TNF-α, IL-10, and IL-12 was measured by ELISA (Fig. 6b). C3H/HeJ Mφ had a greatly reduced response to E. coli LPS. The low levels of TNF-α detected (Fig. 6b) may be due to contamination of the LPS with other bacterial products, such as peptidoglycan, that elicit cytokine responses via other TLRs. Following incubation with MC58, both C3H/HeN and C3H/HeJ Mφ were able to secrete TNF-α, IL-10, and IL-12, but the concentrations produced by C3H/HeJ cells were markedly reduced (Fig. 6b). Incubation with 2F8 to reduce ingestion via SR-A had no effect on cytokine release (not shown). These data show that TLR-4 is a major receptor for cytokine release by Mφ and that ingestion of the bacteria via SR-A is not required for Mφ activation.
DISCUSSION
We have investigated two Mφ PRM, TLR-4 and SR-A, using Mφ obtained from animals selectively deficient for either receptor function. The main findings are that SR-A is a major PRM for N. meningitidis uptake but is not required for cytokine release. Conversely, TLR-4 was not required for meningococcal entry into Mφ, but cells lacking the receptor were unable to secrete high levels of cytokine following exposure to N. meningitidis. Although LPS was required for Mφ cytokine production, it was not the ligand for SR-A. Thus, uptake of meningococci is not essential for cytokine responses, which are, therefore, to a considerable extent independent of SR-A. Our in vitro data provide a firm foundation for further investigation of Mφ in vivo and suggest that Mφ take up N. meningitidis directly via SR-A and may be critical in the innate host immune response to meningococci.
SR-A was the predominant receptor on BMMφ for nonopsonic uptake of meningococci. It was shown previously that the contribution of SR-A to uptake of bacteria varied depending on the strain (25). This suggested that some bacteria might use SR-A almost exclusively while others would be entirely SR-A independent, e.g., various strains of Haemophilus influenzae (L. Peiser and S. Gordon, unpublished data). As our culture system, which includes L-cell conditioned medium containing Mφ colony-stimulating factor, results in up-regulated SR-A expression, it is likely that in other Mφ populations SR-A is not an exclusive receptor for the organism. Indeed, uncharacterized SR in human Mφ contributed approximately 60% of uptake. Other candidate receptors for uptake include molecules reported previously to bind N. meningitidis that are also expressed on Mφ, such as CD46 and CD66 (19, 43). MARCO and CD36 are other candidate SR; however, CD36 does not bind bacteria (24), and MARCO, which binds E. coli and Staphylococcus aureus (4), is normally expressed on only a small subset of Mφ.
The microbial ligand for direct binding of N. meningitidis to SR-A is presently unknown but appears to be remarkably specific for SR-A. Furthermore, the particular requirement for SR-A was conserved over the whole genus Neisseria. Candidate ligands included the negatively charged capsule, pili, LPS, and other outer membrane proteins, but we have found that the presence or absence of capsule, Opa, Opc, or pili did not affect SR-A-dependent uptake. Soluble lipid A moiety has been implicated previously as an SR-A ligand (11) and was the best candidate molecule for SR-A recognition. Lipid A is the active component of endotoxin, and binding to Mφ results in activation and endotoxic shock (29). In intact bacteria, the availability for SR-A binding is unclear, as the buried lipid A may be sterically hindered. However, N. meningitidis has a large number of membrane blebs and outer membrane vesicles that contain outer membrane and lipid A (27), which may also allow access to SR-A for binding. The lpxA mutant of 44/76 engineered by Steeghs and colleagues completely lacks LPS (33), and it was intriguing to find that SR-A recognition of this mutant was unaltered. Preliminary studies of the lpxA mutant surface composition have shown that most major membrane molecules are present but there is increased expression of an Opa protein (33). While further studies are needed to define the actual ligand for SR-A, we can conclude that it is not LPS or a molecule that requires the presence of LPS in the membrane for appropriate expression.
In these studies, the Mφ secretory response to N. meningitidis was uncoupled from cellular ingestion. Survival assays and EM showed that WT Mφ killed most bacteria at early stages of ingestion, and although the level of uptake was dramatically reduced, SR-A−/− Mφ showed a similar pattern of bacterial clearance. The Mφ used here resembled resident, immunologically nonactivated Mφ (8), and although the mode of killing in this system is still unknown, intraphagosomal destruction of N. meningitidis was observed by EM. Similar results were obtained in the presence or absence (not shown) of gentamicin, confirming that the clearance of N. meningitidis was not due to slow killing by the antibiotic, as evidence exists that aminoglycoside antibiotics may not be totally excluded from entering phagocytic cells (22). Mφ possess a number of intra- and extracellular mechanisms capable of destroying pathogens, including the respiratory burst, acid hydrolases, and lysozyme, and further study is required to test their involvement in Mφ-mediated killing of meningococci.
High levels of proinflammatory cytokines can be correlated with meningococcal-disease severity (1, 46), and following contact with N. meningitidis, WT and SR-A−/− cells rapidly produced cytokines. Again, these responses were largely SR-A independent. Intracellular-cytokine assays showed that most WT and SR-A−/− Mφ produced TNF-α irrespective of uptake. SR-A−/− Mφ generally secreted slightly less cytokine, but the difference was variable even over a range of MC58 doses and, in WT Mφ, was not blocked by 2F8 (not shown). Transient surface contacts or shedding of the LPS from the bacterial surface may be responsible for this activation of Mφ signaling. Soluble products in the bacterial preparation were able to elicit cytokines to some extent, and few bacteria were observed within SR-A−/− Mφ, although some extracellular organisms were observed close to the plasma membrane.
We demonstrated that Mφ required the presence of TLR-4 to mediate cytokine secretion. C3H/HeJ Mφ were unable to generate high levels of cytokine following N. meningitidis challenge compared with C3H/HeN controls, but ingestion via the receptor was unaffected. Also, although LPS was not the meningococcal ligand for SR-A, it was required for the induction of proinflammatory cytokines by Mφ. N. meningitidis bacteria lacking LPS were unable to activate WT and SR-A−/− cells, even though ingestion via SR-A was normal. Some TLR-4- and lipid A-independent induction of cytokines was observed, but in most cases it was minor. Interestingly, IL-6 production appeared to have the greatest lipid A-independent component. Previous studies with N. meningitidis lpxA mutants showed that these organisms stimulated proinflammatory cytokine release via TLR-2 (28). The membrane molecules responsible for TLR-4-independent activation have not been identified (17), but candidate molecules include the outer membrane proteins, Opa/Opc and porin, peptidoglycan, and other lipoproteins.
The hallmark of PRM molecules is the ability to recognize conserved motifs on pathogen cell surfaces directly. Although, it is difficult to determine what role these nonopsonic receptors would play in vivo when competing with the opsonic receptors and serum opsonins, animal knockout studies have clearly shown that these receptors, specifically SR-A and TLR-4, are important in other bacterial infections. The significance of our findings remains to be tested in vivo to determine if SR-A is specifically targeted by N. meningitidis to assist in the initial stages of infection before the bacteria become opsonized by complement or antibodies or if this is a Mφ clearance mechanism. Monocytes (as well as neutrophils) lack SR-A, but this receptor is present on most mature tissue Mφ populations (16). Resident Mφ are widely distributed throughout the body, including the nasopharynx, but are also present in the choroid plexus, the liver, and the adrenal gland, where they are in direct contact with CSF and blood (9). They are therefore well placed for clearance and localization of circulating organisms and LPS via SR-A. The survival of bacteria following uptake by resident Mφ may facilitate their dissemination. Once Mφ become primed by immune activation, SR-A may protect the host from LPS-induced septic shock, mediated by SR-A-independent Mφ receptors (12). However, the pathogenesis of meningococcal infection cannot be readily studied in murine models, as N. meningitidis is not a natural pathogen of mice and is quickly cleared when mice are infected without the presence of large doses of iron. We showed that human SR-A transfected in CHO cells was able to bind N. meningitidis, and thus examination of the relative roles of SR-A, other SR, and non-SR in human subjects may provide clues to the variation in susceptibility to the disease and its serious complications.
Acknowledgments
We thank P. van der Ley and L. Steeghs, National Institute of Public Health and the Environment, Bilthoven, The Netherlands, for the kind gift of the 44/76lpxA mutant strain.
L.P. is supported by a Goodger Scholarship, and work in the laboratories of S.G. and E.R.M. is supported by grants from the Medical Research Council, United Kingdom, and the Wellcome Trust. Facilities for microscopy were provided by a grant from the Wellcome Trust.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Brandtzaeg, P. 1995. Pathogenesis of meningococcal infections, p. 71-114. In K. Cartwright (ed.), Meningococcal disease. John Wiley, Chichester, United Kingdom.
- 2.Brandtzaeg, P., L. Osnes, R. Ovstebo, G. B. Joo, A. B. Westvik, and P. Kierulf. 1996. Net inflammatory capacity of human septic shock plasma evaluated by a monocyte-based target cell assay: identification of interleukin-10 as a major functional deactivator of human monocytes. J. Exp Med. 184:51-60. (Erratum, 184:2075.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cartwright, K. 1995. Introduction and historical aspects, p. 1-20. In K. Cartwright (ed.), Meningococcal disease. John Wiley and Sons, Chichester, United Kingdom.
- 4.Elomaa, O., M. Kangas, C. Sahlberg, J. Tuukkanen, R. Sormunen, A. Liakka, I. Thesleff, G. Kraal, and K. Tryggvason. 1995. Cloning of a novel bacteria-binding receptor structurally related to scavenger receptors and expressed in a subset of macrophages. Cell 80:603-609. [DOI] [PubMed] [Google Scholar]
- 5.Fitzgerald, K. A., and L. A. J. O'Neill. 2000. The role of the interleukin-1/Toll-like receptor family in inflammation and host defence. Microb. Infect. 2:933-943. [DOI] [PubMed] [Google Scholar]
- 6.Fraser, I., D. Hughes, and S. Gordon. 1993. Divalent cation-independent macrophage adhesion inhibited by monoclonal antibody to murine scavenger receptor. Nature 364:343-346. [DOI] [PubMed] [Google Scholar]
- 7.Freeman, M., Y. Ekkel, L. Rohrer, M. Penman, N. J. Freedman, G. M. Chisolm, and M. Krieger. 1991. Expression of type I and type II bovine scavenger receptors in Chinese hamster ovary cells: lipid droplet accumulation and nonreciprocal cross competition by acetylated and oxidized low density lipoprotein. Proc. Natl. Acad. Sci. USA 88:4931-4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon, S. 1999. Macrophages and the immune response, p. 533-545. In W. Paul (ed.), Fundamental immunology, 4th ed. Lippincott Raven Publishers, Philadelphia, Pa.
- 9.Gordon, S., L. Lawson, S. Rabinowitz, P. R. Crocker, L. Morris, and V. H. Perry. 1992. Antigen markers of macrophage differentiation in murine tissues, p. 1-37. In S. Russell and S. Gordon (ed.), Macrophage biology and activation, vol. 181. Springer-Verlag, Berlin, Germany. [DOI] [PubMed]
- 10.Gough, P. J., D. R. Greaves, H. Suzuki, T. Hakkinen, M. O. Hiltunen, M. Turunen, S. Y. Herttuala, T. Kodama, and S. Gordon. 1999. Analysis of macrophage scavenger receptor (SR-A) expression in human aortic atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 19:461-471. [DOI] [PubMed] [Google Scholar]
- 11.Hampton, R. Y., D. T. Golenbock, M. Penman, M. Krieger, and C. R. Raetz. 1991. Recognition and plasma clearance of endotoxin by scavenger receptors. Nature 352:342-344. [DOI] [PubMed] [Google Scholar]
- 12.Haworth, R., N. Platt, S. Keshav, D. Hughes, E. Darley, H. Suzuki, Y. Kurihara, T. Kodama, and S. Gordon. 1997. The macrophage scavenger receptor type A is expressed by activated macrophages and protects the host against lethal endotoxic shock. J. Exp. Med. 186:1431-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haziot, A., E. Ferrero, F. Kontgen, N. Hijiya, S. Yamamoto, J. Silver, C. L. Stewart, and S. M. Goyert. 1996. Resistance to endotoxin shock and reduced dissemination of gram-negative bacteria in CD14-deficient mice. Immunity 4:407-414. [DOI] [PubMed] [Google Scholar]
- 14.Heyderman, R. S., C. A. Ison, M. Peakman, M. Levin, and N. J. Klein. 1999. Neutrophil response to Neisseria meningitidis: inhibition of adhesion molecule expression and phagocytosis by recombinant bactericidal/permeability-increasing protein (rBPI21). J. Infect. Dis. 179:1288-1292. [DOI] [PubMed] [Google Scholar]
- 15.Holten, E. 1979. Serotypes of Neisseria meningitidis isolated from patients in Norway during the first six months of 1978. J. Clin. Microbiol. 9:186-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes, D. A., I. P. Fraser, and S. Gordon. 1995. Murine macrophage scavenger receptor: in vivo expression and function as receptor for macrophage adhesion in lymphoid and non-lymphoid organs. Eur J. Immunol. 25:466-473. [DOI] [PubMed] [Google Scholar]
- 17.Ingalls, R. R., E. Lien, and D. T. Golenbock. 2001. Membrane-associated proteins of a lipopolysaccharide-deficient mutant of Neisseria meningitidis activate the inflammatory response through toll-like receptor 2. Infect. Immun. 69:2230-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaeger, H. 1895. Zur aetiologie der meningitis cerebrospinalis epidemica. Zeitschr. Hyg. Infect. 19:351-370. [Google Scholar]
- 19.Kallstrom, H., M. K. Liszewski, J. P. Atkinson, and A. B. Jonsson. 1997. Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic Neisseria. Mol. Microbiol. 25:639-647. [DOI] [PubMed] [Google Scholar]
- 20.Landmann, R., B. Muller, and W. Zimmerli. 2000. CD14, new aspects of ligand and signal diversity. Microbes Infect. 2:295-304. [DOI] [PubMed] [Google Scholar]
- 21.Lien, E., N. B. Liabakk, A. C. Johnsen, U. Nonstad, A. Sundan, and T. Espevik. 1995. Polymorphonuclear granulocytes enhance lipopolysaccharide-induced soluble p75 tumor necrosis factor receptor release from mononuclear cells. Eur. J. Immunol. 25:2714-2717. [DOI] [PubMed] [Google Scholar]
- 22.Mandell, G. L. 1973. Interactions of intraleukocytic bacteria with antibiotics. J. Clin. Investig. 52:1673-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muzio, M., and A. Mantovani. 2000. Toll-like receptors. Microbes Infect. 2:251-255. [DOI] [PubMed] [Google Scholar]
- 24.Peiser, L., and S. Gordon. 2001. The function of scavenger receptors expressed by macrophages and their role in the regulation of inflammation. Microbes Infect. 3:149-159. [DOI] [PubMed] [Google Scholar]
- 25.Peiser, L., P. J. Gough, T. Kodama, and S. Gordon. 2000. Macrophage class A scavenger receptor-mediated phagocytosis of Escherichia coli: role of cell heterogeneity, microbial strain, and culture conditions in vitro. Infect. Immun. 68:1953-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pollard, A. J., and C. Frasch. 2001. Development of natural immunity to Neisseria meningitidis. Vaccine 19:1327-1346. [DOI] [PubMed] [Google Scholar]
- 27.Poolman, J. T., P. A. van der Ley, and J. Tommassen. 1995. Surface structures and secreted products of meningococci, p. 21-34. In K. Cartwright (ed.), Meningococcal disease. John Wiley, Chichester, United Kingdom.
- 28.Pridmore, A. C., D. H. Wyllie, F. Abdillahi, L. Steeghs, P. van der Ley, S. K. Dower, and R. C. Read. 2000. A lipopolysaccharide-deficient mutant of Neisseria meningitidis elicits attenuated cytokine release by human macrophages and signals via Toll-like receptor (TLR) 2 but not via TLR4/MD2. J. Infect. Dis. 183:89-96. [DOI] [PubMed] [Google Scholar]
- 29.Raetz, C. R. H. 1990. Biochemistry of endotoxins. Annu. Rev. Biochem. 59:129-170. [DOI] [PubMed] [Google Scholar]
- 30.Read, R. C., A. Fox, K. Miller, T. Gray, N. Jones, R. Borrows, D. M. Jones, and R. G. Finch. 1995. Experimental infection of human nasal mucosal explants with Neisseria meningitidis. J. Med. Microbiol. 42:353-361. [DOI] [PubMed] [Google Scholar]
- 31.Read, R. C., S. Zimmerli, C. Broaddus, D. A. Sanan, D. S. Stephens, and J. D. Ernst. 1996. The (α2→8)-linked polysialic acid capsule of group B Neisseria meningitidis modifies multiple steps during interaction with human macrophages. Infect. Immun. 64:3210-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ross, S. C., P. J. Rosenthal, H. M. Berberich, and P. Densen. 1987. Killing of Neisseria meningitidis by human neutrophils: implications for normal and complement-deficient individuals. J. Infect. Dis. 155:1266-1275. [DOI] [PubMed] [Google Scholar]
- 33.Steeghs, L., R. den Hartog, A. den Boer, B. Zomer, P. Roholl, and P. van der Ley. 1998. Meningitis bacterium is viable without endotoxin. Nature 392:449-450. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki, H., Y. Kurihara, M. Takeya, et al. 1997. The multiple roles of macrophage scavenger receptors (MSR) in vivo: resistance to atherosclerosis and susceptibility to infection in MSR knockout mice. J. Atheroscler. Thromb. 4:1-11. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki, H., Y. Kurihara, M. Takeya, et al. 1997. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature 386:292-296. [DOI] [PubMed] [Google Scholar]
- 36.Tabrizi, S. N., and R. M. Robins-Browne. 1993. Elimination of extracellular bacteria by antibiotics in quantitative assays of bacterial ingestion and killing by phagocytes. J. Immunol. Methods 158:201-206. [DOI] [PubMed] [Google Scholar]
- 37.Thomas, C. A., Y. Li, T. Kodama, H. Suzuki, S. C. Silverstein, and J. El Khoury. 2000. Protection from lethal gram-positive infection by macrophage scavenger receptor-dependent phagocytosis. J. Exp. Med. 191:147-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Ley, P., M. Kramer, L. Steeghs, et al. 1996. Identification of a locus involved in meningococcal lipopolysaccharide biosynthesis by deletion mutagenesis. Mol. Microbiol. 19:1117-1125. [DOI] [PubMed] [Google Scholar]
- 39.van Deuren, M., P. Brandtzaeg, and J. W. van der Meer. 2000. Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin. Microbiol. Rev. 13:144-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Virji, M., H. Kayhty, D. J. Ferguson, C. Alexandrescu, J. E. Heckels, and E. R. Moxon. 1991. The role of pili in the interactions of pathogenic Neisseria with cultured human endothelial cells. Mol. Microbiol. 5:1831-1841. [DOI] [PubMed] [Google Scholar]
- 41.Virji, M., H. Kayhty, D. J. Ferguson, C. Alexandrescu, and E. R. Moxon. 1991. Interactions of Haemophilus influenzae with cultured human endothelial cells. Microb. Pathog. 10:231-245. [DOI] [PubMed] [Google Scholar]
- 42.Virji, M., K. Makepeace, D. J. Ferguson, et al. 1993. Meningococcal Opa and Opc proteins: their role in colonization and invasion of human epithelial and endothelial cells. Mol. Microbiol. 10:499-510. [DOI] [PubMed] [Google Scholar]
- 43.Virji, M., K. Makepeace, D. J. Ferguson, and S. M. Watt. 1996. Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for Opa proteins of pathogenic Neisseriae. Mol. Microbiol. 22:941-950. [DOI] [PubMed] [Google Scholar]
- 44.Virji, M., K. Makepeace, I. R. Peak, D. J. Ferguson, M. P. Jennings, and E. R. Moxon. 1995. Opc- and pilus-dependent interactions of meningococci with human endothelial cells: molecular mechanisms and modulation by surface polysaccharides. Mol. Microbiol. 18:741-754. [DOI] [PubMed] [Google Scholar]
- 45.Vogel, U., and M. Frosch. 1999. Mechanisms of neisserial serum resistance. Mol. Microbiol. 32:1133-1139. [DOI] [PubMed] [Google Scholar]
- 46.Waage, A., A. Halstensen, and T. Espevik. 1987. Association between tumour necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet i:355-357. [DOI] [PubMed]
- 47.Ziegler-Heitbrock, H. W., and R. J. Ulevitch. 1993. CD14: cell surface receptor and differentiation marker. Immunol. Today 14:121-125. [DOI] [PubMed] [Google Scholar]