Abstract
Background:
Previous studies of inflammation in allergic rhinitis using nasal irrigation have been unsatisfactory because of 1) poor reproducibility; 2) the tendency of irrigation to overdilute mediators; and 3) the failure of this technique to evaluate interstitial concentrations of relevant mediators. For this study we used filter paper as a matrix to collect nasal secretions in patients undergoing nasal antigen challenge.
Objective:
To evaluate inflammatory mediators of allergen-induced rhinitis during a clinical trial of fexofenadine.
Methods:
Subjects evaluated at a referral medical center were placed on traditional dosing of fexofenadine at 60 mg, twice daily, or placebo in a double-blind, crossover fashion for 1 week before the nasal challenge. Nasal challenge was performed with nasal insufflation of either 1,000 AU timothy or 0.1 mL ragweed (1:100 wt/vol) extract outside the pollen season. Nasal secretions were collected at baseline and then at 2, 4, and 6 hours after nasal challenge. Secretions were evaluated for expression of the cellular adhesion molecule-1, tumor necrosis factor (TNF)-α, interleukin (IL)-4, IL-10, macrophage inflammatory protein (MIP)-1α, and granulocyte-macrophage colony-stimulating factor (GM-CSF) using commercially available enzyme-linked immunoadsorbent assay kits. Patients’ symptom scores were evaluated during the nasal challenge.
Results:
Significantly (P < 0.05) increased peak levels of TNF-α, IL-4, IL-10, and MIP-1α were detected after antigen challenge as compared with baseline levels. There was a nonsignificant trend toward an increase in GM-CSF after antigen challenge (P = 0.07). There was no difference in the peak levels of TNF-α, IL-4, IL-10, MIP-1α, or GM-CSF measured when patients were on fexofenadine versus placebo. Finally, there were no significant differences in patients’ symptom scores during antigen challenge when subjects were on fexofenadine versus placebo.
Conclusions:
Collection of nasal secretions using a filter paper matrix provides a reproducible model for accurately detecting and evaluating changes in cytokine levels after nasal challenge. Cytokine levels tend to peak 3 to 4 hours after antigen challenge. Standard doses of fexofenadine do not seem to have a mitigating effect on the production of these cytokines. Symptoms of allergic rhinitis using this type of antigen challenge did not differ from treatment with fexofenadine versus placebo.
INTRODUCTION
The typical early or immediate phase allergic response reflects the interaction of allergen with immunoglobulin (Ig)E on the surface of mast cells, inducing the release of preformed biochemical mediators (eg, histamine, tryptase) and rapidly generated mediators of inflammation (leukotrienes, prostaglandins). Histamine has a variety of clinically important effects associated with the allergic response, including rhinorrhea, mucosal edema, neutrophil and eosinophil chemotactic effects, and increased permeability of the bronchial epithelium and vascular endothelium. In conjunction with this immediate response, many patients also demonstrate a late inflammatory-phase of reactivity, both upon natural exposure, as would occur during a typical allergen season, or upon exposure via nasal provocation. The late-phase reaction results from the production of newly synthesized protein mediators by mast cells (cytokines and chemokines) and from activation by allergen of inflammatory cells including T-helper lymphocytes, mononuclear cells, and others. This late-phase response is accompanied by the release of a variety of cytokines and chemokines, including interleukin (IL)-1β, IL-5, IL-6, IL-8, granulocyte-macrophage colony-stimulating factor (GM-CSF), tumor necrosis factor (TNF)-α, macrophage inflammatory protein (MIP)-1α, and RANTES, and the recruitment of eosinophils, basophils, neutrophils, and other inflammatory cells. Antihistamines, particularly the newer nonse-dating molecules, have antiallergy, mast cell-stabilizing properties in addition to their ability to act as histamine H1 receptor antagonists.1,2 These antiallergy effects have been identified in skin, nasal, lung, and ocular challenge studies.1,3,4 Inhibition of inflammatory mediator release by mast cells, including cytokines and chemokines, may be predicted to reduce inflammation, although this has not been satisfactorily proven.
Nasal provocation tests are a useful investigative method to not only induce acute clinical symptoms of sneezing, rhinorrhea, and nasal congestion, but also to evaluate the immune response by measurement of mediators in nasal secretions.5,6 After nasal challenge, terfenadine was reported to decrease or inhibit histamine, kinins, mast cell tryptase, cysteinyl leukotrienes, and albumin in nasal fluid.3,7 Terfenadine had a significant protective effect on the early-phase cellular and clinical events of conjunctival reaction induced by conjunctival provocation testing.8 Terfenadine also reduced the late bronchoconstriction responses to inhaled allergens or to exercise in allergic asthmatic patients.9 Terfenadine and other H1 receptor antagonists, including loratadine and cetirizine, have been reported to modulate the expression of the cellular adhesion molecules (sICAM),10–13 inflammatory cell influx,12–15 and cytokine production.16
The purpose of this study was to examine the effects of twice-daily fexofenadine (60 mg) on the late-phase nasal reaction after nasal allergen provocation. Our primary interest was the effect of fexofenadine on the appearance of proinflammatory cytokines and chemokines in the nasal secretions during the late-phase nasal response. The effect of nasal challenge after fexofenadine and placebo were evaluated for the following cytokines: IL-4, TNF-α, MIP-1α, IL-10, and GM-CSF, and for sICAM-1.
METHODS
Patient Selection
Ten patients between the ages of 18 to 65 years of age were selected for the study. Females were of non-childbearing potential or using appropriate contraception and had negative urinary β-human chorionic gonadotropin before entry into the study. Subjects were selected that had at least a 2-year history of seasonal allergic rhinitis during the grass or ragweed pollen seasons but were currently asymptomatic. Subjects with perennial allergic rhinitis were excluded from the study. All subjects demonstrated a positive percutaneous allergen skin test response (≥4-mm wheal) to ragweed (1/10 wt/ vol) or timothy (100,000 BAU/mL) extract. Patients were excluded from the study if they had a history of an upper respiratory infection or acute sinusitis within 30 days before the screening visit, had current symptoms of rhinitis, had a history of smoking in the past year, had received immunotherapy in the past 3 years, or had used any of the following drugs within the specified period of time: parenteral corticosteroids within 90 days; oral corticosteroids within 30 days; nasal or inhaled corticosteroids within 14 days; nasal or inhaled nedocromil or cromolyn sodium within 14 days; β blockers within 14 days; loratadine, cetirizine, fexofenadine, and other antihistamines (oral, ophthalmic, inhaled) within 7 days; anticholinergic agents within 3 days; tricyclic antidepressants within 14 days; antipsychotics within 14 days; oral decongestants, decongestant nasal sprays or drops, including all over-the-counter preparations (cough/cold preparations and sleep aids) within 3 days; nasal decongestants within 2 days; or H2 blockers within 1 day.
Design
This randomized, double-blind, placebo-controlled, crossover study was conducted outside the patient’s pollen season, January through February. After the screening visit all patients received either fexofenadine or placebo, which they took for 7 days. At the end of the week they were challenged with the appropriate antigen as described below. After the challenge they underwent a 1-week washout period and were then crossed over to the other medication. They were challenged again in identical fashion at the end of another 7 days of medication. The order of the treatment regiments before the challenges were randomized to mitigate the small possibility that the initial exposure may have had a “priming” influence on the subsequent challenge.
Medication
Patients were supplied with either fexofenadine 60-mg tablets or identical placebo tablets which were placed in bottles labeled only “A” or “B.” Bottles were collected at each visit and remaining pills were counted to ensure compliance.
Nasal Challenge
Nasal challenges and sample collections were adapted from the methodology of Alam et al.17 Nasal provocation tests were performed at the end of each treatment period beginning 2 hours after the ingestion of the last dose of that treatment group. Initially, the nares were lavaged three times with normal saline solution to decrease the preexisting level of mediators in the nasal secretions. To mitigate the development of mucosal edema after the allergen challenge and assist with subsequent specimen collections, 0.1 mL oxymetazoline (0.05%) was applied to each nostril. Subjects were then challenged with the diluent for the allergen extract (phenol-buffered saline) to establish the level of nonspecific reactivity. This was followed by bilateral insufflation of appropriate pollen allergen extract into each nostril (1,000 AU timothy [Hollister-Stier, Spokane, WA] and 0.01 mL ragweed 1:10 wt/vol [Hollister-Stier]). Nasal secretion collection was performed bilaterally with filter paper strips (7 × 30 mm Whatman No. 42, Whatman, Clifton, NJ). Three filter paper strips were sequentially placed on each anterior portion of the inferior turbinate for a total of six filter paper strips. The filter paper strips were left in place for 10 minutes. Filter paper strips were placed 15 minutes after diluent challenge and 2, 4, and 6 hours after antigen challenge. Filter paper strips were air dried after challenge then stored at −86° C until assays were performed. Sneezes, symptoms of nasal obstruction, rhinorrhea, and pruritus were recorded hourly using a scoring system summarized in Table 1. Filter paper strips were cut into small pieces and then eluted overnight in a cold room. Elution buffer consisted of 0.1 M Tris, pH 7.4 with 0.3% human serum albumin, 0.01% sodium azide, and 0.002% Tween.
Table 1.
Nasal Symptom Scoring
| Sneezing (15 minutes) | |
| 3–4 sneezes | 1 |
| 5 or more | 2 |
| Nasal blockage | |
| Patient can only breathe with difficulty | 1 |
| One nostril blocked | 2 |
| Both nostrils blocked | 3 |
| Rhinorrhea | |
| Minor anterior | 1 |
| Posterior | 2 |
| Anterior and posterior | 3 |
| Pruritus | |
| Nose | 1 |
| Palate and/or ear | 2 |
Assays
All assays were performed using commercial enzyme-linked immunoadsorbent assay (ELISA) kits with IL-4, TNF-α, MIP-1α, IL-10, and GM-CSF ELISA from Intergen (Purchase, NY) and sICAM-1 from R&D Systems (Minneapolis, MN) performed according to the manufacturer’s directions. ELISAs were performed on an EL808 Microplate Reader (Bio-Tex Instruments, Winooski, VT). The Intergen cytokine kits were ultrasensitive competitive ELISAs designed to identify cytokines in the presence of soluble receptors, naturally occurring anticytokine antibodies or other cytokine-binding proteins. The sensitivity of these kits was: IL-4, <2 pg/mL; TNF-α, <5 pg/mL; MIP-1α, 9.10 pg/mL; IL-10, <3 pg/mL; and GM-CSF, <2.0 pg/ mL. Sensitivity of the sICAM-1 ELISA was <0.35 ng/mL.
Statistics
Data are presented as mean ± SE of the replicate samples. Each subject was challenged in a crossover fashion after administration of placebo and control. Paired t test statistical analyses were performed on a Macintosh G4 computer using JMP 3.2 software (Cary, NC).
RESULTS
Allergen Challenge
All subjects were completely asymptomatic at the time of each allergen challenge. Allergen challenges were associated with dramatic induction of nasal symptoms in all subjects. Using our symptom score system (Table 1), subjects developed an average peak symptom score of 6.0 ± 0.5 of a possible maximum score of 10 (Fig 1). Symptoms peaked at 15 minutes and gradually improved through the 6 hours of study, without a distinct recurrent late-phase response. At 6 hours the average total symptom score was 3.2 ± 0.6.
Figure 1.
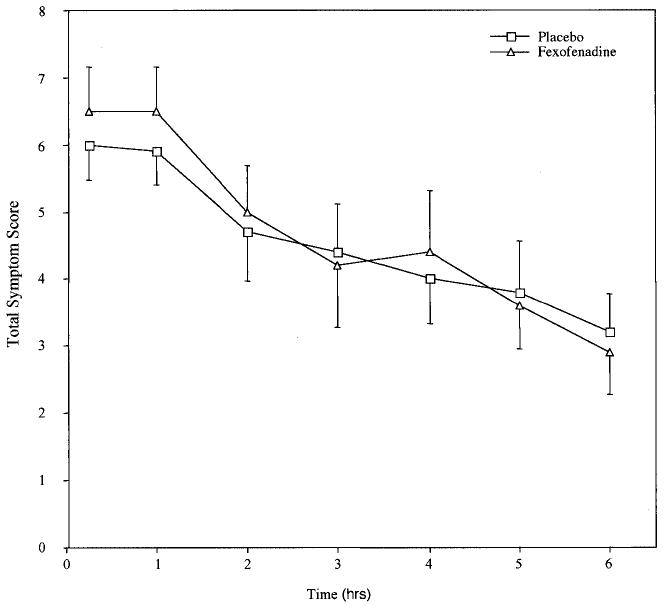
Nasal symptom scores after allergen challenge with and without pretreatment with fexofenadine. Subjects received either placebo or fexofenadine for 7 days at the end of which period they underwent a nasal allergen challenge with ragweed or timothy. Symptoms were scored for 6 hours after the challenge as described. After a 1-week washout period, subjects were crossed over to the other medication and a second identical challenge was performed at the end of another 7 days of medication.
Cytokine production
Significantly increased peak levels of TNF-α (1.2 ± 0.2 ng at baseline to 1.9 0.4 after allergen challenge; P = 0.02), IL-4 (8.1 ± 1.1 ng to 12.6 ± 2.3; P = 0.01), IL-10 (2.8 ± 0.5 ng to 4.8 ± 1.0; P = 0.02), and MIP-1α (8.1 ± 1.8 ng to 11.2 ± 1.7; P = 0.05) were detected after antigen challenge as compared with baseline levels (Fig 2a–e). There was a nonsignificant trend toward an increase in GM-CSF after antigen challenge (12.7 ± 1.8 ng to 14.0 ± 1.7; P = 0.07). Soluble ICAM-1 could not be detected in the nasal secretions.
Figure 2.
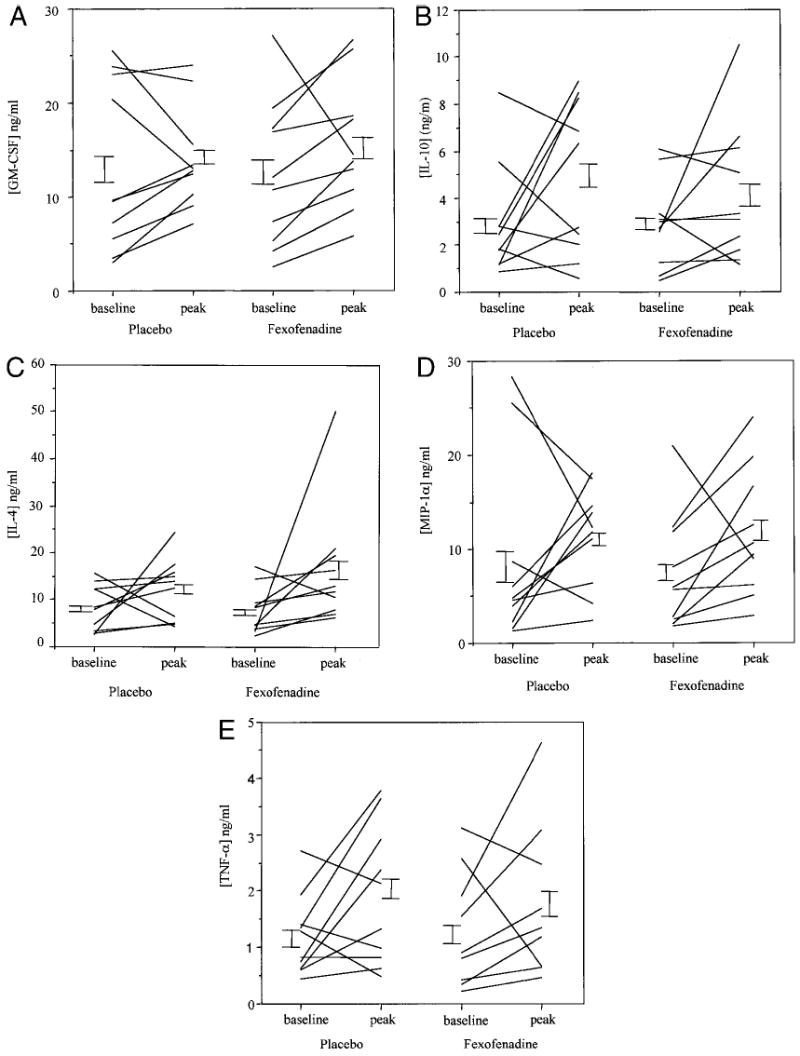
Nasal cytokine concentrations after allergen challenge with and without pretreatment with fexofenadine. After the nasal allergen challenges, nasal secretions were collected with filter paper strips placed on the inferior turbinate at baseline and at 2, 4, and 6 hours. Cytokines were eluted from the filter paper and concentrations determined by ELISA. Data represent baseline and peak cytokine concentrations. Figure 2a: GM-CSF, 2b: IL-10, 2c: IL-4, 2d: MIP-1α, 2e: TNF-α.
Fexofenadine
Fexofenadine had no influence on concentrations of cytokines or chemokines measured in the baseline samples. There was no difference in the peak levels of any of the cytokines measured while patients were on fexofenadine versus placebo (TNF-α, 1.9 ± 0.4 ng on placebo and 1.8 ± 0.4 after fexofenadine), IL-4 (12.6 ± 2.3 ng and 16.4 ± 4.1), IL-10 (4.8 ± 1.0 ng and 4.1 ± 0.9), MIP-1α (11.2 ± 1.7 ng and 11.6 ± 2.1), or GM-CSF (14.0 ± 1.7 ng and 15.5 ± 2.2; Fig 2a–e). The average time after nasal challenge to peak levels of TNF-α, IL-4, IL-10, MIP-1α, and GM-CSF were respectively 3.4, 3.1, 3.7, 3.1, and 3.4 hours; this was not influenced by the use of fexofenadine. Finally, there were no significant differences in patients’ symptom scores during antigen challenge while subjects were on fexofenadine versus placebo (Fig 1). Mean peak symptom scores were 6.0 ± 0.5 on placebo and 6.5 ± 0.6 while taking fexofenadine. Fexofenadine had no influence on severity of symptoms, time to onset of symptoms, or the duration of symptoms.
DISCUSSION
The objective of our study was to evaluate inflammatory mediators of allergen-induced rhinitis during a clinical trial of fexofenadine. Fexofenadine (Allegra, Aventis, Bridgewater, NJ) is a nonsedating H1 antihistamine that has been approved for the relief of symptoms of seasonal allergic rhinitis. Fexofenadine is the active carboxylic acid metabolite of terfenadine and has terfenadine’s H1 receptor blocking properties, but does not have the rare cardiovascular effects (increased QT intervals) associated with terfenadine. Adverse event rates reported with fexofenadine are similar to placebo. Approximately 6,000 subjects have received fexofenadine as an investigational drug in clinical trials. The overall data indicate that fexofenadine is safe and well tolerated across the dose range studied (10 to 800 mg in single-dose studies, and 20 to 690 mg, twice daily, in chronic-dose studies).18–22
Inhibition of H1 receptors is responsible for the ability of these antagonists to block symptoms of rhinorrhea, sneezing, and pruritus. However, antihistamines have antiallergy, mast cell-stabilizing activities. Inhibition of release of cysteinyl leukotrienes and cytokines by mast cells may be predicted to produce anti-inflammatory effects; however, this has never been satisfactorily demonstrated. Our study was unable to find any anti-inflammatory influences of fexofenadine on cytokine or chemokine production after a nasal allergen challenge. Our data are in contrast to previous studies that have suggested anti-inflammatory influences of antihistamines in allergic rhinitis.1,2 However, these studies, as well as other studies evaluating inflammation in allergic rhinitis, may be unsatisfactory because of their use of nasal irrigation. Nasal lavage has proven unsatisfactory because of its poor reproducibility, the tendency of the irrigation to overdilute mediators to below the threshold of many of the assays, and the failure of this technique to evaluate interstitial concentrations of relevant mediators. The application of filter paper on the surface of the inferior turbinate allows the paper to “absorb” mediators from the underlying interstitium. This is in contrast to lavage-derived samples which collect mediators that have been “flushed” into the nares as part of an allergic reaction. Such secretions represent a combination of mediators released from leaky vasculature, nasal glands, and goblet cells. Thus, studies dependent on irrigation are more likely to reflect influences of antihistamines on these secretory processes and may not represent interstitial inflammatory processes. The consistent reproducibility of the data derived from subjects on placebo and on fexofenadine for each of our measured parameters in the face of our conclusion that antihistamines have no influence on cytokine production is an important finding that validates our technique for performing nasal challenges and evaluating inflammation.
An alternative explanation for our negative data regarding fexofenadine is that H1 receptor antagonists vary in their mast cell-stabilizing effectiveness and, as such, a different antihistamine may have shown anti-inflammatory efficacy. For example, cetirizine and ketotifen seem to have a greater antiallergenic effect in the skin than in the nose.1,2,13,23 Using the nasal challenge model, cetirizine did not reduce the concentration of antigen-induced histamine relative to placebo.23 In this same model terfenadine significantly lowered concentrations of antigen-induced histamine and tosyl arginine methyl ester-esterase in lavage fluid, but loratadine did not.3
In contrast to previous studies,10–12 we were unable to measure sICAM-1 in nasal secretions. This may also reflect the differences in mediators eluted out of tissue using our filter paper technique as opposed to those present in secretions flushed into the nasal cavities. It is plausible that sICAM-1 is not widely present in inflamed interstitial spaces.
A surprising result from this study was the lack of effectiveness of fexofenadine on nasal symptom scores. As part of our experimental design we challenged all subjects with identical doses of allergen to normalize as much as possible the intensity of the subsequent inflammatory response and to optimize our ability to induce cytokine production. Our concern was that if we titrated allergen challenges to severity of symptoms, the inherent variability in such a subjective index would attenuate the inflammatory response in subjects more subjectively affected by their symptoms. A supraphysiologic dose of allergen was required in these studies to elicit measurable quantities of cytokines in nasal cytokines. The disadvantage of our approach was that all of our subjects developed an extremely intense response, which may have contributed to the failure of fexofenadine to demonstrate efficacy in attenuating symptom scores. Fexofenadine may have shown clinical efficacy at a lower dose of allergen. For example, our data are in contrast to those of Day et al,24 in which fexofenadine at 60 mg significantly reduced allergic rhinitis symptoms in an allergen challenge setting using much lower concentrations of allergen. These contrasting results invite further investigation as they may be relevant to clinical observations demonstrating the clinical effectiveness of antihistamines in mild, intermittent allergic rhinitis but reporting their frequent loss of efficacy as the condition becomes more chronic or more severe. Mild, intermittent disease, and similarly low-dose challenges, may represent largely allergen/ IgE-induced mast cell degranulation with histamine release and may therefore be generally responsive to antihistamines. More severe, chronic disease, and similarly our high-dose challenges, may represent a progression to a more inflammatory process with T cell activation, cytokine production, and inflammatory cell recruitment and activation. This process may be less likely to respond to antihistamines.
CONCLUSION
We describe an effective, reproducible, and valid technique for measuring inflammatory mediators in nasal tissue after an allergen challenge. Our data demonstrate consistent and reproducible induction of IL-4, TNF-α, IL-10, and MIP-1 α synthesis after an allergen challenge. The use of fexofenadine 60 mg, twice daily, for 7 days before the allergen challenge was not associated with any attenuation of this inflammatory response.
Footnotes
Supported by private funds and NIH AI01793 and AI/HL47737
References
- 1.Simons FE. The antiallergic effects of antihistamines (H1-receptor antagonists) J Allergy Clin Immunol. 1992;90:705–715. doi: 10.1016/0091-6749(92)90156-v. [DOI] [PubMed] [Google Scholar]
- 2.Walsh GM. The anti-inflammatory effects of cetirizine. Clin Exp Allergy. 1994;24:81–85. doi: 10.1111/j.1365-2222.1994.tb00921.x. [DOI] [PubMed] [Google Scholar]
- 3.Naclerio RM. Inhibition of mediator release during the early reaction to antigen. J Allergy Clin Immunol. 1992;90:715–719. doi: 10.1016/0091-6749(92)90157-w. [DOI] [PubMed] [Google Scholar]
- 4.Townley RG. Antiallergic properties of the second-generation H1 antihistamines during the early and late reactions to antigen. J Allergy Clin Immunol. 1992;90:720–725. doi: 10.1016/0091-6749(92)90158-x. [DOI] [PubMed] [Google Scholar]
- 5.Naclerio RM, Meier HL, Kagey-Sobotka A, et al. Mediator release after nasal airway challenge with allergen. Am Rev Respir Dis. 1983;128:597–602. doi: 10.1164/arrd.1983.128.4.597. [DOI] [PubMed] [Google Scholar]
- 6.Naclerio RM, Kagey-Sobotka A, Lichtenstein LM, et al. Terfenadine, an H1 antihistamine, inhibits histamine release in vivo in the human. Am Rev Respir Dis. 1990;142:162–171. doi: 10.1164/ajrccm/142.1.167. [DOI] [PubMed] [Google Scholar]
- 7.de Weck AL, Derer T, Bischoff SC, Takafuji S. The effect of terfenadine on the immediate and late-phase reactions mediated by immunoglobulin E. Arch Allergy Immunol. 1993;101:326–332. doi: 10.1159/000236472. [DOI] [PubMed] [Google Scholar]
- 8.Ciprandi G, Buscaglia S, Iudice A, Canonica GW. Protective effect of terfenadine at different dosage on conjunctival provocation test. Allergy. 1992;47:309–312. doi: 10.1111/j.1398-9995.1992.tb02059.x. [DOI] [PubMed] [Google Scholar]
- 9.Rafferty P, Holgate ST. Terfenadine (Seldane) is a potent and selective H1 receptor antagonist in asthmatic airways. Am Rev Respir Dis. 1987;135:181–184. doi: 10.1164/arrd.1987.135.1.181. [DOI] [PubMed] [Google Scholar]
- 10.Vignola AM, Crampette L, Mondain M, et al. Inhibitory activity of loratadine and descarboethoxyloratadine on expression of ICAM-1 and HLA-DR by nasal epithelial cells. Allergy. 1995;50:200–203. doi: 10.1111/j.1398-9995.1995.tb01133.x. [DOI] [PubMed] [Google Scholar]
- 11.Ciprandi G, Pronzato C, Ricca V, et al. Terfenadine exerts antiallergic activity reducing ICAM-1 expression on nasal epithelial cells in patients with pollen allergy. Clin Exp Allergy. 1995;25:871–878. doi: 10.1111/j.1365-2222.1995.tb00030.x. [DOI] [PubMed] [Google Scholar]
- 12.Ciprandi G, Pronzato C, Ricca V, et al. Loratadine treatment of rhinitis due to pollen allergy reduces epithelial ICAM-1 expression. Clin Exp Allergy. 1997;27:1175–1183. [PubMed] [Google Scholar]
- 13.Ciprandi G, Tosca M, Ricca V, et al. Cetirizine treatment of rhinitis in children with pollen allergy: evidence of its antiallergic activity. Clin Exp Allergy. 1997;27:1160–1166. [PubMed] [Google Scholar]
- 14.Smith RE, Casanova-Roig R, Wells DE. The effect of antihistamines on nasal smear eosinophils in patients with allergic rhinitis. Ann Allergy. 1968;26:80–82. [PubMed] [Google Scholar]
- 15.Baroody FM, Lim MC, Proud D, et al. Effects of loratadine and terfenadine on the induced nasal allergic reaction. Arch Otolaryngol Head Neck Surg. 1996;122:309–316. doi: 10.1001/archotol.1996.01890150083015. [DOI] [PubMed] [Google Scholar]
- 16.Lippert U, Kruger-Krasagakes S, Moller A, et al. Pharmacological modulation of IL-6 and IL-8 secretion by the H1-antagonist decarboethoxy-loratadine and dexamethasone by human mast and basophilic cell lines. Exp Dermatol. 1995;4:272–276. doi: 10.1111/j.1600-0625.1995.tb00257.x. [DOI] [PubMed] [Google Scholar]
- 17.Alam R, Sim TC, Hilsmeier KA, Grant JA. Development of a new technique for recovery of cytokines from inflammatory sites in situ. J Immunol Methods. 1992;155:25–29. doi: 10.1016/0022-1759(92)90267-w. [DOI] [PubMed] [Google Scholar]
- 18.Bernstein DI, Schoenwetter WF, Nathan RA, et al. Efficacy and safety of fexofenadine hydrochloride for treatment of seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 1997;79:443–448. doi: 10.1016/S1081-1206(10)63041-4. [DOI] [PubMed] [Google Scholar]
- 19.Bronsky EA, Falliers CJ, Kaiser HB, et al. Effectiveness and safety of fexofenadine, a new nonsedating H1-receptor antagonist, in the treatment of fall allergies. Allergy Asthma Proc. 1998;19:135–141. doi: 10.2500/108854198778604112. [DOI] [PubMed] [Google Scholar]
- 20.Casale TB, Andrade C, Qu R. Safety and efficacy of once-daily fexofenadine HCl in the treatment of autumn seasonal allergic rhinitis. Allergy Asthma Proc. 1999;20:193–198. doi: 10.2500/108854199778553046. [DOI] [PubMed] [Google Scholar]
- 21.Sussman GL, Mason J, Compton D, et al. The efficacy and safety of fexofenadine HCl and pseudoephedrine, alone and in combination, in seasonal allergic rhinitis. J Allergy Clin Immunol. 1999;104:100–106. doi: 10.1016/s0091-6749(99)70120-x. [DOI] [PubMed] [Google Scholar]
- 22.Prenner BM, Capano D, Harris AG. Efficacy and tolerability of loratadine versus fexofenadine in the treatment of seasonal allergic rhinitis: a double-blind comparison with crossover treatment of nonresponders. Clin Ther. 2000;22:760–769. doi: 10.1016/S0149-2918(00)90009-2. [DOI] [PubMed] [Google Scholar]
- 23.Naclerio RM, Proud D, Kagey-Sobotka A, et al. The effect of cetirizine on the early allergic response. Laryngoscope. 1989;99:596–599. doi: 10.1288/00005537-198906000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Day JH, Briscoe MP, Welsh A, et al. Onset of action, efficacy, and safety of a single dose of fexofenadine hydrochloride for ragweed allergy using an environmental exposure unit. Ann Allergy Asthma Immunol. 1997;79:533–540. doi: 10.1016/S1081-1206(10)63062-1. [DOI] [PubMed] [Google Scholar]


