Abstract
The field of epidermal stem cells has dramatically advanced in the last decade, leading to a better understanding of the molecular factors, signalling pathways and cellular events that identify and characterize stem cells, thus revealing their immense potential for therapeutic use. Furthermore, multipotent epidermal stem cells present the major advantage of easy accessibility with the discovery of their specific location within the bulge of the hair follicle. This review focuses on the most recent findings on epidermal stem cells, and their potential role in initial epidermal commitment, differentiation and wound healing processes in the skin.
Keywords: differentiation, epidermis, hair follicle, stem cell, wound healing
Abbreviations used: EDC, epidermal differentiation complex; EGF, epidermal growth factor; GFP, green fluorescent protein; HSE, human skin equivalent; IFE, interfollicular epidermis; IFNγ, interferon γ; IL-1 (etc.); interleukin-1 (etc.); LRC, label-retaining cell; SC, stem cell; TA, transit amplifying (cell); TNFα, tumour necrosis factor α; TGFβ, transforming growth factor β
Introduction
Skin is composed of an underlying dermis, of mesodermal embryonic origin, separated by a basement membrane from the multilayered overlaying epidermis, of ectodermal origin. Epidermis is a stratified squamous epithelium primarily composed of keratinocytes: cells that expresses the characteristic intermediate filament known as keratin. Stratification of the epidermis commences during embryonic development and is a process that continues to occur throughout the life of the organism. The process entails the outward movement of the proliferative basal cells that are adjacent to the basement membrane towards the surface of the skin. The stratification is concurrent with a Ca2+-dependent differentiation process and the layer-specific expression of structural and enzymatic markers, with the basal cell differentiating first to a spinous cell, then to a granular cell, to ultimately terminally differentiate as a cornified, anucleated cell (Figure 1).
Figure 1. Stem cells in the interfollicular stratified epidermis and the bulge region of the hair follicle.
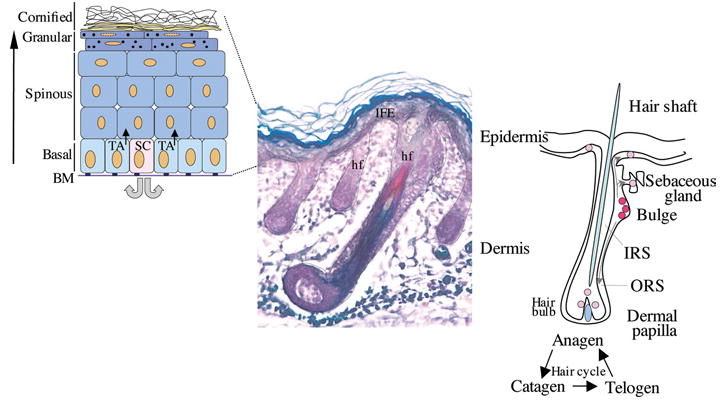
Left-hand panel, schematic representation of the stratified layers of the epidermis (basal, spinous, granular and cornified layers). The proliferative basal layer is adjacent to the basement membrane (BM). Stem cells (SC) generate transit amplifying (TA) cells which will differentiate to form the stratified layers. Middle panel, section of mouse skin stained to distinguish the different compartments of the hair follicle (hf) (Magnification 10×). Right-hand panel, schematic representation of a hair follicle and hair cycle, with the multipotent SCs (red) localizing to the bulge region. These cells migrate to populate the bulb region of the follicle, the sebaceous gland and the interfollicular epidermis (IFE; pink). IRS, inner root sheath; ORS, outer root sheath.
Studies over the last two decades have demonstrated that epidermal-specific genes expressed in keratinocytes are regulated in a temporal and spatial manner during differentiation (reviewed in Presland and Dale, 2000). In humans, many of these epidermal genes, which encode putative cornified envelope precursors, are closely linked in the epidermal differentiation complex (EDC), a 2.5 Mbp region located on chromosome 1q21 (Volz et al., 1993); in mice a syntenic region is on chromosome 3. Three types of proteins are clustered in the EDC: (1) proteins characterized by relatively small sizes with short tandem peptide repeats in the central region, (2) proteins termed fused-type which have Ca2+-binding EF-hand domains at the N-terminal region followed by multiple tandem repeats, and (3) members of the S100 family, characterized by presenting only Ca2+-binding EF-hand domains (Kalinin et al., 2002; Eckert et al., 2004). Recently, a second cluster of epidermal-specific genes has been described on the human chromosome 19q13.12 and in the syntenic region of chromosome 7 in the mouse (Park et al., 2002; Michibata et al., 2004; Moffat et al., 2004).
The outermost layer of the epidermis is the product of covalent cross-links of envelope precursor proteins by Ca2+-dependent transglutaminases and attachment of lipid molecules (Madison, 2003). This cornified layer provides the protective and water barrier functions between the body and the environment (Kalinin et al., 2002) (Figure 1). The differentiation of keratinocytes and subsequent generation of a protective cornified layer are processes that must be accomplished continuously, thus requiring the contribution of the primary stem cells (SCs) for the renewing epidermis. Additionally, in mammals, the epidermis gives rise to a specialized, derived structure, the hair follicle.
During embryonic development and epidermis formation, a series of signals between the surface epithelial cells and the underlying dermal cells generate hair placodes in the epidermis (reviewed by Hardy, 1992; Paus and Cotsarelis, 1999; Millar, 2002; Rogers, 2004). These placodes subsequently give rise to the lineages for all the hair follicle layers that ultimately differentiate into the hair shaft and inner root sheath (Hardy, 1992; Figure 1). The hair follicles vary in size and shape depending on their location. The cycling and regeneration of each hair follicle depend on specialized mesenchymal dermal papilla cells and proliferating matrix cells located at the base of the follicle. During normal development, each hair follicle goes through three stages: growth (anagen), involution (catagen) and rest (telogen) (Paus and Cotsarelis, 1999; Figure 1). After the resting telogen period, signals from the dermal papilla cells initiate the regeneration of a new hair.
SCs in the epidermis
An important question that has been the focus of great interest is where do the multipotent SCs reside within the epidermis and what molecular markers can be used for their detection? Reports have shown there are a number of SC repositories within the undamaged adult epidermis: cells from the bulge region of the hair follicle, keratinocytes of the interfollicular epidermis (IFE) and sebaceous gland (Ghazizadeh and Taichman, 2001).
Several lines of evidence indicate that the primary source of multipotent SCs in the epidermis that ultimately replenishes the hair, IFE and sebaceous lineages rests within the bulge region of the hair follicle (Taylor et al., 2000; Oshima et al., 2001). Several reports have demonstrated the enrichment of a population with label-retaining cells (LRCs) or SC-like cells based on their slow cycling, hence their ability to retain bromodeoxyuridine or [3H]thymidine labelling (Cotsarelis et al., 1990; Morris and Potten, 1999). The identification and characterization of multipotent murine hair follicle SCs, as well as what regulates their commitment and maintenance, was shown utilizing transgenic methodologies that allow for labelling, tracking and isolation of the slow-cycling subpopulation of cells from the bulge region. Tumbar et al. (2004) accomplished this by generating transgenic lines: a line expressing histone H2B–green fluorescent protein (GFP) controlled by tetracycline-responsive element to be crossed with a second line expressing a tetracycline repressor–V16 under the control of a keratinocyte-specific keratin 5 (K5) promoter. Morris et al. (2004), used the well-characterized keratin 15 (K15) promoter (Liu et al., 2003) to express the Cre recombinase or the GFP marker in the bulge cells to allow for lineage analysis and isolation. In vitro studies, performed with the isolated putative SCs, demonstrated their ability to reconstitute all epithelial cell types within the skin (Morris et al., 2004). Clonal analysis of individual bulge cells will be necessary to determine if there is a mix of multiple types of unipotent SCs or if all the SCs that localize to the bulge are multipotent.
The secondary SCs dispersed throughout the basal layer in the IFE are somewhat less effective and multi-potential than the bulge SCs (Costarelis et al., 1999; Watt, 2001; Fuchs and Raghavan, 2002). Within the basal proliferative layer of the IFE there are subpopulation cell types that differ in their capacity for self-renewal and ability to undergo terminal differentiation (Barrandon and Green, 1987; Jones and Watt, 1993). The slow-cycling IFE SCs that have high self-renewal capacity give rise to non-SCs. These non-SCs are known as transit amplifying cells (TAs, Figure 1), and are characterized by their limited proliferative capacity, dividing only a few times before undergoing terminal differentiation.
Epidermal SC markers
Although several characteristics such as capacity of self-renewal and multipotency have been defined for the epidermal SCs, the determination of a specific marker, or combination of markers, that would assist in their direct identification within the epidermis is still focus of much interest. Much as surface markers for SCs in the haematopoietic system (e.g. CD34 and CD38) and for neural SCs (e.g. CD133) have aided in their isolation and purification, several studies have characterized putative epidermal SC markers. Based on recent reports (Tumbar et al., 2004 and Morris et al., 2004), the comparison of the transcriptional profiles of SCs identified several genes (i.e. ESP8, COL18A1, PKD2) common to hair follicle, neural, haematopoietic and embryonic SCs (Turksen, 2004). It remains to be determined if these genes can be used as a ‘footprint’ to indicate the ‘stemness’ potential of the epidermal or other particular cell population.
Below we summarize results on specific factors and signalling pathways that have been used in the enrichment of a cell population with epidermal SCs.
Integrins
Notwithstanding the important structural and regulatory roles that integrins (β1 and α6β4) have in the epidermis, there are questions with regard to their specific role as SC markers. In the epidermis, basal cells are maintained in a spatio-temporal orientation by attachment to the basal lamina through hemidesmosomes, anchorages formed by laminin 5 from the basal lamina and integrin α6β4 that are intracellularly linked to the keratin cytoskeleton (Borradori and Sonnenberg, 1999). In addition, they also transduce signals from the extracellular matrix to the interior of the cell, thus participating in the overall organization of the cytoskeleton, proliferation, apoptosis, and differentiation (Mainiero et al., 1996; Goldfinger et al., 1999).
Li et al. (1998) reported that high levels of α6 integrin and low expression of the transferrin receptor is a combination that identifies epidermal SCs characterized by a long-term proliferative capacity. However, others did not find a direct correlation between the levels of integrin α6β4 heterodimer and the epidermal proliferation capacity (Jones and Watt, 1993; Jones et al., 1995) and α6β4 integrin-null mice show no defects in proliferation. The phenotype of these mice, characterized by epidermal blistering, is consistent with a role for α6β4 integrin in anchorage (DiPersio et al., 2000).
Another proposed epidermal SC marker is β1 integrin, which is expressed throughout the basal layer of the epidermis, and its expression is downregulated in keratinocytes that have initiated the differentiation program. Utilizing human keratinocytes and IFE, it is possible to enrich for cells with SC qualities by selecting those with high levels of β1 integrin expression (Jones and Watt, 1993; Jones et al., 1995). β1 integrin has a role in maintaining keratinocytes in an undifferentiated state (Levy et al., 2000) and a conditional knockout using K14cre mice lead to defects in epidermal proliferation and basement membrane formation (Raghavan et al., 2000). These findings indicate a prominent role of β1 integrin in the proliferative capacity of keratinocytes, but whether this protein identifies the SC compartment within the epidermis remains to be determined.
Despite the conflicting findings when using β1 or α6β4 integrin as SC markers, several studies indicate that both markers can be used to enrich a population for SCs. Some of the reported differences noted above might be attributed to differences between the mouse and human keratinocytes. Ultimately, it is plausible that a more complex combination of markers will be needed to distinctively profile the epidermal SC.
p63
p63 belongs to the p53 family of transcription factors, with p53 having a well-established role as a tumour suppressor gene (Yang et al., 2002). The pivotal role for p63 in epidermis was demonstrated by ablating it during embryonic development. This leads to prenatal lethality with a lack of all stratified epithelia, including severe defects in epithelial–mesenchymal-derived structures, such as mammary and prostate glands (Yang et al., 1999; Mills et al., 1999). Mutations in p63 are associated with human ectodermal dysplasias (Celli et al., 1999; van Bokhoven et al., 2001). The implications are that functional p63 is essential for commitment of the ectoderm to epidermal lineages (Mills et al., 1999) and/or for maintenance of the epidermal SC population necessary for epithelial morphogenesis and renewal (Yang et al., 1999; Pellegrini et al., 2001).
In contrast with the related p53 protein, p63 is transcribed from two different promoters (TA and ΔN) as alternatively spliced isoforms (α, β and γ). The TA isoforms (TAp63α, β and γ) contain an N-terminal transactivating domain that is absent from the ΔN isoforms (ΔNp63α, β and γ). p63 is expressed mainly in the basal layer of the epidermis, predominantly as the ΔNp63α isoform (Yang et al., 1998); however, other isoforms are expressed at lower levels (Nylander et al., 2002). In the mouse ablation reports mentioned above, both groups disrupted exons common to all isoforms (TA and ΔN), resulting in phenotypes that are a consequence of the loss of all possible gene products (Yang et al., 1999; Mills et al., 1999). Thus it is not possible to determine from these data whether each isoform contributes a distinct function during epidermal determination and differentiation.
In vitro data suggest that ΔN isoforms might act in a dominant-negative fashion versus the TA isoforms (Yang et al., 1998), with ΔNp63α acting as a positive and negative regulator blocking differentiation (King et al., 2003). Findings reported by Koster et al. (2004) showed that TAp63 isoform overexpression blocks keratinocyte differentiation while they are required for the process of commitment and initiation of epithelial stratification during embryogenesis. The authors also showed that the ability of TAp63 to drive commitment extended to non-keratinocyte cell types. There are several potential reasons for the different findings, including the influences of genetic backgrounds and/or variation in methods of isolation of cells to be studied in vitro. Nonetheless, the functional analysis of the specific isoforms should provide critical data to unravel their role in the processes of epithelial SC determination and maintenance.
Wnt signalling, β-catenin and c-Myc
β-Catenin is a multifunctional protein that plays important roles during embryonic development and neoplasia as a mediator of the Wnt signalling pathway. When the Wnt pathway is quiescent, β-catenin participates only in adherens junctions. When β-catenin moves into the cytoplasmic compartment it gets phosphorylated and targeted for ubiquination and degradation. Under certain circumstances, such as the activation of the Wnt pathway, phosphorylation of cytoplasmic β-catenin is inhibited, leading to an accumulation of stabilized cytoplasmic protein. As a consequence, β-catenin translocates to the nucleus where it binds Tcf/Lef transcription factors and regulates transcription (Merrill et al., 2001; Jamora and Fuchs, 2002; Alonso and Fuchs, 2003; Jamora et al., 2003).
Using diverse mouse models has demonstrated that the Wnt pathway is involved in regulating SC fate decisions, with the level of β-catenin correlating with different epidermal SC fates (Huelsken et al., 2001). One of the downstream targets of the β-catenin transcriptional pathway is the oncogene c-Myc (Honeycutt and Roop, 2004). c-Myc is required for transition from the G1 to the S phase of the cell cycle and it promotes the proliferation of epidermal TA cells. However, deregulation of c-Myc transcription depletes epidermal SCs, with the consequent inability of SCs to react to injury (Waikel et al., 1999, Arnold and Watt, 2001, Frye et al., 2003). Furthermore, targeted overexpression of c-Myc in basal keratinocytes leads to impairment of keratinocyte migration and inhibition of wound healing (Waikel et al., 2001). The majority of genes suppressed by c-Myc in epidermis encode cell adhesion and cytoskeleton proteins, resulting in profound changes in keratinocyte adhesion and motility. Lastly, c-Myc can redirect epidermal cells to exit the SC compartment and differentiate into sebocytes and IFE at the expense of the hair lineages (Frye et al., 2003), uncovering an additional role for c-Myc in epidermal SC fate determination.
As highlighted in this section, the determination of specific epidermal SC marker(s) remains a challenge; however, crucial progress has been made in recent years: (1) the detection of the major repository for SCs in the bulge, (2) the determination of the plasticity of the adult epidermal SC, and (3) the identification of factors and pathways involved in the commitment and maintenance of the epidermal SC population. These discoveries should prove essential in the usage of SCs in therapeutic procedures.
Response to injury: the keratinocyte activation cycle
The normal process of keratinocyte differentiation is an invaluable biological programme that constantly restores an important barrier between the organism and its environment. However, this perpetual programme of self-renewal is interrupted when an injury occurs. Keratinocytes represent the body’s ‘first line of defence’ from the outside environment, and as such they are the first responders to injury. After injury, keratinocytes become activated (Figure 2A). This means that they secrete various cytokines and growth factors and, at the same time, respond to them. Therefore, these responses alter the keratinocyte phenotype from basal/differentiated to what is generally called the activated keratinocyte (Tomic-Canic et al., 1998).
Figure 2. Schematic representation of keratinocyte phenotypes in acute (A) and chronic (B) wounds.
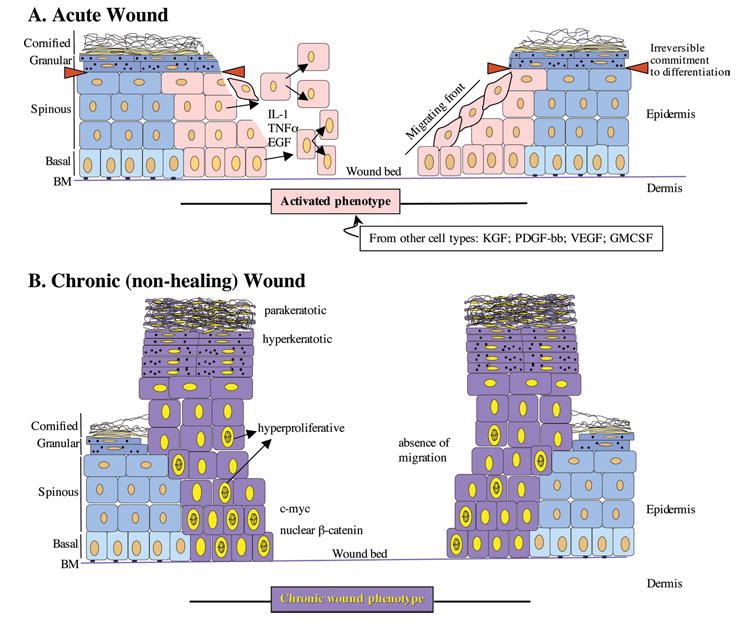
(A) In the acute wound, keratinocytes become activated (pink), and release/respond to various pro-inflammatory cytokines and growth factors. In response to these stimuli, keratinocytes from the basal and suprabasal layers start proliferating and migrating. Red triangles demarcate the point of irreversible commitment of keratinocytes to differentiation after which they are unable to participate in the wound healing response. (B) Chronic wound keratinocytes (purple) are hyperproliferative (indicated by mitotically active cells present throughout the suprabasal layers), hyperkeratotic (indicated by thick cornified layer) and parakeratotic (indicated by presence of nuclei in the cornified layer). Chronic wound keratinocytes are unable to migrate.
Given that there are different types of possible injuries, there are multiple ways in which keratinocytes are equipped to respond to them. Upon UV radiation, differentiation of keratinocytes is stimulated, possibly because enhanced cornification would provide additional protection (Li et al., 2001; Sesto et al., 2002). After mechanical injury, keratinocytes will start migrating and proliferating instead (Tomic-Canic et al., 2004). Upon interferon γ (IFNγ) stimuli, keratinocytes will activate the expression of tight-junction genes thus augmenting their junctions in order to prevent paracellular penetration of viral particles (Banno et al., 2003). Therefore, keratinocytes possess biological tools to first assess the damage and then to appropriately respond to it. How is this accomplished?
Upon mechanical injury, keratinocytes release pre-stored interleukin-1 (IL-1), which is the first signal upon wounding and has a dual function: to activate keratinocytes and to signal-alert the surrounding tissues. The keratinocyte activation cycle is governed by extracellular signals including tumour necrosis factor α(TNFα), transforming growth factor β(TGFβ), epidermal growth factor (EGF), HG-EGF, IL-8 and IFNγ, and is characterized by changes in expression of keratin proteins. One of the markers of activated epidermal keratinocytes is the expression of keratins K6 and K16. K6 is activated by cytokines and growth factors such as IL-1, EGF and TNFα (Jiang et al., 1993; Komine et al., 2000; 2001). It has been proposed that cytoskeletal changes that include the expression of K6 and K16 keratin filaments provide plasticity and flexibility while maintaining resilience of the intracellular scaffold that is important for migration (Coulombe, 1997; Wong and Coulombe, 2003). Once the activation cycle is completed, keratinocytes become deactivated and revert to normal differentiation.
Very little is known about which signals participate in ending the activation cycle. One possibility is that once the activation signals cease, as a consequence, so does the activation cycle. Alternatively, factors such as corticosteroids may participate in inhibition of the activation cycle (Lee and Tomic-Canic, 2002). Corticosteroids are known to participate in the inhibition of wound healing, but the molecular mechanism of this inhibition is not understood (Muller-Decker et al., 2002). Preliminary studies indicate that corticosteroids act as dominant inhibitors of EGF leading to suppression of K6/K16 expression as well as keratinocyte migration (B. Lee, C. Vouthounis, O. Stojadinovic, H. Brem, M.J. Im and M. Tomic-Canic, unpublished work).
The process of wound healing
During the process of tissue repair, keratinocytes undergo significant changes of their junctions and adhesion molecules: hemidesmosomes have to be dissolved in order to allow for keratinocyte migration. The migrating keratinocytes produce a different set of membrane molecules, such as vitronectin, fibronectin receptors and integrin α5β1, and they replace the collagen receptor allowing keratinocytes to migrate (Cavani et al., 1993; Haapasalmi et al., 1996; Singer and Clark, 1999). Many factors active during wound healing such as EGF, keratinocyte growth factor (KGF) and TGFβ, are potential regulators of these processes (Werner et al., 1994; Zambruno et al., 1995; Abraham and Klagsburn, 1996; Nanney and King, 1996).
The activated keratinocyte phenotype is broadly defined, but which keratinocyte phenotypes can become activated? Based on early studies it was believed that because of their mitotic ability, basal keratinocytes rather than keratinocytes already committed to differentiation take part during wound healing (Galvin et al., 1989). However, the use of conditional knockouts, which allow the targeting of genes specifically in epidermis, and viral transductions with biomarkers such as GFP or β-galactosidase, permit a much ‘closer look and better resolution’, therefore revisiting this question. Epidermal SCs, TA cells and early differentiated cells have the ability to form fully differentiated epidermis both in vivo and in vitro (Li et al., 2004). Although each of these keratinocyte types has different capacity and matrix requirements, all of them can reproduce stratified epidermis (Figure 2A) (Li et al., 2004). If early differentiating keratinocytes possess regenerative capacity, the question is at which point do they become irreversibly committed, i.e. what is the ‘point of no return’? Although there is no conclusive answer, one can speculate that once a differentiating keratinocyte is unable to respond to activating signals it is irreversibly committed to terminal differentiation. Once keratinocytes process profilaggrin into filaggrin they undergo specific changes that mark late (terminal) differentiation (Presland et al., 2001), and perhaps this process demarcates the ‘line of regenerative potency’ among the differentiating keratinocyte population (Figure 2A).
Failure to heal: chronic wounds from an epidermal prospective
Under certain circumstances a wound fails to heal, thus leading to the development of a chronic wound (ulcer). Incidences of chronic wounds are higher among the elderly and diabetic, as well as among people with vasculature problems; therefore failure to heal may have various underlying pathologies. The epidermis of a chronic wound has a typical appearance (Figures 2B and 3). It is thick and hyperproliferative, with mitotically active cells located in the suprabasal (differentiated) layers. Furthermore, the cornified layer is hyperkeratotic (thick cornified layer) and parakeratotic (presence of nuclei in the cornified layer). Keratinocytes on a chronic wound edge are capable of proliferating but are unable to migrate properly. In the need to restore the ‘injured’ barrier, keratinocytes keep differentiating but the presence of nuclei in the cornified layer (parakeratosis) indicates incomplete differentiation. Taken together, the histology of epidermis at the non-healing edge of chronic wounds suggests that chronic wound keratinocytes do not successfully complete either of the two possible pathways: activation or differentiation (Figures 2B and 3). Instead, keratinocytes are caught in a ‘loop’ of trying, but not succeeding, to accomplish either of the two processes. Results from our laboratory have shown that non-healing keratinocytes of the chronic wound edge are marked by induction of c-Myc and nuclearization of β-catenin, which may contribute to the inhibition of migration (O. Stojadinovic, C. Vouthounis, B. Lee, H. Brem, A. Merchant, J. Fallon, M.R. Stallcup and M. Tomic-Canic, unpublished work). These results are consistent with reports in transgenic animals, in which the persistent activation of c-Myc in basal keratinocytes led to inhibition of keratinocyte migration and development of a chronic wound (Waikel et al., 2001; Arnold and Watt, 2001). Furthermore, overexpression of c-Myc has been implicated in promoting the cycling of SCs that leads to depletion of the local epidermal SCs (Ganderillas and Watt, 1997; Waikel et al., 2001; reviewed by Honeycutt and Roop, 2004). Given that keratinocytes of the wound edge are hyperproliferative and express c-Myc, our current hypothesis is that local depletion of epidermal SCs at the non-healing edge of a chronic wound may be responsible for the persistence of a wound.
Figure 3. Histology of human epidermis in normal skin (left-hand panel) and at a non-healing edge of a chronic ulcer (right-hand panel).
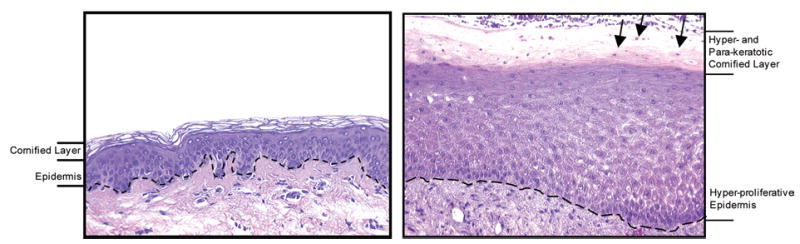
In the chronic ulcer, the epidermis is hyperproliferative, as indicated by increased thickness. The cornified layer is both thicker and contains cell nuclei (arrows). Dashed line: basement membrane. Magnification 20×.
One of the major issues in chronic wound treatments is how to revert the chronic wound keratinocyte phenotype to a proper differentiating phenotype. One possible approach is to surgically remove (debride) non-healing keratinocytes (purple in Figure 2B) and allow the freshly wounded keratinocytes to become exposed and permissive to the activation signals. The intent is that these cells will resume their activation cycle followed by proper differentiation. Another important issue is to provide and maintain the source of the activation signals in a chronic wound environment. In the past decade many clinical trials have utilized a variety of growth factors and cytokines in potential treatment of chronic wounds (Martin, 1997). Currently, platelet-derived growth factor (PDGF-BB) and human skin equivalents (HSEs), containing either both dermis and epidermis or only dermis, are the only Food and Drug Administration (FDA)-approved therapy for chronic wounds (Brem et al., 2003; Nagai and Embril, 2002; Sibbald, 1998). HSEs may consist of either living fibroblasts suspended in a native collagen matrix (resembling dermis), or, in addition, may have their surface covered with viable, stratified keratinocytes (dermis and epidermis). These are viable cells fully capable of secreting and responding to a variety of growth factors/cytokines, similarly to normal skin (Ehrlich, 2004). Although there are many questions that remain to be answered on the molecular mechanism of how HSE works as therapy of chronic wounds, it is believed that it acts as temporal cell therapy that provides local sustained release of stimulating factors (Brem et al., 2003; Ehrlich, 2004). The chronic wound environment is very complex and involves pathogenic changes in underlying tissues such as dermis, endothelium and peripheral nerves, all of which also contribute to keratinocyte impairment. Therefore, the appropriate treatments should have a broad and complex approach, and will require the targeting of more than one cell population.
Epidermal SCs and chronic wounds
Using HSE as a therapy for chronic wounds also raises a question of the use of SCs as a therapeutic approach in wound healing. The intriguing question is: what is the fate of epidermal SCs provided by HSE therapy? As mentioned above, both epidermal SCs and TA cells have the ability to fully restore epidermis (Li et al., 2004). Epidermal SCs have the potential to regenerate epidermis, and if stimulated adequately, develop different cell types and tissues (Liang and Bickenback, 2002). Embryonic SCs can form an epidermal equivalent when seeded on extracellular matrix in the presence of bone morphogenic protein-4 (BMP-4) or ascorbate (Coraux et al., 2003). Furthermore, the potency of skin SCs in tissue repair is not limited to epidermis. Recent reports indicate that human skin-derived SCs are capable of neural differentiation (Belicchi et al., 2004; Joannides et al., 2004). Conversely, bone-marrow-derived cells also are able to incorporate and differentiate into skin structures, suggesting that bone marrow might be a valuable source of SCs for skin repair (Badavias et al., 2003).
Conclusions
Although many questions remain to be answered, it is clear that because of their plasticity and accessibility, epidermal SCs provide a major resource for therapeutic applications in tissue repair and regeneration, as well as mediators for gene therapy approaches. In addition to their multi-potency, SCs can be genetically engineered using viral vector constructs (Ghazizadeh and Taichman, 2001), which allows for usage in gene therapy. The recent advances in biotechnology, such as genomics, proteomics, in vivo epidermal targeting, GFP labelling and adeno- and retro-viral gene transfers, will allow for much better understanding of SC biology. Questions such as how specific SCs respond and create appropriate niche(s) are now being successfully answered (Brouard and Barrandon, 2003; Gambardella and Barrandon, 2003; Blanpain et al., 2004; Fuchs et al., 2004). Lastly, these novel technological approaches will allow for identification of specific molecular markers which will aid further in the isolation of SCs, thus opening unlimited possibilities for therapeutic approaches.
Acknowledgments
We thank members of the Morasso and Tomic laboratories, especially Dr Olivera Stojadinovic. Special thanks to Dr Harold Brem and Dr John Fallon for chronic wound histology, and Mr John Ward for photography assistance. We apologize to our colleagues whose contributions were not cited due to the space limitations. Our research is supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases (M.M.) and by the National Institutes of Health grants AR45974, NR08029 (M.T.-C.).
Footnotes
EF-hand domain: A helix-loop-helix (HLH) structural motif in the calcium-binding site of calcium-binding proteins.
S100 family: EF-hand-containing proteins of 10–14 kDa, in which calcium-binding motifs function to transmit calcium-dependent regulatory signals.
References
*Articles of special interest
- Abraham, J.A. and Klagsburn, M. (1996) Modulation of wound repair by members of the fibroblast growth factor family. In the molecular and cellular biology of wound repair (C.R.A., ed.), pp. 195–248, Plenum Press, New York
- Alonso L, Fuchs E. Stem cells in the skin: waste not, Wnt not. Genes Dev. 2003;17:1189–2000. doi: 10.1101/gad.1086903. [DOI] [PubMed] [Google Scholar]
- Arnold I, Watt FM. c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and differentiation of their progeny. Curr Biol. 2001;11:558–568. doi: 10.1016/s0960-9822(01)00154-3. [DOI] [PubMed] [Google Scholar]
- Badavias EV, Abedi M, Butmarc J, Falanga V, Quesenberry P. Participation of bone marrow-derived cells in cutaneous wound healing. J Cell Physiol. 2003;196:245–250. doi: 10.1002/jcp.10260. [DOI] [PubMed] [Google Scholar]
- Banno T, Adachi M, Mukkamala L, Blumenberg M. Unique keratinocyte-specific effects of interferon-γ that protect skin from viruses identified using transcriptional profiling. Antiviral Ther. 2003;8:541–554. [PubMed] [Google Scholar]
- Barrandon Y, Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci USA. 1987;84:2302–2306. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belicchi M, Pisati F, Lopa R, Poretti L, Fortunato F, Sironi M, Scalamogna M, Parati EA, Bresolin N, Torrente YJ. Human skin-derived stem cells migrate throughout forebrain and differentiate into astrocytes after injection into adult mouse brain. Neurosci Res. 2004;77:475–486. doi: 10.1002/jnr.20151. [DOI] [PubMed] [Google Scholar]
- *Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell (Cambridge, Mass) 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Borradori L, Sonnenberg A. Structure and function of hemidesmosomes: more than simple adhesion complexes. J Invest Dermatol. 1999;112:411–418. doi: 10.1046/j.1523-1747.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- Brem H, Young J, Tomic-Canic M, Isaacs C, Ehrlich HP. Clinical efficacy and mechanism of bilayered living human skin equivalent (HSE) in treatment of diabetic foot ulcers. Surg Tech Int. 2003;11:23–31. [PubMed] [Google Scholar]
- Brouard M, Barrandon Y. Controlling skin morphogenesis: hope and despair. Curr Opin Biotechnol. 2003;14:520–525. doi: 10.1016/j.copbio.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Cavani A, Zambruno G, Marconi A, Manca V, Marchetti M, Giannetti A. Distinctive integrin expression in the newly forming epidermis during wound healing in humans. J Invest Dermatol. 1993;101:600–604. doi: 10.1111/1523-1747.ep12366057. [DOI] [PubMed] [Google Scholar]
- Celli J, Duijf P, Hamel BC, Bamshad M, Kramer B, Smits AP, Newbury-Ecob R, Hennekam RC, Van Buggenhout G, van Haeringen A, et al. Heterozygous germline mutations in the p53 homolog p63 are the cause of EEC syndrome. Cell (Cambridge, Mass) 1999;99:143–153. doi: 10.1016/s0092-8674(00)81646-3. [DOI] [PubMed] [Google Scholar]
- Coulombe PA. Towards a molecular definition of keratinocyte activation after acute injury to stratified epithelia. Biochem Biophys Res Commun. 1997;236:231–238. doi: 10.1006/bbrc.1997.6945. [DOI] [PubMed] [Google Scholar]
- Coraux C, Hilmi C, Rouleau M, Spadafora A, Hinnrasky J, Ortonne JP, Dani C, Aberdam D. Reconstituted skin from murine embryonic stem cells. Curr Biol. 2003;13:849–853. doi: 10.1016/s0960-9822(03)00296-3. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of the pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell (Cambridge, Mass) 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Kaur P, Dhouailly D, Hengge U, Bickenbach J. Epithelial stem class in the skin: definition, markers, localization and functions. Exp Dermatol. 1999;8:80–88. doi: 10.1111/j.1600-0625.1999.tb00351.x. [DOI] [PubMed] [Google Scholar]
- DiPersio CM, van der Neut R, Georges-Labouesse E, Kreidberg JA, Sonnenberg A, Hynes RO. α3β1 and α6β4 integrin receptors for laminin-5 are not essential for epidermal morphogenesis and homeostasis during skin development. J Cell Sci. 2000;113:3051–3062. doi: 10.1242/jcs.113.17.3051. [DOI] [PubMed] [Google Scholar]
- Eckert RL, Broome AM, Ruse M, Robinson N, Ryan D, Lee K. S100 proteins in the epidermis. J Invest Dermatol. 2004;123:23–33. doi: 10.1111/j.0022-202X.2004.22719.x. [DOI] [PubMed] [Google Scholar]
- Ehrlich HP. Understanding experimental biology of skin equivalent: from laboratory to clinical use in patients with burns and chronic wounds. Am J Surg. 2004;187:29S–33S. doi: 10.1016/S0002-9610(03)00301-5. [DOI] [PubMed] [Google Scholar]
- Frye M, Gardner C, Li ER, Arnold I, Watt FM. Evidence that Myc activation depletes the epidermal stem cell compartment by modulating adhesive interactions with the local microenvironment. Development. 2003;130:2793–2808. doi: 10.1242/dev.00462. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Raghavan S. Getting under the skin of epidermal morphogenesis. Nat Rev Genet. 2002;3:199–209. doi: 10.1038/nrg758. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell (Cambridge, Mass) 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- Galvin S, Loomis C, Manabe M, Dhouailly D, Sun TT. The major pathways of keratinocyte differentiation as defined by keratin expression: an overview. Adv Dermatol. 1989;4:277–299. [PubMed] [Google Scholar]
- Gambardella L, Barrandon Y. The multifaceted adult epidermal stem cell. Curr Opin Cell Biol. 2003;15:771–777. doi: 10.1016/j.ceb.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Ganderillas A, Watt FM. c-Myc promotes differentiation of human epidermal stem cells. Genes Dev. 1997;11:2869–2882. doi: 10.1101/gad.11.21.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazizadeh S, Taichman LB. Multiple classes of stem cells in cutaneous epithelium; a lineage analysis of adult mouse skin. EMBO J. 2001;20:1215–1222. doi: 10.1093/emboj/20.6.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfinger LE, Hopkinson SB, deHart GW, Collawn S, Couchman JR, Jones JC. The α3 laminin subunit, α6β4 and α3β1 integrin coordinately regulate wound healing in cultured epithelial cells and in the skin. J Cell Sci. 1999;112:2615–2629. doi: 10.1242/jcs.112.16.2615. [DOI] [PubMed] [Google Scholar]
- Haapasalmi K, Zhang K, Tonnesen M, Olerud J, Sheppard D, Salo T, Kramer R, Clark RA, Uitto VJ, Larjava H. Keratinocytes in human wounds express αv β6 integrin. J Invest Dermatol. 1996;106:42–48. doi: 10.1111/1523-1747.ep12327199. [DOI] [PubMed] [Google Scholar]
- Hardy M. The secret life of the hair follicle. Trends Genet. 1992;8:55–61. doi: 10.1016/0168-9525(92)90350-d. [DOI] [PubMed] [Google Scholar]
- Honeycutt KA, Roop DR. c-Myc and epidermal stem cell fate determination. J Dermatol. 2004;31:368–375. doi: 10.1111/j.1346-8138.2004.tb00687.x. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Costarelis G, Birchmeier W. B-catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell (Cambridge, Mass) 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Jamora C, Fuchs E. Intercellular adhesion, signalling and the cytoskeleton. Nature Cell Biol. 2002;4:101–108. doi: 10.1038/ncb0402-e101. [DOI] [PubMed] [Google Scholar]
- Jamora C, DasGupta R, Kocieniewski P, Fuchs E. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature (London) 2003;422:317–322. doi: 10.1038/nature01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang CK, Magnaldo T, Ohtsuki M, Freedberg IM, Bernerd F, Blumenberg M. Epidermal growth factor and transforming growth factor α specifically induce the activation-and hyperproliferation-associated keratins 6 and 16. Proc Natl Acad Sci USA. 1993;90:6786–6790. doi: 10.1073/pnas.90.14.6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joannides A, Gaughwin P, Schwiening C, Majed H, Sterling J, Compston A, Chandran S. Efficient generation of neural precursors from adult human skin: astrocytes promote neurogenesis from skin-derived stem cells. Lancet. 2004;364:172–178. doi: 10.1016/S0140-6736(04)16630-0. [DOI] [PubMed] [Google Scholar]
- Jones PH, Watt FM. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell (Cambridge, Mass) 1993;73:713–724. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- Jones PH, Harper S, Watt FM. Stem-cell patterning and fate in human epidermis. Cell (Cambridge, Mass) 1995;80:83–93. doi: 10.1016/0092-8674(95)90453-0. [DOI] [PubMed] [Google Scholar]
- Kalinin A, Kajava AV, Steinert PM. Epithelial barrier function: assembly and structural features of the cornified cell envelope. Bioessays. 2002;24:789–800. doi: 10.1002/bies.10144. [DOI] [PubMed] [Google Scholar]
- King KE, Ponnamperuma RM, Yamashita T, Tokino T, Lee LA, Young MF, Weinberg WC. δNp63α functions as both a positive and a negative transcriptional regulator and blocks in vitro differentiation of murine keratinocytes. Oncogene. 2003;22:3635–3644. doi: 10.1038/sj.onc.1206536. [DOI] [PubMed] [Google Scholar]
- Komine M, Rao LS, Kaneko T, Tomic-Canic M, Tamaki K, Freedberg IM, Blumenberg M. Inflammatory versus proliferative processes in epidermis. Tumor necrosis factor α induces K6b keratin synthesis through a transcriptional complex containing NFκB and C/EBPβ. J Biol Chem. 2000;275:32077–32088. doi: 10.1074/jbc.M001253200. [DOI] [PubMed] [Google Scholar]
- Komine M, Rao LS, Freedberg IM, Simon M, Milisavljevic V, Blumenberg M. Interleukin-1 induces transcription of keratin K6 in human epidermal keratinocytes. J Invest Dermatol. 2001;116:330–338. doi: 10.1046/j.1523-1747.2001.01249.x. [DOI] [PubMed] [Google Scholar]
- Koster MI, Huntzinger KA, Roop DR. Epidermal differentiation: transgenic/knockout mouse models reveal genes involved in stem cell fate decisions and commitment to differentiation. J Invest Dermatol Symp Proc. 2002;7:41–45. doi: 10.1046/j.1523-1747.2002.19639.x. [DOI] [PubMed] [Google Scholar]
- *Koster MI, Kim S, Mills AA, DeMayo FJ, Roop DR. p63 is the molecular switch for initiation of an epithelial stratification program. Genes and Dev. 2004;18:126–131. doi: 10.1101/gad.1165104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy L, Broad S, Diekmann D, Evans RD, Watt FM. β1 Integrins regulate keratinocyte adhesion and differentiation by distinct mechanisms. Mol Biol Cell. 2000;11:453–466. doi: 10.1091/mbc.11.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, B. and Tomic-Canic, M. (2002) Tissue specificity of steroid action: Glucocorticoids in epidermis. In Molecular Mechanisms of action of steroid hormone receptors. (Krstic-Dermonacos, M. and Demonacos, C., eds.), , pp. 1–25, Research Signpost Co.
- Li A, Simmons PJ, Kaur P. Identification and isolation of candidate human keratinocyte stem-cells based on cell-surface phenotype. Proc Natl Acad Sci USA. 1998;95:3902–3907. doi: 10.1073/pnas.95.7.3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Li A, Normand P, Redvers R, Kaur P. Extensive tissue-regenerative capacity of neonatal human keratinocyte stem cells and their progeny. J Clin Invest. 2004;113:390–400. doi: 10.1172/JCI19140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Turi TG, Schuck A, Freedberg IM, Khitrov G, Blumenberg M. Rays and arrays: the transcriptional program in the response of human epidermal keratinocytes to UVB illumination. FASEB J. 2001;15:2533–2535. doi: 10.1096/fj.01-0172fje. [DOI] [PubMed] [Google Scholar]
- Liang L, Bickenbach JR. Somatic epidermal stem cells can produce multiple cell lineages during development. Stem Cells. 2002;20:21–31. doi: 10.1634/stemcells.20-1-21. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lyle S, Yang X, Cotsarelis G. Keratin 15 promoter targets putative epithelial stem cells in the hair follicle bulge. J Invest Dermatol. 2003;121:963–968. doi: 10.1046/j.1523-1747.2003.12600.x. [DOI] [PubMed] [Google Scholar]
- Madison KC. Barrier function of the skin: ‘La raison d’etre’ of the epidermis. J Invest Dermatol. 2003;121:231–241. doi: 10.1046/j.1523-1747.2003.12359.x. [DOI] [PubMed] [Google Scholar]
- Mainiero F, Pepe A, Yeon M, Ren Y, Giancotti FG. The intracellular functions of α6β4 integrin are regulated by EGF. J Cell Biol. 1996;134:241–253. doi: 10.1083/jcb.134.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. Wound healing-aiming for perfect skin regeneration. Science (Washington, DC) 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- Merrill BJ, Gat U, DasGupta R, Fuchs E. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 2001;15:1688–1705. doi: 10.1101/gad.891401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michibata H, Chiba H, Wakimoto K, Seishima M, Kawasaki S, Okubo K, Mitsui H, Torii H, Imai Y. Identification and characterization of a novel component of the cornified envelope, cornifelin. Biochem Biophys Res Commun. 2004;318:803–813. doi: 10.1016/j.bbrc.2004.04.109. [DOI] [PubMed] [Google Scholar]
- Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol. 2002;118:216–225. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature (London) 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- Moffatt P, Salois P, St-Amant N, Gaumond MH, Lanctot C. Identification of a conserved cluster of skin-specific genes encoding secreted proteins. Gene. 2004;334:123–131. doi: 10.1016/j.gene.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Morris RJ, Potten CS. Highly persistent label-retaining cells in the hair follicles of mice and their fate following induction of anagen. J Invest Dermatol. 1999;112:470–475. doi: 10.1046/j.1523-1747.1999.00537.x. [DOI] [PubMed] [Google Scholar]
- *Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nature Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- Muller-Decker K, Hirschner W, Marks F, Furstenberger G. The effects of cyclooxygenase isozyme inhibition on incisional wound healing in mouse skin. J Invest Dermatol. 2002;119:1189–1195. doi: 10.1046/j.1523-1747.2002.19501.x. [DOI] [PubMed] [Google Scholar]
- Nagai MK, Embil JM. Becaplermin: recombinant platelet-derived growth factor, a new treatment for healing diabetic foot ulcers. Exp Opin Biol Ther. 2002;2:211–218. doi: 10.1517/14712598.2.2.211. [DOI] [PubMed] [Google Scholar]
- Nanney, L.B. and King, L.E.J. (1996) Epidermal growth factor and transforming growth factor α. In The molecular and cellular biology of wound repair (C.R.A., ed.), pp. 171–194, Plenum Press, New York
- Nylander K, Vojtesek B, Nenutil R, Lindgren B, Roos G, Zhanxiang W, Sjostrom B, Dahlqvist A, Coates PJ. Differential expression of p63 isoforms in normal tissues and neoplastic cells. J Pathol. 2002;198:417–427. doi: 10.1002/path.1231. [DOI] [PubMed] [Google Scholar]
- Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell (Cambridge, Mass) 2001;104:233–245. doi: 10.1016/s0092-8674(01)00208-2. [DOI] [PubMed] [Google Scholar]
- Park GT, Lim SE, Jang SI, Morasso MI. Suprabasin, a novel epidermal differentiation marker and potential cornified envelope precursor. J Biol Chem. 2002;277:45195–45202. doi: 10.1074/jbc.M205380200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341:491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, Pozin D, McKeon F, De Luca M. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci USA. 2001;98:3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presland RB, Dale BA. Epithelial structural proteins of the skin and oral cavity: function in health and disease. Crit Rev Oral Biol Med. 2000;11:383–408. doi: 10.1177/10454411000110040101. [DOI] [PubMed] [Google Scholar]
- Presland RB, Kuechle MK, Lewis SP, Fleckman P, Dale BA. Regulated expression of human filaggrin in keratinocytes results in cytoskeletal disruption, loss of cell–cell adhesion, and cell cycle arrest. Exp Cell Res. 2001;270:199–213. doi: 10.1006/excr.2001.5348. [DOI] [PubMed] [Google Scholar]
- Raghavan S, Bauer C, Mundschau G, Li Q, Fuchs E. Conditional ablation of β1 integrin in the skin: severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol. 2000;150:1149–1169. doi: 10.1083/jcb.150.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers GE. Hair follicle differentiation and regulation. Int J Dev Biol. 2004;48:163–170. [PubMed] [Google Scholar]
- Sesto A, Navarro M, Burslem F, Jorcano JL. Analysis of the ultraviolet B response in primary human keratinocytes using oligonucleotide microarrays. Proc Natl Acad Sci USA. 2002;99:2965–2970. doi: 10.1073/pnas.052678999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibbald RG. Apligraf living skin equivalent for healing venous and chronic wounds. J Cutan Med Surg. 1998;3:24–28. [PubMed] [Google Scholar]
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell (Cambridge, Mass) 2000;102:451–461. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- Tomic-Canic M, Komine M, Freedberg IM, Blumenberg M. Epidermal signal transduction and transcription factor activation in activated keratinocytes. J Dermatol Sci. 1998;17:167–181. doi: 10.1016/s0923-1811(98)00016-4. [DOI] [PubMed] [Google Scholar]
- Tomic-Canic, M., Agren, M.S. and Alvarez, O.M. (2004) Epidermal repair and the chronic wound. In The epidermis in wound healing, (Rovee, D., Maibach H., eds.), pp. 25–57, CRC Press, U.S.A.
- *Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl MM, Fuchs E. Defining the epithelial stem cell niche in skin. Science (Washington, DC) 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turksen K. Revisiting the bulge. Dev Cell. 2004;6:454–456. doi: 10.1016/s1534-5807(04)00105-4. [DOI] [PubMed] [Google Scholar]
- van Bokhoven H, Hamel BC, Bamshad M, Sangiorgi E, Gurrieri F, Duijf PH, Vanmolkot KR, van Beusekom E, van Beersum SE, Celli J, et al. p63 Gene mutations in EEC syndrome, limb-mammary syndrome, and isolated split hand-split foot malformation suggest a genotype-phenotype correlation. Am J Hum Genet. 2001;69:481–492. doi: 10.1086/323123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz A, Korge BP, Compton JG, Ziegler A, Steinert PM, Mischke D. Physical mapping of a functional cluster of epidermal differentiation genes on chromosome 1q21. Genomics. 1993;18:92–99. doi: 10.1006/geno.1993.1430. [DOI] [PubMed] [Google Scholar]
- Waikel RL, Wang XJ, Roop DR. Targeted expression of c-Myc in the epidermis alters normal proliferation, differentiation and UV-B induced apoptosis. Oncogene. 1999;18:4870–4878. doi: 10.1038/sj.onc.1203040. [DOI] [PubMed] [Google Scholar]
- Waikel RL, Kawachi Y, Waikel PA, Wang XJ, Roop DR. Deregulated expression of c-Myc depletes epidermal stem cells. Nat Genet. 2001;28:165–168. doi: 10.1038/88889. [DOI] [PubMed] [Google Scholar]
- Watt FM. Stem cell fate and patterning in mammalian epidermis. Curr Opin Genet Dev. 2001;11:410–417. doi: 10.1016/s0959-437x(00)00211-2. [DOI] [PubMed] [Google Scholar]
- Werner S, Smola H, Liao X, Longaker MT, Krieg T, Hofschneider PH, Williams LT. The function of KGF in morphogenesis of epithelium and reepithelialization of wounds. Science (Washington, DC) 1994;266:819–822. doi: 10.1126/science.7973639. [DOI] [PubMed] [Google Scholar]
- Wong P, Coulombe PA. Loss of keratin 6 (K6) proteins reveals a function for intermediate filaments during wound repair. J Cell Biol. 2003;163:327–337. doi: 10.1083/jcb.200305032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, Andrews NC, Caput D, McKeon F. p63, a p53 homolog at 3q27–3q29, encodes multiple products with transactivating, death-inducing and dominant-negative activities. Mol Cell. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- Yang AA, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, Tabin C, Sharpe A, Caput D, Crum C, McKeon F. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature (London) 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- Yang A, Kaghad M, Caput D, McKeon F. On the shoulders of giants: p63, p73 and the rise of p53. Trends Genet. 2002;18:90–95. doi: 10.1016/s0168-9525(02)02595-7. [DOI] [PubMed] [Google Scholar]
- Zambruno G, Marchisio PC, Marconi A, Vaschieri C, Melchiori A, Giannetti A, De Luca M. Transforming growth factor-β1 modulates β1 and β5 integrin receptors and induces the de novo expression of the αvβ6 heterodimer in normal human keratinocytes: implications for wound healing. J Cell Biol. 1995;129:853–865. doi: 10.1083/jcb.129.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]


