Abstract
The alternative sigma factor σΕ (RpoE) is involved in the response to extracytoplasmic stress and plays a role in the virulence of a variety of different bacteria. To assess the role of σΕ in Vibrio cholerae pathogenesis, a ΔrpoE mutant was constructed and analyzed using the infant mouse model. The results here show that σΕ contributes significantly to the virulence of V. cholerae. The ΔrpoE mutant was highly attenuated with a 50% lethal dose more than 3 logs higher than that for the parental strain, and its ability to colonize the intestine was reduced approximately 30-fold. A time course of infection revealed that the number of CFU of the ΔrpoE mutant was approximately 1 log lower than that of the parental strain by 12 h postinoculation and decreased further by 24 h. The defect in virulence in the ΔrpoE mutant thus appears to be a diminished ability to survive within the intestinal environment. The results here also show that σΕ is not required for growth and survival of V. cholerae in vitro at high temperatures but is required under other stressful conditions, such as in the presence of 3% ethanol. As in Escherichia coli, the expression of rpoE in V. cholerae is dependent upon two promoters located upstream of the gene, P1 and P2. P1 appears to be σ70 dependent, whereas the downstream promoter, P2, is positively autoregulated by σΕ.
Vibrio cholerae is an intestinal pathogen that causes the life-threatening diarrheal disease cholera. The organism is acquired by oral ingestion of contaminated food or water, and once it passes through the gastric acid barrier of the stomach, it colonizes the epithelium of the small intestine using the toxin-coregulated pilus (TCP) (32) and produces cholera toxin. Cholera toxin is a potent ADP-ribosylating exotoxin that is responsible for the profuse diarrhea associated with the disease (17). The expression of TCP and cholera toxin in V. cholerae is regulated by a complex transcriptional cascade that occurs in response to stimuli in the environment (for reviews, see references 3 and 31).
In order for pathogenic bacteria to survive and grow within living host tissues, they must be able to monitor changes in their extracellular environment and to respond accordingly by altering the expression of various genes. Alternative sigma factors provide one common means of regulating gene expression in response to various extracellular changes, and a number of these sigma factors have been shown to contribute to virulence (reviewed in references 6 and 8). For example, the alternative sigma factor σS (rpoS), which is involved in the general stress response and which is required for survival upon exposure to starvation conditions, low pH, and oxidative stress, plays an important role in the virulence of Salmonella enterica serovar Typhimurium (7, 11).
Two additional alternate sigma factors, σ32 (rpoH) and σΕ (rpoE), are also involved in the response to environmental stress, and their activities are specifically increased by exposure to conditions such as high temperature or ethanol (reviewed in references 10 and 36). Whereas σ32 responds to the presence of misfolded proteins in the cytoplasm and directs the transcription of a number of heat shock genes that function to assist in the folding or unfolding of proteins, σΕ controls a less-well-defined extracytoplasmic response that is signaled by the accumulation of immature outer membrane protein precursors in the periplasmic space (20). σΕ belongs to a growing family of extracytoplasmic sigma factors that regulate diverse functions in bacteria such as alginate and carotenoid biosynthesis, cobalt and nickel efflux, as well as virulence (reviewed in reference 25). With respect to virulence, σΕ has been shown to play a role in the survival of a number of pathogenic bacteria within their hosts. For example, serovar Typhimurium rpoE mutants are unable to survive and proliferate in mice and as a result are highly attenuated (16, 33), and Mycobacterium tuberculosis rpoE mutants are defective in their ability to grow and survive inside macrophages (19). The Pseudomonas aeruginosa σΕ homologue AlgU confers resistance to phagocytic killing (35) and plays a role in the development of cystic fibrosis since it is responsible for the conversion to mucoidy (6).
To assess whether rpoE contributes to the virulence of V. cholerae within its host, a ΔrpoE mutant was constructed and analyzed using the infant mouse model. In this report, we show that V. cholerae ΔrpoE mutants are unable to kill infant mice and exhibit a defect in intestinal colonization. These phenotypes appear to be due to the reduced ability of the ΔrpoE mutant to survive within the intestinal environment. We also show here that similar to the situation in E. coli (28, 29), the expression of rpoE in V. cholerae is dependent upon two promoters located upstream of the gene. The upstream promoter P1, appears to be σ70 dependent, whereas the downstream promoter, P2, is σΕ dependent, thereby creating a regulatory loop in which σΕ positively controls its own expression.
MATERIALS AND METHODS
Construction of bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. The classical biotype ΔrpoE plasmid pKAS175 was constructed by PCR amplifying two 500-bp fragments from O395 using primer SE-Eco (5′-GATCGGAATTCAATTCCCAAACTGTAGCGAG) with SE-NotI (5′-GATCGGCGGCCGCACTCGTTCATTCGAGCGGTCAC) and SE-Not2 (5′-GATCGGCGGCCGCCTTCTGTAACGCAAATTCCG) with SE-Bam (5′-GATCGGGATCCTCACGAGTCAAACTCACTGG). The resulting fragments were ligated into pKAS32 (30). An E. coli lacZ fragment was inserted between the two fragments in pKAS175 to generate pKAS177, and this ΔrpoE-lacZ mutation was introduced from S17-1 λ pir into CG842 by allelic exchange generating KSK1665. The ΔlacZ plasmid pKAS180 was constructed by PCR amplifying two 500-bp fragments flanking the lacZ gene from O395 using primer CHR-1 (5′-GATCGGCGGCCGCGATATCATGCTAACGATTTTTAGAAC) with CHR-2 (5′-GATCGGAATTCGACCGCCACCAAACACTAAG) and primer GAL-1 (5′-GATCGGCGGCCGCGCCAGAGAGCCTTAAGGCTC) with GAL-2 (5′-GATCGGGATCCACGCCAACGAGGTAAAAACG). The resulting fragments were ligated into pKAS32. An rpoE promoter-lacZ fragment was digested out of pKAS177 and subsequently ligated between the two fragments of homology in pKAS180. The fusion in the resulting plasmid, pKAS183, was then introduced into CG842, generating KSK1686. The ΔrpoE mutation was introduced into KSK1686 with pKAS175, generating KSK1695. The El Tor biotype ΔrpoE plasmid pKAS188 was constructed using the same primers as for the classical biotype, except the products were amplified from C6706 str2 and ligated into pKAS154, a derivative of pKAS32 in which the Ampr gene was replaced by a Kanr gene to allow cloning into the BglII site. The σΕ overproducing plasmid pGKK220 was constructed by amplifying the coding region of rpoE from the classical biotype with primers SE-11 (5′-CACCATGAACGAGCAACTGACCGATC) and SE-Sph (5′-GATCGGCATGCCGGAATTTGCGTTACAGAAG), and the resulting fragment was inserted into pBAD22 (12).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or phenotype | Reference or source |
|---|---|---|
| Strains | ||
| V. cholerae | ||
| O395 | Classical Ogawa Smr | Laboratory collection |
| C6706 str2 | El Tor Inaba Smr | Laboratory collection |
| CG842 | O395 ΔlacZ | 2 |
| KSK1665 | CG842 ΔrpoE-lacZ | This work |
| KSK1686 | CG842 ΔlacZ::rpoEP1 + P2-lacZ rpoE+ | This work |
| KSK1695 | KSK1686 ΔrpoE | This work |
| KSK1747 | CG842 ΔlacZ::rpoEP1-lacZ rpoE+ | This work |
| KSK1748 | CG842 ΔlacZ::rpoEP2-lacZ rpoE+ | This work |
| KSK1769 | C6706 str2 ΔrpoE | This work |
| E. coli S17-1 λ pir | Tpr SmrrecA thi pro hsdR−M+ [RP4-2-Tc::Mu::Kmr Tn7] (λ pir) | 5 |
| Plasmids | ||
| pKAS175 | ΔrpoE (classical) in pKAS32 | This work |
| pKAS177 | ΔrpoE-lacZ in pKAS32 | This work |
| pKAS180 | ΔlacZ in pKAS32 | This work |
| pKAS183 | rpoE-lacZ in pKAS180 | This work |
| pKAS188 | ΔrpoE (El Tor) in pKAS154 | This work |
| pGKK220 | rpoE+ in pBAD22 | This work |
| pGKK230 | rpoEP1-lacZ (ΔP2) in pKAS180 | This work |
| pGKK231 | rpoEP2-lacZ (ΔP1) in pKAS180 | This work |
Construction of the chromosomal rpoE ΔP1 and ΔP2 mutations.
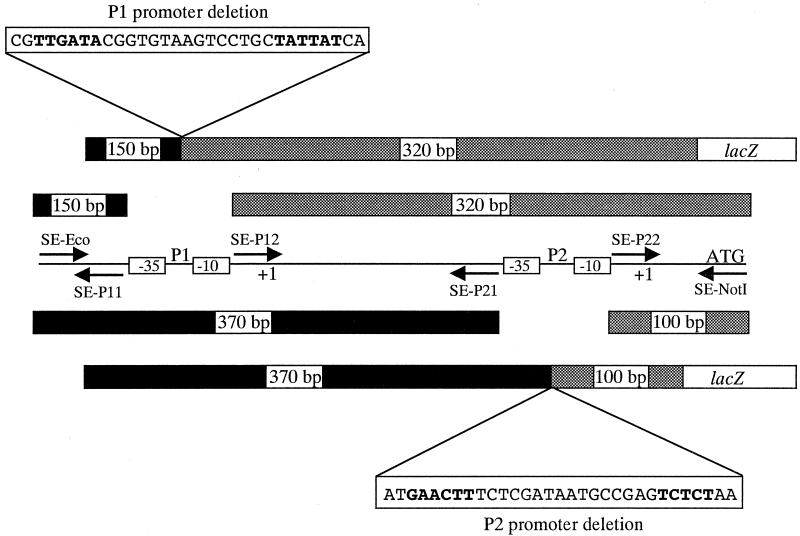
Oppositely oriented overlapping primers were designed which flank the −35 and −10 regions of the rpoE P1 and P2 promoters (see Fig. 5) and which contain restriction sites for the type IIS enzyme EarI. For the P1 deletion, amplification with the upstream primer SE-P11 (5′-GATCGCTCTTCGCGAATCCGAATTTTTCGACAATTTC) and SE-Eco produced a 150-bp fragment, and amplification with the downstream primer SE-P12 (5′-GATCGCTCTTCGTCGCGCTAAATGCAATAGTTTG) and SE-NotI produced a 320-bp fragment. For the P2 deletion, PCR amplification with the upstream primer SE-P21 (5′-GATCGCTCTTCGCCTTATTGGGTGAAATATTTTAC) and SE-Eco produced a 370-bp fragment, and amplification with the downstream primer SE-P22 (5′-GATCGCTCTTCGAGGTGCTCATGCAAGTAGTGG) and SE-NotI produced a 100-bp fragment. For each promoter, ligation of the two resulting fragments into pKAS32 generated deletions of 31 bp. After sequencing, a promoterless lacZ gene was introduced into these constructs in the proper orientation. The fusions were then transferred into pKAS180, generating pGKK230 and pGKK231, prior to allelic exchange into CG842.
FIG. 5.
Construction of the chromosomal rpoE ΔP1 and ΔP2 mutations. PCR was carried out with oppositely oriented overlapping primers which flank the −35 and −10 regions of the rpoE P1 and P2 promoters and which contain restriction sites for the type IIS enzyme EarI. For the P1 promoter deletion mutant (top), SE-P11 and SE-Eco produced a 150-bp upstream fragment (black bar) and SE-P12 and SE-NotI produced a 320-bp downstream fragment (grey bar). For the P2 promoter deletion mutant (bottom), SE-P21 and SE-Eco produced a 370-bp upstream fragment (black bar) and SE-P22 and SE-NotI produced a 100-bp downstream fragment (grey bar). For each promoter, ligation of the two resulting fragments together into pKAS32 generated deletions of 31 bp. A promoterless lacZ gene was then introduced into these constructs in the proper orientation, and the fusions were transferred into pKAS180 prior to allelic exchange into CG842.
Growth conditions.
Strains were maintained at −70°C in Luria-Bertani (LB) medium (23) containing 30% (vol/vol) glycerol. The following antibiotics were used at the indicated concentrations in LB medium unless otherwise noted: ampicillin, 100 μg/ml; kanamycin 45 μg/ml; streptomycin, 100 μg/ml, except when selecting for loss of plasmid integrants, when it was used at 1 mg/ml. X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) was used in LB agar at 40 μg/ml.
Colonization and LD50 assays.
The infant mouse competition assays were performed essentially as described (32). Three- to five-day-old suckling CD-1 mice (Charles River) were inoculated orally, and the total CFU were obtained from the small intestine of four to six mice at the given times by plating intestinal homogenates on streptomycin-containing X-Gal plates. The competitive index is defined as the difference between the input and output ratios of the strains. The 50% lethal doses (LD50s) were determined by oral challenge with various doses of viable bacteria. Four mice were used per dose, and the results were analyzed after 48 h. Prior to inoculation, all strains were grown in LB medium pH 6.5 at 30°C.
Identification of the rpoE transcriptional start sites.
Total RNA was isolated from O395 after growth for 4 h in LB medium, pH 6.5, at 30°C using RNA-WIZ (Ambion). The RNA was subjected to 5′ rapid amplification of cDNA ends (RACE) according to the procedures described by Gibco BRL. Briefly, first-strand cDNA synthesis was carried out with the rpoE-specific primer SER-2 (5′-GCCATCAAGCTCGCGCAACG). The resulting cDNA was purified on a PCR purification column (Qiagen), and poly(dC) [or poly(dA) if the start site potentially begins with G] tails were added to the 3′ ends using terminal deoxynucleotidyl transferase. For the poly(dA)-tailed cDNA, it was necessary to perform a second-strand cDNA synthesis using the 3′ RACE adapter primer [5′-GGCCACGCGTCGACTAGTAC(T)17] prior to PCR. PCR of the cDNA was carried out initially using the 5′ RACE-abridged anchor primer (5′-GGCCACGCGTCGACTAGTACGGGIIGGGIIGGGIIG) for the poly(dC)-tailed cDNA and the abridged universal amplification primer (5′-GGCCACGCGTCGACTAGTAC) for the poly(dA)-tailed cDNA. The first and second nested primers for PCR were SER-4 (5′-TGCCGTTTTTAAATCATCCG) and SER-6 (5′-AGTCTTCTTCAACTCTTTGG), respectively.
β-Galactosidase assays.
β-Galactosidase assays (23) with V. cholerae rpoE-lacZ fusions were carried out with cultures grown in LB medium pH 6.5 at 30°C for 4 h with aeration.
RESULTS
Inactivation of the rpoE gene in V. cholerae.
An in-frame deletion in the rpoE gene was constructed by PCR amplifying two 500-bp fragments flanking rpoE in the V. cholerae chromosome and inserting them into the positive selection vector pKAS32 (30). An E. coli lacZ fragment was subsequently inserted between the two fragments, and the resulting plasmid, pKAS177, was used for allelic exchange with CG842 to generate strain KSK1665.
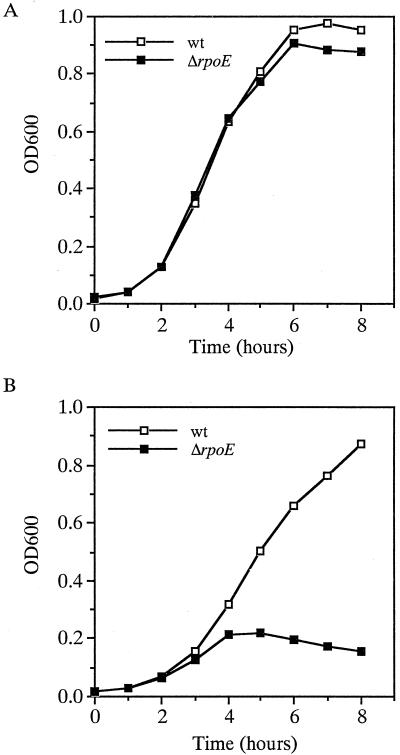
The rpoE gene is essential for the growth and survival of E. coli, but not S. enterica serovar Typhimurium, at high temperatures (13, 16). To assess whether rpoE is required for growth of V. cholerae at high temperatures, O395 and the ΔrpoE mutant KSK1665 were grown at 43°C over an 8-h period. As shown in Fig. 1A, growth remained similar for the wild type and the ΔrpoE mutant over a 4-h period and then declined only slightly thereafter for the mutant. This is in contrast to the pattern observed in E. coli, in which growth of the rpoE mutant declined sharply after 60 min (13), and in serovar Typhimurium, in which the mutant grew but exhibited a longer lag phase throughout the entire growth curve (16). These results show that rpoE is not required for high-temperature growth of V. cholerae.
FIG. 1.
Growth of O395 and the ΔrpoE derivative KSK1665 in LB medium at 43°C (A) and in the presence of 3% ethanol at 37°C (B). Open boxes, O395 (wild type wt]); closed boxes, KSK1665.
Ethanol closely mimics the effects of heat as an inducer of the heat shock response (26) and, unlike the situation observed at 43°C, the rpoE gene is required for the growth of V. cholerae in LB medium containing 3% ethanol. As shown in Fig. 1B, O395 grew in a culture containing 3% ethanol at 37°C at a slightly reduced rate compared to 42°C. In contrast, the ΔrpoE mutant lysed after 4 h in the presence of 3% ethanol indicating that σΕ is required for its survival under this condition. The effect of stress on the V. cholerae rpoE mutant appears to be specific, however, since the mutant was not any more sensitive than the wild-type strain to the presence of bile salts (2%), H2O2 (0.5%), the antimicrobial peptide polymyxin B (5 U/ml), or changes in osmolarity (5 to 800 mM), or pH (5.5 to 10) (data not shown).
V. cholerae rpoE mutants are attenuated for virulence and defective for colonization.
To determine if σΕ influences virulence in V. cholerae, the ability of the ΔrpoE mutant KSK1665 to kill infant mice was assessed. For these experiments, the mutant and its parental strain CG842 were grown overnight in LB medium, pH 6.5, at 30°C and fed orally to 3- to 5-day-old CD-1 mice. After 48 h, the LD50s were determined. For the ΔrpoE mutant, the LD50 was found to be >1.0 × 108, whereas it was approximately 1.0 × 105 for CG842. These results indicate that the ΔrpoE mutant is significantly attenuated for virulence compared to wild-type V. cholerae.
To address whether the reduced virulence of the ΔrpoE mutant was due to a defect in its ability to colonize the infant mouse intestines, the ΔrpoE mutant KSK1665 was subjected to competition against CG842 in infant mouse colonization assays. For these experiments, the mutant and parental strains were grown overnight as described above, mixed equally, and fed orally to the infant mice. After 24 h the mice were euthanized, and dilutions of intestinal homogenates were plated on agar containing selective antibiotic and X-Gal. The ΔrpoE mutant was found to be reduced for colonization relative to CG842, with a mean competitive index of 3.3 × 10−2. In an in vitro competition assay, there was no observable difference between the mutant and parental strains (data not shown). The attenuation that is observed with the ΔrpoE mutant thus appears to be the result of its reduced ability to colonize the infant mouse intestine. This reduced colonization is not restricted to the classical biotype of V. cholerae, since a ΔrpoE mutant in the El Tor biotype strain C6706 str2 (KSK1769) had a mean competitive index of 2.4 × 10−2 relative to its parent and also showed no difference when subjected to competition against its parent in vitro. The reduction in colonization observed with the ΔrpoE mutants is not due to their inability to produce TCP, since both biotype strains showed wild-type levels of TCP under in vitro inducing conditions (data not shown).
V. cholerae rpoE mutants have a reduced ability to persist within the small intestine.
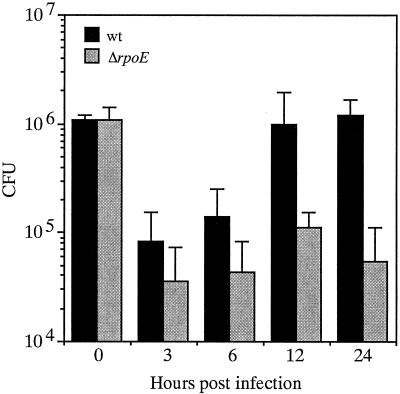
Given the importance of σΕ in the response to environmental stress, it seemed possible that the reduced colonization of the ΔrpoE mutant might be due to a diminished ability to survive within the infant mouse small intestine. To assess this, a time course of colonization was performed. For these experiments, CG842 and the ΔrpoE mutant KSK1665 were mixed equally and administered to infant mice as described above except that at various times over a 24-h period, three mice were sacrificed and the total number of intestinal CFU recovered from each mouse for each strain was determined and averaged. As shown in Fig. 2, the ΔrpoE mutant was able to colonize and grow within the small intestine, but at a reduced rate compared to the parental strain. By 12 h postinoculation, the number of CFU of the ΔrpoE mutant was approximately 1 log lower than that of the parental strain, and by 24 h this number was reduced even further. These results indicate that the ΔrpoE mutant has a defect in its ability to survive within the small intestine.
FIG. 2.
CFU of CG842 and the ΔrpoE derivative KSK1665 at various times postinoculation from infant mice. Black bars, CG842; grey bars, KSK1665. Using the Prism program, a nonpaired two-tailed t test with the values from the 24-h time point were statistically significant (P = 0.01). Error bars, standard deviations.
Identification of the transcriptional start sites of rpoE.
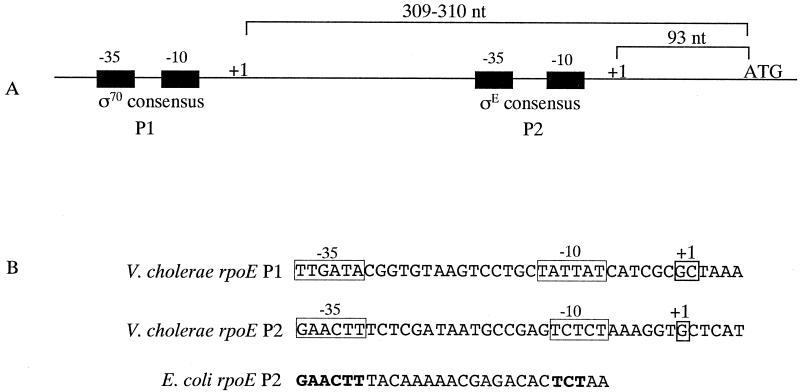
The expression of the E. coli rpoE gene is controlled by two promoters located upstream of the gene, P1 (174 nucleotides [nt] upstream) and P2 (75 nt upstream), and expression from P2 is positively autoregulated by σΕ (28, 29). To determine if the V. cholerae rpoE gene has a similar regulatory arrangement, its transcriptional start sites were identified using 5′ RACE (9). For this procedure, RNA was isolated from a mid-log culture of O395 grown in LB, pH 6.5, at 30°C. cDNA was initially synthesized from the RNA using an antisense primer within the rpoE gene, and a homopolymeric tail was added to the resulting 3′ end. The cDNA was then amplified by PCR using tail-specific primers and nested rpoE primers. Sequencing of the major resulting PCR product revealed a transcriptional start site (G) 93 nt upstream of the ATG start codon (Fig. 3A). This promoter appears to be analogous to the E. coli P2 promoter, since it exhibited high similarity with other σΕ regulated promoters (29). The −35 sequence, GAACTT, was a perfect match with the E. coli P2 promoter, and the −10 sequence, TCTCT showed only two mismatches from the TCTAA of E. coli (Fig. 3B). In addition, the spacing between the two regions, 16 bp, was the same for both promoters.
FIG. 3.
Organization of the V. cholerae rpoE P1 and P2 promoters. (A) The distances between the transcriptional start sites and the rpoE start codon (not to scale) are shown. (B) The nucleotide sequences of the −35 and −10 regions of the promoters are shown. The base pairs within the E. coli rpoE P2 −35 and −10 regions that are identical to those of the V. cholerae rpoE P2 promoter are shown in bold.
In addition to the primary PCR product from 5′ RACE, a minor band was also observed, and sequencing of it revealed a second transcriptional start site (G or C) 309 to 310 nt upstream of the ATG start codon (Fig. 3A). This promoter did not match the σΕ consensus, suggesting it is analogous to the E. coli P1 promoter. However, unlike the E. coli P1 promoter, this promoter does appear to closely match the consensus for σ70. The −35 sequence, TTGATA, has only one mismatch from the σ70 consensus TTGACA, and the −10 sequence, TATTAT, has only one mismatch from the consensus TATAAT (Fig. 3B).
Transcriptional regulation of rpoE expression in V. cholerae.
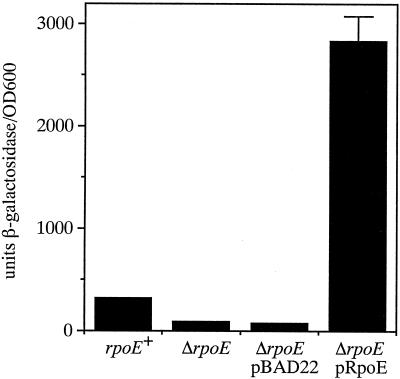
The finding that the promoter immediately upstream of rpoE in V. cholerae exhibited high similarity with σΕ-regulated promoters in E. coli suggested that σΕ also positively regulates its own expression in V. cholerae. To assess this, an rpoE-lacZ reporter fusion was constructed in the V. cholerae chromosome at the lacZ locus so that a functional copy of rpoE could remain in the strain. To introduce the rpoE-lacZ reporter fusion into the V. cholerae lacZ locus, we developed a suicide vector, pKAS180, specifically for this purpose. pKAS180 contains portions of the chrA and galR genes which flank lacZ in the V. cholerae chromosome (27) to serve as regions of homology for integration but lacks the entire lacZ gene. The rpoE-lacZ reporter fusion was inserted between the chrA and galR genes in pKAS180, generating pKAS183, and the fusion was introduced into the ΔlacZ locus of CG842, generating KSK1686. The ΔrpoE mutation was subsequently introduced into this fusion strain at the rpoE locus, generating KSK1695. As shown in Fig. 4, the rpoE+ rpoE-lacZ reporter strain KSK1686 produced approximately 300 U of β-galactosidase in mid-log LB medium, pH 6.5, cultures at 30°C, and the ΔrpoE mutation in this background reduced β-galactosidase production approximately fourfold under these conditions. Complementation of the ΔrpoE mutation with the σΕ-overexpressing plasmid pGKK220 resulted in a 37-fold increase in β-galactosidase production, raising the level almost 10-fold higher than that of the rpoE+ strain. These results indicate that σΕ positively regulates its own expression in V. cholerae as in E. coli.
FIG. 4.
Autoregulation of rpoE expression in V. cholerae. Cultures were grown in LB medium, pH 6.5, at 30°C. From left to right, strains are KSK1686 (rpoE+), KSK1695 (ΔrpoE), KSK1695 pBAD22, and KSK1695 pGKK220 (pRpoE). The cultures with plasmids contained 0.05% arabinose. Error bars, standard deviations.
To assess the relative contributions of the P1 and P2 promoters to the expression of the rpoE-lacZ reporter fusion in KSK1686, chromosomal deletions of either promoter upstream of the fusion were constructed. This was accomplished using overlapping primers which flank each −35 and −10 region such that ligating the resulting PCR products together created a seamless 31-bp deletion of each promoter (Fig. 5). After inserting a promoterless lacZ gene into the constructs, the fusions were transferred into pKAS180 and introduced into the ΔlacZ locus of CG842. The resulting strains, KSK1747 and KSK1748, are identical to KSK1686 except that instead of having two functional rpoE promoters (P1 and P2) contributing to the expression of lacZ, each strain has a deletion of one of them. As shown in Table 2, the ΔrpoE P2 strain KSK1747 showed an approximately 80% reduction in β-galactosidase production relative to the strain containing both promoters, whereas the ΔrpoE P1 strain KSK1748 showed only a slight, approximately 20%, but reproducible reduction in β-galactosidase production. These results demonstrate that the P2 promoter plays a larger role in rpoE expression in V. cholerae than P1 and are consistent with the findings above that the rpoE P1 product obtained from 5′ RACE was considerably reduced compared to that of P2. Furthermore, the σΕ-overexpressing plasmid pGKK220 increased β-galactosidase production only in the ΔrpoE P1 strain KSK1748 (Table 2), thus confirming its role at the P2 promoter. It is noteworthy that the expression of rpoE-lacZ in neither the wild-type nor the promoter deletion strains was significantly responsive to changes in environmental conditions. For example, there was virtually no change in rpoE expression observed upon shifting the cultures from 30 to 50°C (data not shown).
TABLE 2.
Activation of the rpoE-P2 promoter by RpoE
| Strain | Mean β-Galactosidase activity ± SDa |
|---|---|
| KSK1686 (ΔlacZ::rpoEP1 + P2-lacZ rpoE+) | 336 ± 1 |
| KSK1747 (ΔlacZ::rpoEP1-lacZ rpoE+) | 84 ± 4 |
| KSK1747 pBAD22b | 107 ± 1 |
| KSK1747 pGKK220 (RpoE) | 124 ± 1 |
| KSK1748 (ΔlacZ::rpoEP2-lacZ rpoE+) | 277 ± 11 |
| KSK1748 pBAD22 | 412 ± 3 |
| KSK1748 pGKK220 (RpoE) | 2,407 ± 96 |
Units per optical density at 600 nm.
All plasmids induced with 0.05% arabinose.
DISCUSSION
Pathogenic bacteria optimize survival and growth within the host environment by inducing the expression of genes to help them cope with the myriad of stresses they encounter during infection. The expression of many genes that confer resistance to environmental stress are under the control of alternative sigma factors, and some of these, such as σS and σΕ, have clear roles in both the stress response and virulence. The results presented here show that in V. cholerae, σΕ plays a significant role in both of these processes. Although σΕ is not required in V. cholerae for survival at high temperatures, in contrast to the situation in E. coli, V. cholerae rpoE mutants are incapable of surviving for long periods under other kinds of environmental stress, such as exposure to 3% ethanol. E. coli rpoE mutants are known to be sensitive to a variety of other stresses, such as chemicals that disrupt the outer membrane, suggesting they may have a defective cell envelope (29). However, they still maintain the ability to respond normally to other types of stress, such as that induced by changes in osmolarity or oxidative damage (29), and this appears to be the case for V. cholerae as well.
The rpoE gene contributes significantly to the virulence of V. cholerae. Administration of undiluted overnight cultures of the ΔrpoE mutant KSK1665 to infant mice resulted in no deaths or any evidence of sickness. This ΔrpoE mutant was also impaired for colonization approximately 30-fold in infant mice. This attenuation of virulence appears to be due to a reduced ability of the mutant to survive within the intestinal environment, since by 12 h postinoculation the CFU of the ΔrpoE mutant recovered from mice was approximately 1 log lower than that for the parental strain, and its numbers decreased even further by 24 h. Whether this diminished ability to survive within the intestine is solely responsible for the reduced lethality observed here is not yet known. In vitro, the ΔrpoE mutation does not influence the expression of the tcp or ctx genes themselves. Assuming that the expression of tcp or ctx is not influenced in vivo in the ΔrpoE mutant, its significant attenuation suggests that some gene or genes regulated by σΕ play an important role in the intestinal survival of this organism. At present, none of the promoters that σΕ regulates in V. cholerae have been identified. Known targets of σΕ in other bacteria include the P3 promoter of rpoH (σ32); htrA (degP), which encodes a periplasmic protease (28, 29); and fkpA, a peptidyl-prolyl-isomerase involved in protein folding in the periplasm (25). In serovar Typhimurium, both htrA and fkpA play a role in virulence, since mutants lacking either gene have a reduced ability to survive intracellularly (1, 14).
An analysis of rpoE expression in V. cholerae revealed that it is dependent upon two promoters located upstream of the gene. The upstream promoter P1, appears to be σ70 dependent, whereas the downstream promoter, P2, is σΕ dependent, thereby establishing a positive autoregulatory loop. This transcriptional arrangement is similar to the situation in E. coli except that P1 is not transcribed by RNA polymerase containing σ70 (28, 29). In addition, the contribution of the P1 promoter to rpoE expression in V. cholerae appears to be smaller than that in E. coli (28). The lower levels of P1 product relative to P2 obtained from 5′ RACE suggested that this transcript was less abundant in the cell and most likely explains why poly(A) tailing could not be performed to discern whether the actual start is the G or the C for this promoter. In V. cholerae, the expression of rpoE was not dramatically responsive to shifts in temperature from 30 to 50°C or the addition of ethanol. A similar situation has been observed in E. coli (28).
The σΕ-mediated response is specifically induced by the generalized effectors heat and ethanol as well as by the accumulation of immature outer membrane precursors in the periplasmic space (20, 29). This regulation occurs primarily through a posttranscriptional mechanism involving anti-sigma factors which sequester the σ in a nonfunctional state in the absence of the proper stimulus (reviewed in reference 15). Induction by the proper stimulus results in a rapid increase in the activity of the sigma factor by either removal or inactivation of its cognate anti-sigma factor. In E. coli, rpoE is the first gene in a four-gene operon; the three downstream genes encode its specific regulators RseA, RseB, and RseC (4, 24). RseA and RseB exhibit anti-σE activity, and RseC may play a role as an inhibitor of RseA/RseB activity. The V. cholerae rpoE gene also appears to be the first in a four-gene operon, and the three downstream open reading frames bear a strong sequence similarity to the negative regulators described in E. coli. This finding suggests that the posttranscriptional regulatory mechanisms of σE are similar between the two organisms.
It is not yet known what effectors are encountered in the infant mouse intestine that induce the activity of σΕ in V. cholerae, but this induction clearly is necessary for the survival of the organism within this niche. Presumably, it is encountering some stress that is causing immature outer membrane protein precursors to accumulate in the periplasm, and it has a reduced ability to survive without a mechanism for alleviating this. Serovar Typhimurium and M. tuberculosis rpoE mutants have an increased susceptibility to oxidative stress, and it has been suggested that the σΕ regulon plays an important antioxidant role in these pathogens during infection (16, 19, 33). The ΔrpoE mutant KSK1665 did not appear to be any more sensitive to oxidative stress than the wild-type strain. It is likely that the σΕ regulon plays different roles in pathogenesis depending on the particular niches inhabited by the pathogens.
V. cholerae possesses other systems in addition to σΕ that contribute to its stress survival and influence pathogenesis. For example, V. cholerae has an acid tolerance response system that influences intestinal colonization (22). The alternative sigma factor σS controls the expression of a large number of genes in V. cholerae that enhance the ability of the organism to survive diverse environmental stresses (34), but it does not appear to play a significant role in intestinal colonization (18, 21, 34). Clearly, additional work is required to determine what signals are encountered by V. cholerae within its host and how the stress response systems they induce coordinately contribute to its pathogenesis.
Acknowledgments
This work was supported by Public Health Service grant AI-41558 from the National Institutes of Health to K.S.
We thank Ronald Taylor for critical reading of the manuscript and Bill Wade for assistance with the statistical analyses.
Editor: V. J. DiRita
REFERENCES
- 1.Bäumler, A. J., J. G. Kusters, I. Stojiljkovic, and F. Heffron. 1994. Salmonella typhimurium loci involved in survival within macrophages. Infect. Immun. 62:1623-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiang, S. L., R. K. Taylor, M. Koomey, and J. J. Mekalanos. 1995. Single amino acid substitutions in the N terminus of Vibrio cholerae TcpA affect colonization, autoagglutination, and serum resistance. Mol. Microbiol. 17:1133-1142. [DOI] [PubMed] [Google Scholar]
- 3.Cotter, P. A., and V. J. DiRita. 2000. Bacterial virulence gene regulation: an evolutionary perspective. Annu. Rev. Microbiol. 54:519-565. [DOI] [PubMed] [Google Scholar]
- 4.De Las Peñas, A., L. Connolly, and C. A. Gross. 1997. The σΕ−mediated response to extracytoplasmic stress in Escherichia coli is transduced by RseA and RseB, two negative regulators of σΕ. Mol. Microbiol. 24:373-385. [DOI] [PubMed] [Google Scholar]
- 5.de Lorenzo, V., and K. N. Timmis. 1994. Analysis and construction of stable phenotypes in Gram-negative bacteria with Tn5- and Tn10-derived minitransposons. Methods Enzymol. 235:386-405. [DOI] [PubMed] [Google Scholar]
- 6.Deretic, V., M. J. Schurr, J. C. Boucher, and D. W. Martin. 1994. Conversion of Pseudomonas aeruginosa to mucoidy in cystic fibrosis: environmental stress and regulation of bacterial virulence by alternative sigma factors. J. Bacteriol. 176:2773-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang, F. C., S. J. Libby, N. A. Buchmeier, P. C. Loewen, J. Switala, J. Harwood, and D. G. Guiney. 1992. The alternative σ factor KatF (RpoS) regulates Salmonella virulence. Proc. Natl. Acad. Sci. USA 89:11978-11982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finlay, B. B., and S. Falkow. 1997. Common themes in microbial pathogenicity revisited. Microbiol. Mol. Biol. Rev. 61:136-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frohman, M. A., M. K. Dush, and G. R. Martin. 1988. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc. Natl. Acad. Sci. USA 85:8998-9002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gross, C. A. 1996. Function and regulation of the heat shock proteins, p. 1382-1399. In F. C. Neidhardt, R. I. Curtiss, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 11.Guiney, D. G., S. Libby, F. C. Fang, M. Krause, and J. Fierer. 1995. Growth-phase regulation of plasmid virulence genes in Salmonella. Trends Microbiol. 3:275-279. [DOI] [PubMed] [Google Scholar]
- 12.Guzman, L.-M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose Pbad promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiratsu, K., M. Amemura, H. Nashimoto, H. Shinagawa, and K. Makino. 1995. The rpoE gene of Escherichia coli, which encodes σΕ, is essential for bacterial growth at high temperature. J. Bacteriol. 177:2918-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horne, S. M., T. J. Kottom., L. K. Nolan., and K. D. Young. 1997. Decreased intracellular survival of an fkpA mutant of Salmonella typhimurium Copenhagen. Infect. Immun. 65:806-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes, K. T., and K. Mathee. 1998. The anti-sigma factors. Annu. Rev. Microbiol. 52:231-286. [DOI] [PubMed] [Google Scholar]
- 16.Humphreys, S., A. Stevenson, A. Bacon, A. B. Weinhardt, and M. Roberts. 1999. The alternative sigma factor, σΕ, is critically important for the virulence of Salmonella typhimurium. Infect. Immun. 67:1560-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaper, J. B., J. G. Morris, Jr., and M. M. Levine. 1995. Cholera. Clin. Microbiol. Rev. 8:48-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klose, K. E., and J. J. Mekalanos. 1998. Distinct roles of an alternative sigma factor during both free-swimming and colonizing phases of the Vibrio cholerae pathogenic cycle. Mol. Microbiol. 28:501-520. [DOI] [PubMed] [Google Scholar]
- 19.Manganelli, R., M. I. Voskuil, G. K. Schoolnik, and I. Smith. 2001. The Mycobacterium tuberculosis ECF sigma factor σΕ: role in global gene expression and survival in macrophages. Mol. Microbiol 41:423-437. [DOI] [PubMed] [Google Scholar]
- 20.Mecsas, J., P. E. Rouviere, J. W. Erickson, T. J. Donohue, and C. A. Gross. 1993. The activity of σΕ, an Escherichia coli heat-inducible σ-factor, is modulated by expression of outer membrane proteins. Genes Dev. 7:2618-2628. [DOI] [PubMed] [Google Scholar]
- 21.Merrell, D. S., A. D. Tischler, S. H. Lee, and A. Camilli. 2000. Vibrio cholerae requires rpoS for efficient intestinal colonization. Infect. Immun. 68:6691-6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merrell, D. S., D. L. Hava, and A. Camilli. 2002. Identification of novel factors involved in colonization and acid tolerance of Vibrio cholerae. Mol. Microbiol. 43:1471-1491. [DOI] [PubMed] [Google Scholar]
- 23.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 24.Missiakas, D., M. P. Mayer, M. Lemaire, C. Georgopoulos, and S. Raina. 1997. Modulation of the Escherichia coli σΕ (RpoE) heat-shock transcription-factor activity by the RseA, RseB and RseC proteins. Mol. Microbiol. 24:355-371. [DOI] [PubMed] [Google Scholar]
- 25.Missiakas, D., and S. Raina. 1998. The extracytoplasmic function sigma factors: role and regulation. Mol. Microbiol. 28:1059-1066. [DOI] [PubMed] [Google Scholar]
- 26.Neidhardt, F. C., and R. A. VanBogelen. 1987. Heat shock response, p. 1334-1345. In F. C. Neidhardt, J. L. Ingraham, K. B Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology. ASM Press, Washington, D.C.
- 27.Parsot, C. 1992. Identification of a lacZ gene in Vibrio cholerae. Res. Microbiol. 143:27-36. [DOI] [PubMed] [Google Scholar]
- 28.Raina, S., D. Missiakas, and C. Georgopoulos. 1995. The rpoE gene encoding the σΕ (σ24) heat shock sigma factor of Escherichia coli. EMBO J. 14:1043-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rouviere, P. E., A. De Las Peñas, J. Mecsas, C. Z. Lu, K. E. Rudd, and C. A. Gross. 1995. rpoE, the gene encoding the second heat-shock sigma factor σΕ in Escherichia coli. EMBO J. 14:1032-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skorupski, K., and R. K. Taylor. 1996. Positive selection vectors for allelic exchange. Gene 169:47-52. [DOI] [PubMed] [Google Scholar]
- 31.Skorupski, K., and R. K. Taylor. 1997. Control of the ToxR virulence regulon in Vibrio cholerae by environmental stimuli. Mol. Microbiol. 25:1003-1009. [DOI] [PubMed] [Google Scholar]
- 32.Taylor, R. K., V. L. Miller, D. B. Furlong, and J. J. Mekalanos. 1987. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc. Natl. Acad. Sci. USA 84:2833-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Testerman, T. L., A. Vazquez-Torres, Y. Xu, J. Jones-Carson, S. J. Libby, and F. C. Fang. 2002. The alternative sigma factor σΕ controls antioxidant defences required for Salmonella virulence and stationary-phase survival. Mol. Microbiol. 43:771-782. [DOI] [PubMed] [Google Scholar]
- 34.Yildiz, F. H., and G. K. Schoolnik. 1998. Role of rpoS in stress survival and virulence of Vibrio cholerae. Infect. Immun. 180:773-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu, H., J. C. Boucher, N. S. Hibler, and V. Deretic. 1996. Virulence properties of Pseudomonas aeruginosa lacking the extreme-stress sigma factor AlgU (σΕ). Infect. Immun. 64:2774-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yura, T., H. Nagai, and H. Mori. 1993. Regulation of the heat-shock response in bacteria. Annu. Rev. Microbiol. 47:321-350. [DOI] [PubMed] [Google Scholar]