Abstract
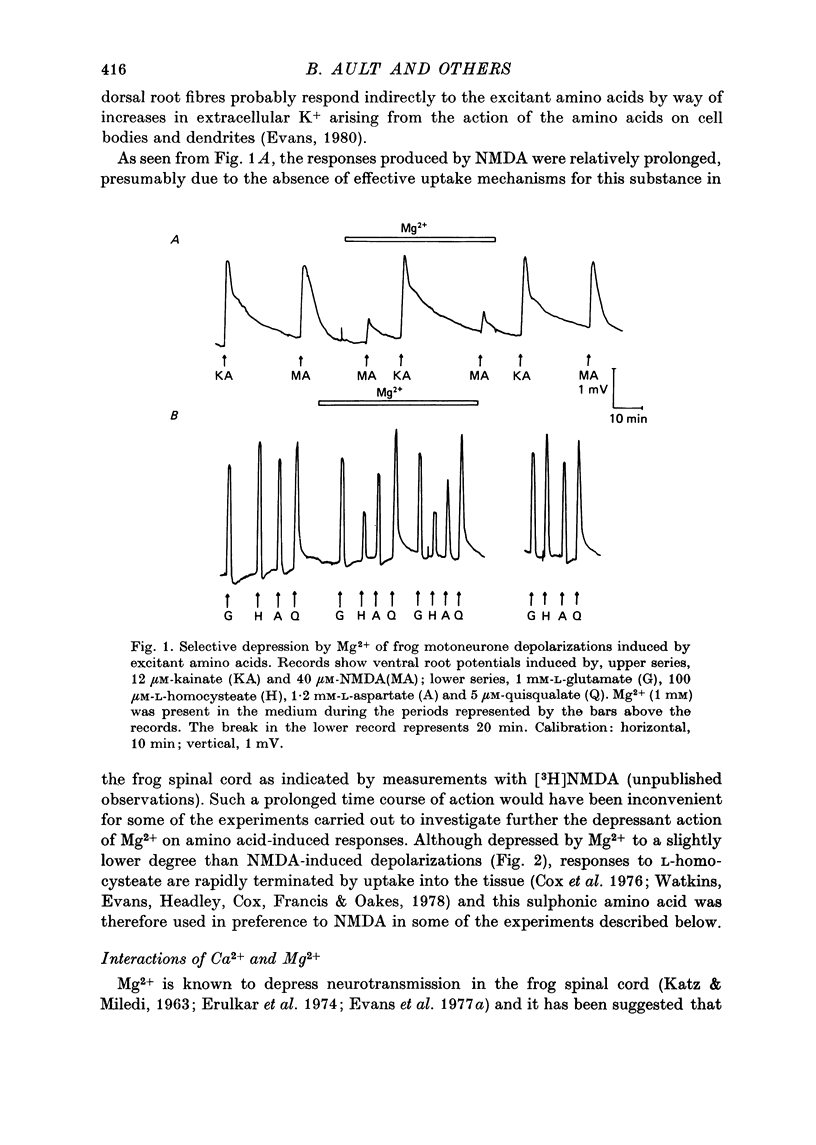
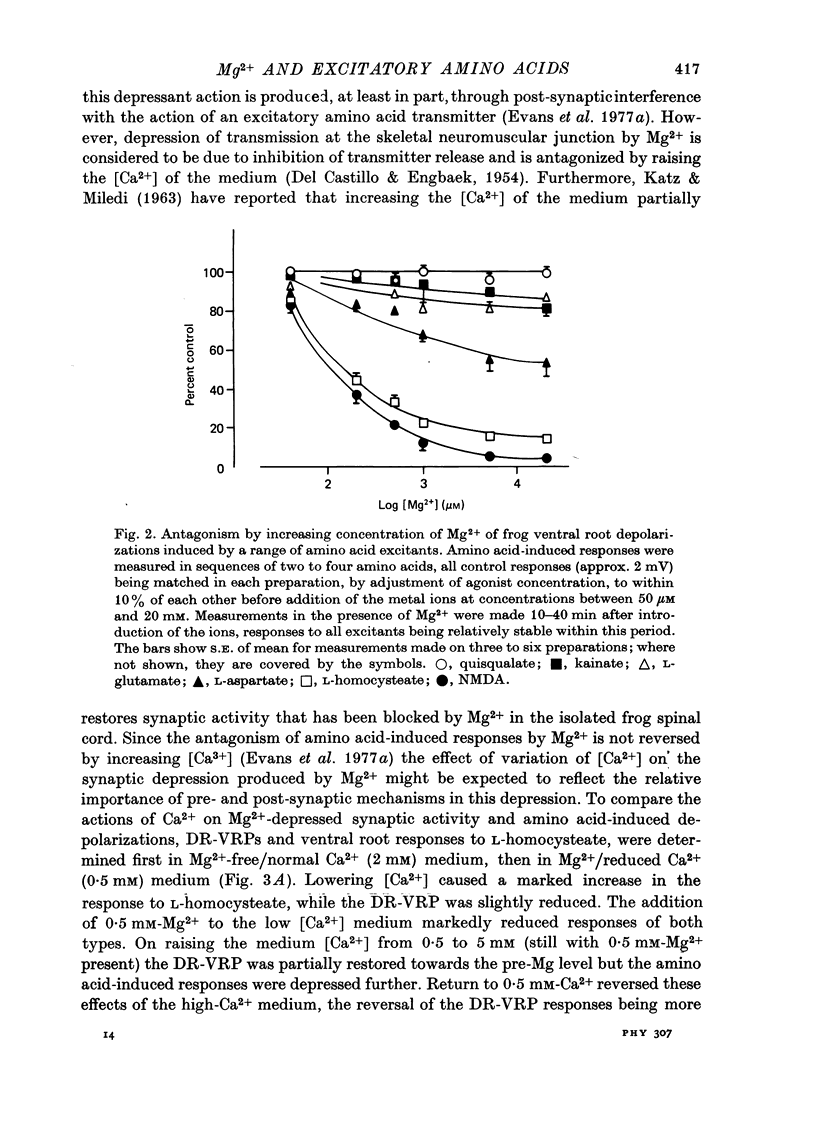
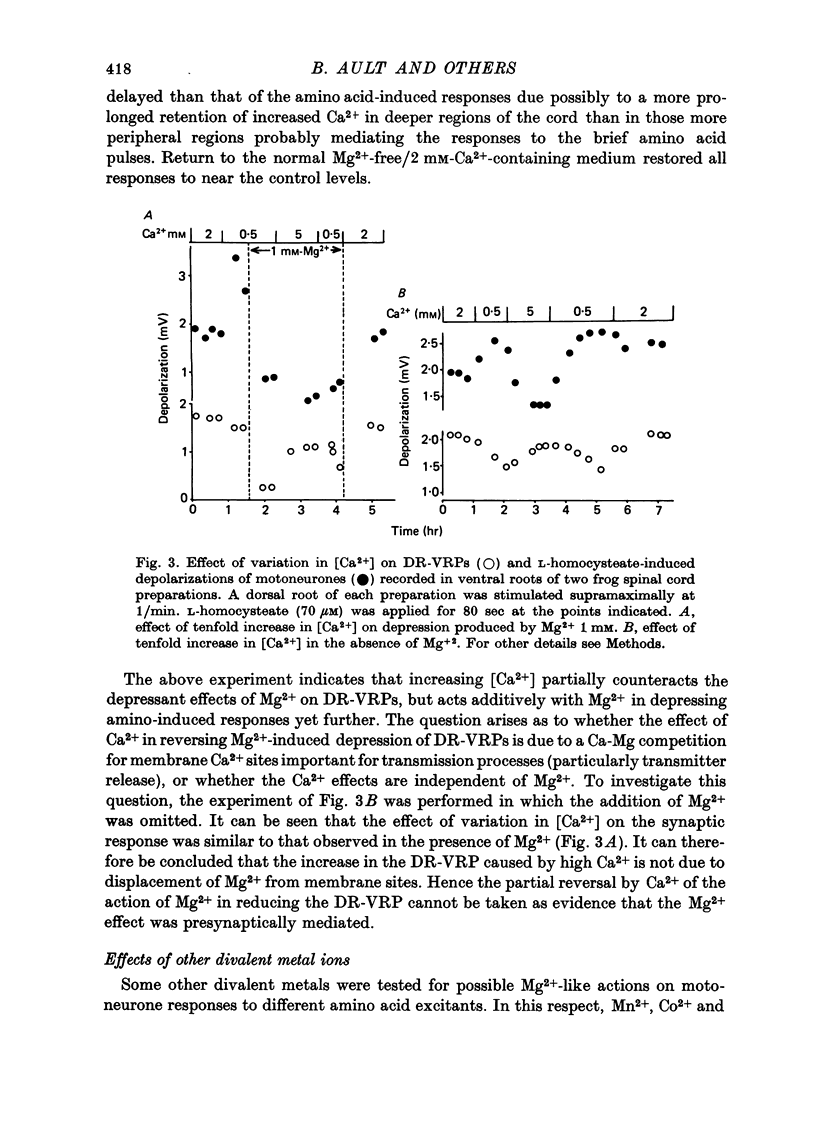
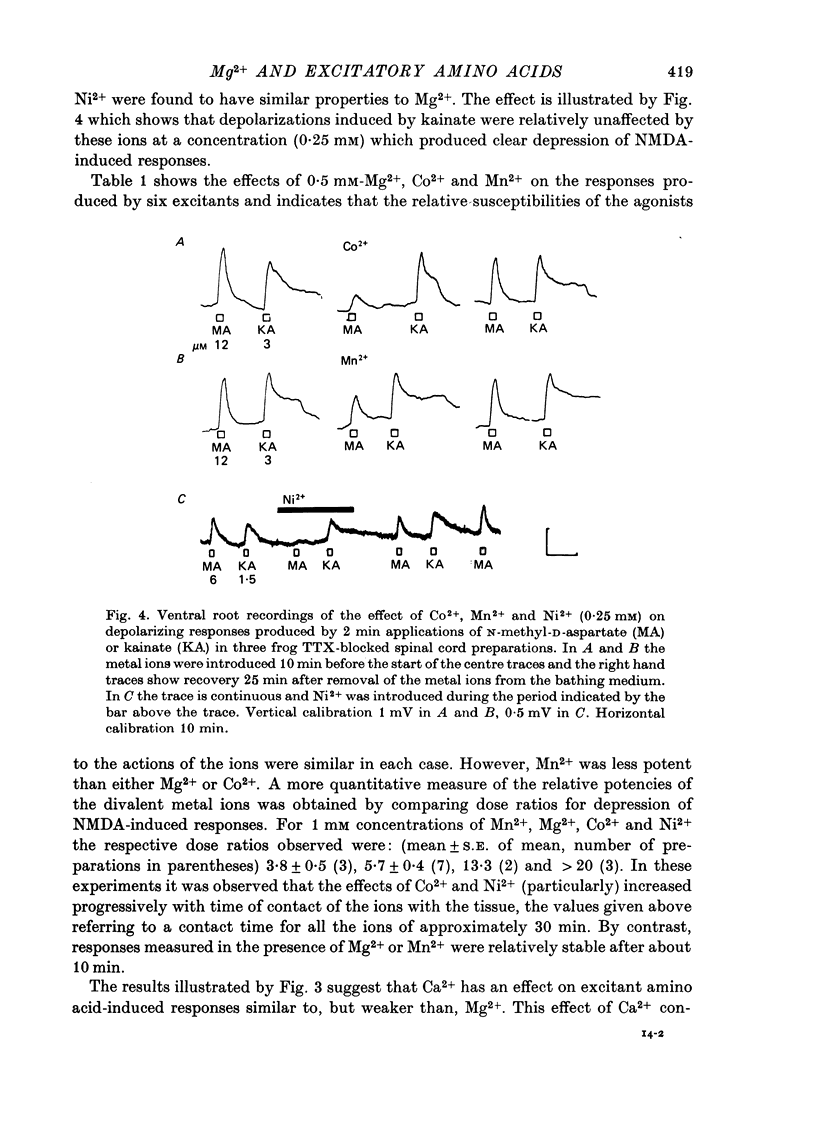
1. The depressant actions of Mg2+ and a range of other divalent ions on synaptic excitation and on responses produced by excitatory amino acids and other putative transmitters have been investigated in hemisected isolated spinal cords of frogs and neonatal rats. Some comparative studies were also made using the rat isolated superior cervical ganglion. 2. At concentrations above 10 microM, Mg2+ selectively antagonized N-methyl-D-aspartate (NMDA)-induced motoneurone depolarization as recorded from ventral roots of tetrodotoxin-blocked spinal cords. Depolarization evoked by quisqualate (unaffected by 20 mM-Mg2+) was resistant to the depressant action of these ions, while depolarizations evoked by other excitant amino acids were depressed to intermediate degrees. 3. Mn2+, Co2+ and Ni2+ had qualitatively similar actions to Mg2+; Mn2+ was somewhat less potent and Co2+ and Ni2+ more potent than Mg2+. The alkaline earth metal ions, Ca2+, Sr2+ and Ba2+, had very weak Mg2+-like actions. Ca2+ and Mg2+ acted additively in depressing amino acid-induced responses. 4. Mg2+ also depressed motoneurone responses evoked by noradrenaline, substance P and carbachol in the neonatal rat isolated spinal cord. However, none of these effects were as marked as the depression of NMDA-induced responses by Mg2+ in this preparation. Mg2+ did not depress motoneurone depolarization produced by 5-HT in the rat spinal cord or the depolarizing action of GABA on primary afferent terminals of the isolated frog spinal cord. 5. At concentrations producing marked depression of NMDA-induced responses, Mg2+ also depressed synaptic transmission in spinal cords in the absence of an effect on ganglionic transmission. At the same concentrations, Mn2+, Co2+ and Ni2+ depressed synaptic transmission in both preparations. 6. From the similarity in action between Mg2+ and the D-alpha-aminoadipate group of NMDA antagonists, it is suggested that the central depressant action of low concentrations of Mg2+ involves predominantly a postsynaptically mediated interference with the action of an excitatory amino acid transmitter.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez-Leefmans F. J., De Santis A., Miledi R. Effects of some divalent cations on synaptic transmission in frog spinal neurones. J Physiol. 1979 Sep;294:387–406. doi: 10.1113/jphysiol.1979.sp012936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., Nicoll R. A., Padjen A. Studies on convulsants in the isolated frog spinal cord. I. Antagonism of amino acid responses. J Physiol. 1975 Mar;245(3):521–536. doi: 10.1113/jphysiol.1975.sp010859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Davies J., Dray A., Evans R. H., Martin M. R., Watkins J. C. D-alpha-aminoadipate, alpha, epsilon-diominopimelic acid and HA-966 as antagonists of amino acid-induced and synpatic excitation of mammalian spinal neurones in vivo. Brain Res. 1978 Jun 16;148(2):543–548. doi: 10.1016/0006-8993(78)90745-x. [DOI] [PubMed] [Google Scholar]
- CURTIS D. R., PERRIN D. D., WATKINS J. C. The excitation of spinal neurones by the ionophoretic application of agents which chelate calcium. J Neurochem. 1960 Aug;6:1–20. doi: 10.1111/j.1471-4159.1960.tb13443.x. [DOI] [PubMed] [Google Scholar]
- Cox D. W., Headley M. H., Watkins J. C. Actions of L- and D-homocysteate in rat CNS: a correlation between low-affinity uptake and the time courses of excitation by microelectrophoretically applied L-glutamate analogues. J Neurochem. 1977 Sep;29(3):579–588. doi: 10.1111/j.1471-4159.1977.tb10707.x. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., ENGBAEK L. The nature of the neuromuscular block produced by magnesium. J Physiol. 1954 May 28;124(2):370–384. doi: 10.1113/jphysiol.1954.sp005114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Effect of magnesium ions on the responses of spinal neurones to excitatory amino acids and acetylcholine. Brain Res. 1977 Jul 15;130(2):364–368. doi: 10.1016/0006-8993(77)90284-0. [DOI] [PubMed] [Google Scholar]
- Evans R. H. Cholinoceptive properties of motoneurons of the immature rat spinal cord maintained in vitro. Neuropharmacology. 1978 Apr-May;17(4-5):277–279. doi: 10.1016/0028-3908(78)90112-0. [DOI] [PubMed] [Google Scholar]
- Evans R. H. Evidence supporting the indirect depolarization of primary afferent terminals in the frog by excitatory amino acids. J Physiol. 1980 Jan;298:25–35. doi: 10.1113/jphysiol.1980.sp013064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. H., Francis A. A., Hunt K., Oakes D. J., Watkins J. C. Antagonism of excitatory amino acid-induced responses and of synaptic excitation in the isolated spinal cord of the frog. Br J Pharmacol. 1979 Dec;67(4):591–603. doi: 10.1111/j.1476-5381.1979.tb08706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. H., Francis A. A., Watkins J. C. Effects of monovalent cations on the responses of motoneurones to different groups to amino acid excitants in frog and rat spinal cord. Experientia. 1977 Feb 15;33(2):246–248. doi: 10.1007/BF02124092. [DOI] [PubMed] [Google Scholar]
- Evans R. H., Francis A. A., Watkins J. C. Mg2+-like selective antagonism of excitatory amino acid-induced responses by alpha, epsilon-diaminopimelic acid, D-alpha-aminoadipate and HA-966 in isolated spinal cord of frog and immature rat. Brain Res. 1978 Jun 16;148(2):536–542. doi: 10.1016/0006-8993(78)90744-8. [DOI] [PubMed] [Google Scholar]
- Evans R. H., Francis A. A., Watkins J. C. Selective antagonism by Mg2+ of amino acid-induced depolarization of frog and rat spinal neurones [proceedings]. J Physiol. 1977 Feb;265(1):42P–43P. [PubMed] [Google Scholar]
- Evans R. H. The effects of amino acids and antagonists on the isolated hemisected spinal cord of the immature rat. Br J Pharmacol. 1978 Feb;62(2):171–176. doi: 10.1111/j.1476-5381.1978.tb08442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. H., Watkins J. C. Dual sites for antagonism of excitatory amino acid actions on central neurones [proceedings]. J Physiol. 1978 Apr;277:57P–57P. [PubMed] [Google Scholar]
- Evans R. H., Watkins J. C. Specific antagonism of excitant amino acids in the isolated spinal cord of the neonatal rat. Eur J Pharmacol. 1978 Jul 15;50(2):123–129. doi: 10.1016/0014-2999(78)90007-9. [DOI] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. A STUDY OF SPONTANEOUS MINIATURE POTENTIALS IN SPINAL MOTONEURONES. J Physiol. 1963 Sep;168:389–422. doi: 10.1113/jphysiol.1963.sp007199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRNJEVIC K., PHILLIS J. W. Iontophoretic studies of neurones in the mammalian cerebral cortex. J Physiol. 1963 Feb;165:274–304. doi: 10.1113/jphysiol.1963.sp007057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato G., Kelly J. S., Krnjević K., Somjen G. Anaesthetic action of magnesium ions. Can Anaesth Soc J. 1968 Nov;15(6):539–544. doi: 10.1007/BF03004350. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. S., Krnjević K., Somjen G. Divalent cations and electrical properties of cortical cells. J Neurobiol. 1969;1(2):197–208. doi: 10.1002/neu.480010207. [DOI] [PubMed] [Google Scholar]
- Kita H., Van der Kloot W. Action of Co and Ni at the frog neuromuscular junction. Nat New Biol. 1973 Sep 12;245(141):52–53. doi: 10.1038/newbio245052a0. [DOI] [PubMed] [Google Scholar]
- Konishi S., Otsuka M. Excitatory action of hypothalamic substance P on spinal motoneurones of newborn rats. Nature. 1974 Dec 20;252(5485):734–735. doi: 10.1038/252734a0. [DOI] [PubMed] [Google Scholar]
- Meiri U., Rahamimoff R. Neuromuscular transmission: inhibition by manganese ions. Science. 1972 Apr 21;176(4032):308–309. doi: 10.1126/science.176.4032.308. [DOI] [PubMed] [Google Scholar]
- Otsuka M., Konishi S. Electrophysiology of mammalian spinal cord in vitro. Nature. 1974 Dec 20;252(5485):733–734. doi: 10.1038/252733a0. [DOI] [PubMed] [Google Scholar]
- Rozear M., DeGroof R., Somjen G. Effects of micro-iontophoretic administration of divalent metal ions on neurons of the central nervous system of cats. J Pharmacol Exp Ther. 1971 Jan;176(1):109–118. [PubMed] [Google Scholar]
- Rubin R. P. The role of calcium in the release of neurotransmitter substances and hormones. Pharmacol Rev. 1970 Sep;22(3):389–428. [PubMed] [Google Scholar]
- Shapovalov A. I., Shiriaev B. I., Velumian A. A. Mechanisms of post-synaptic excitation in amphibian motoneurones. J Physiol. 1978 Jun;279:437–455. doi: 10.1113/jphysiol.1978.sp012355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATKINS J. C. THE SYNTHESIS OF SOME ACIDIC AMINO ACIDS POSSESSING NEUROPHARMACOLOGICAL ACTIVITY. J Med Pharm Chem. 1962 Nov;91:1187–1199. doi: 10.1021/jm01241a010. [DOI] [PubMed] [Google Scholar]
- Weakly J. N. The action of cobalt ions on neuromuscular transmission in the frog. J Physiol. 1973 Nov;234(3):597–612. doi: 10.1113/jphysiol.1973.sp010363. [DOI] [PMC free article] [PubMed] [Google Scholar]


