Summary
Opioids alone sometimes provide insufficient postoperative analgesia. Co-administration of drugs may reduce opioid use and to improve opioid efficacy. We therefore tested the hypothesis that administration of ketamine or nefopam, to postoperative patients with pain only partly alleviated by morphine, limits the amount of subsequent opioid necessary to produce adequate analgesia. Patients (n=77) recovering from major surgery were given up to 9 mg intravenous morphine. Those still suffering from pain were randomly assigned to blinded administration of: 1) isotonic saline (Control, n=21); 2) ketamine 10 mg (Ketamine, n=22); or, 3) nefopam 20 mg (Nefopam, n=22). Three-mg morphine boluses were subsequently given at 5-minute intervals until adequate analgesia was obtained, or 60 minutes elapsed after the beginning of the study drug administration, or ventilation became insufficient (respiratory rate < 10 breath/minute or saturation by pulse oxymetery < 95%). Supplemental morphine (i.e., after test drug administration) requirements were significantly greater in the Control group [17 ± 10 (SD) mg] than in the Nefopam (10 ± 5 mg, P < 0.005) or Ketamine (9 ± 5 mg, P < 0.001) groups. Morphine titration was successful in all Ketamine and Nefopam patients, but failed in four Control patients (two from respiratory toxicity and two from persistent pain). Tachycardia and profuse sweating were more frequent in patients given nefopam and sedation was greater with ketamine; however, the incidence of other potential complications did not differ between groups.
Implications
We conclude that ketamine 10 mg and nefopam 20 mg comparably potentiate opioid analgesia, each reducing opioid need by approximately 40%. Ketamine administration was associated with sedation whereas nefopam produced tachycardia and sweating. However, none of the side effects was serious. Either drug can thus be used to potentiate opioid analgesia.
Keywords: ketamine, nefopam, analgesics, balanced analgesia, morphine titration
Introduction
Intravenous morphine titration usually provides rapid and effective analgesia in postoperative patients. However, side effects sometimes occur and may necessitate discontinuation of morphine titration before sufficient pain relief is obtained (1). These cases can be considered a failure of morphine titration.
The combination of non-opioid analgesics with morphine provides a morphine sparing-effect and may decrease dose-limiting toxicity. This concept is the basis of multimodal analgesia (2). The morphine requirement reduction during morphine titration have the double advantage to obtain more rapidly a desired level of analgesia and to decrease the risk of morphine titration failure. Better postoperative analgesia may decrease the amount of time spent by nurses titrating morphine; similarly, it may reduce the amount of time patients needed to stay in the PACU.
Small-dose ketamine possesses N-methyl-d-aspartate (NMDA) receptor non-competitive antagonist properties through a use-dependent channel blockade (3). Several studies demonstrate that it improves opioid analgesia (4). It decreases perioperative opioid analgesic requirements, facilitates passive knee mobilization after arthroscopic anterior ligament repair (5), and improves mobilization after arthroscopic meniscectomy by alleviating provoked pain (6). Potentiation between opioids and ketamine was demonstrated in an animal study (7) and suggested in a study in volunteers (8), whereas another volunteer report favoured for only an additive association (9). Furthermore, ketamine attenuates the development of acute analgesic tolerance to opioids in rats (10) and suppress the rebound hyperalgesia observed after opioid exposure in volunteers (11). A recently published study demonstrated that the combined administration of small-dose ketamine and morphine promptly and satisfactorily resolved pain that was unresponsive to intravenous morphine alone (12).
Nefopam (Acupan®, Laboratory Biocodex, France) is a centrally acting non-opioid analgesic. It has been available since 1976 in most Western Europe countries for intravenous and oral administration and has been available for intravenous administration in France since 1996. Intravenous nefopam quickly produces potent inhibition of the nociceptive flexion reflex in human (13). Nefopam 20 mg is equipotent with morphine 6-12 mg (14,15). It provides morphine-sparing effects when given to postoperative surgical patients (16,17). We therefore tested the hypothesis that administration of ketamine or nefopam to postoperative patients resistant to morphine speeds onset of adequate analgesia while triggering fewer opioid side effects than continued administration of morphine alone.
Materials and Methods
After informed consent and with institutional approval, we enrolled 77 patients who were ASA physical status I or II, aged 18–65 yr, and scheduled for major elective open abdominal (colectomy by laparotomy), urologic (nephrectomy by lombotomy), or orthopedic surgery (hip or knee arthroplasty) under general anesthesia. Exclusion criteria included surgery performed with regional anesthesia, history of chronic pain, regular medication with analgesics, drug or alcohol abuse, psychiatric disorders, morbid obesity, and cardiovascular, renal, or hepatic diseases.
Measurements
Postoperative pain was evaluated with a five-point verbal rating scale (VRS): 0 = no pain, 1 = light pain, 2 = moderate pain, 3 = intense pain, 4 = severe pain. Use of this scale was explained to participating patients at the preanesthetic visit the evening before surgery. Sedation was scored with a numeric scale of 0–3: 0 = patient fully awake, 1 = patient somnolent and responsive to verbal commands, 2 = patient somnolent and responsive to tactile stimulation, 3 = patient asleep and responsive to painful stimulation.
Routine anesthetic safety monitors were used throughout surgery and postoperative recovery. These included respiratory rate, oxygen saturation (SpO2), mean arterial pressure, heart rate. Values were recorded at five-minute intervals, along with pain and sedation scores. Tachycardia was defined by a heart rate that exceeded 100 beat.min−1 for five continuous minutes. Side effects were also recorded, including the incidence of nausea, vomiting, pruritis, dysphoria including hallucinations and nightmares, diplopia, dizziness, dry mouth, or profuse sweating.
Protocol
Patients were given lorazepam 1 mg orally the night before surgery, but no premedication was given on the day of the surgery. General anesthesia was induced with thiopental and atracurium, and maintained with isoflurane in 50% nitrous oxide and sufentanil 0.3–1.3 μg.kg−1.min−1. No analgesic other than sufentanil were used intraoperatively. All patients were mechanically ventilated. Both isoflurane and sufentanil administration were discontinued 15–30 minutes before the end of surgery. Neuromuscular relaxation was antagonized at skin closure and patients were extubated before the transfer to the postanesthesia care unit. Postoperatively, patients were given oxygen supplementation at a rate of five liters per minute via a face mask.
When patients were sufficiently alert and complained of pain (VRS ≥ 2), postoperative analgesia was provided by titrating morphine in 3-mg increments every five minutes in patients with a VRS until adequate pain relief was obtained. Adequate pain relief was defined as a VRS pain score < 2. Morphine was titrated by a single investigator (BK). Patients who had adequate pain relieve (VRS < 2) five minutes after the third bolus of morphine were excluded from the study.
The remaining patients were randomly assigned to one of three groups: 1) isotonic saline (Control); 2) ketamine 10 mg (Ketamine); or, 3) nefopam 20 mg (Nefopam). Each study drug was given intravenously in a double-blinded fashion over a 12-minute period. Randomization was based on computer-generated codes that were maintained in sequentially numbered opaque envelopes until just before use. Beginning of the study drug infusion was considered elapsed time zero.
Subsequently, morphine titration (3 mg every five minutes) was resumed until the VRS was < 2 or 60 minutes elapsed after time zero. Opioid given after the test drugs (i.e. time zero) was considered supplemental morphine. However, morphine titration was stopped when patients had a respiratory rate < 10 breaths/minute, or oxygen saturation as measured by pulse oximeter (SpO2) < 95%, or a sedation score > 2. Otherwise, they were observed until reappearance of a VRS pain score ≥ 2.
Data Analysis
Our sample size estimate was based on the expected differences in morphine titration dose excluding the initial 9 mg (i.e. supplemental dose) between treated and untreated patients. In a preliminary study of 15 patients who failed to obtain adequate postoperative analgesia from 9 mg morphine, we found that supplemental morphine consumption was 19 ± 8 (SD) mg. Twenty-two patients per group were thus required to provide an 80% power for detecting a 35% difference in supplemental morphine consumption at an alpha level of 0.05. We therefore planned to include 66 patients requiring more than 9 mg of morphine for postoperative pain relief, with 22 being assigned to each treatment group.
Our primary end point was morphine consumption after test drug administration. The secondary endpoints were: 1) failure of morphine titration to produce adequate analgesia after administration of the test drug; and, 2) the delay between the end of morphine titration and reappearance of a VRS pain score ≥ 2 in patients who obtained adequate analgesia from morphine after administration of the test drug. Morphine titration was considered a failure if pain persisted (VRS ≥ 2) sixty minutes after time zero or if morphine administration was stopped because of respiratory depression or inadequate oxyhemoglobin saturation or excessive sedation (sedation score > 2).
In all cases, normality was assessed with the Kolmogorov-Smirnov test. Potential confounding factors and responses in the three treatment groups were compared with ANOVA and Bonferroni – Dunn test for post hoc analysis. Pain and sedation scores, titration failure and adverse events were compared using Chi Square tests. Data are presented as means ± SD unless otherwise indicated; P < 0.05 was considered statistically significant.
Results
Seventy-seven patients were enrolled in the study. Sixty-six patients failed to obtain adequate postoperative pain relief from 9 mg of morphine. One patient was subsequently excluded from the Control group because of a protocol deviation. Consequently, data analysis was restricted to 65 patients.
The three treatment groups were comparable with respect to demographic and morphometric characteristics, the type and length of surgical procedures, and intraoperative sufentanil dose. The interval between the end of sufentanil administration and the beginning of morphine titration was also similar in the three groups (Table 1).
Table 1.
Patient Characteristics, Surgery, and Opioid Management.
| Nefopam | Ketamine | Control | |
|---|---|---|---|
| Age (year) | 52 ± 14 | 51 ± 13 | 49 ± 15 |
| Height (cm) | 170 ± 7 | 172 ± 9 | 171 ± 8 |
| Weight (kg) | 71 ± 13 | 75 ± 12 | 72 ± 11 |
| Sex (M/F) | 13/9 | 14/8 | 14/7 |
| Surgical Procedures | |||
| Abdominal (n) | 17 | 21 | 18 |
| Urologic (n) | 2 | 1 | 2 |
| Hip or Knee Arthroplasty (n) | 3 | 0 | 1 |
| Intraoperative Sufentanil (μg.kg−1.h−1) | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 |
| Surgical Duration (min) | 173 ± 69 | 223± 86 | 188 ± 82 |
| Sufentanil-to-morphine Interval (min) | 77 ± 34 | 84 ± 31 | 71 ± 22 |
Results presented as means ± SDs. There were no statistically significant differences among the groups.
Supplemental morphine requirements (i.e., after test drug administration) were significantly greater in the Control group than in the Nefopam (P < 0.005) or Ketamine (P < 0.001) groups (Table 2). There were also significantly more failures of morphine titration in the Control patients than in patients given ketamine or nefopam. Morphine titration was interrupted in two control patients because of inadequate ventilation; morphine titration failed in two other Control patients because pain remained unacceptable after one hour of treatment. However, there were no significant differences between the ketamine and nefopam groups. Furthermore, the time between obtaining adequate analgesia and return of pain (VRS ≥ 2) was similar in all three groups (Table 2). Tachycardia and profuse sweating were more frequent in patients given nefopam than in the two other groups (Table 3).
Table 2.
Morphine Titration.
| Nefopam (n = 22) | Ketamine (n = 22) | Control (n = 21) | |
|---|---|---|---|
| Supplemental morphine (mg) | 10 ± 5* | 9 ± 5** | 17 ± 10 |
| Morphine-to-VRS Score ≥ 2 Interval (min) | 153 ± 112 | 166 ± 135 | 122 ± 101 |
| Morphine analgesia failure (n) | 0# | 0# | 4 |
Supplemental morphine is that given after administration of the test drug. VRS is a five-point verbal rating scale for pain. Patients in the Control group required more supplemental morphine than those in the treatment groups:
P < 0.005 and
P < 0.001 vs. Control. Furthermore, morphine titration failed more often in the Control patients:
P < 0.05 vs. Control.
Table 3.
Adverse events.
| Nefopam (n = 22) | Ketamine (n = 22) | Control (n = 21) | P | |
|---|---|---|---|---|
| Nausea or Vomiting | 12 | 8 | 4 | NS |
| Tachycardia | 8 | 2 | 3 | < 0.005 |
| Profuse Sweating | 4 | 0 | 0 | < 0.05 |
| Dry Mouth | 4 | 0 | 1 | NS |
| Dysphoria | 0 | 1 | 0 | NS |
| Diplopia | 0 | 2 | 2 | NS |
| Dizziness | 1 | 4 | 1 | NS |
Results presented as number of patients. Tachycardia and profuse sweating were most frequent in the Nefopam group.
Pain relief after starting infusion of the test drug was obtained more rapidly in the Nefopam and Ketamine groups than in the Control group (Fig. 1). Sedation scores were greater in the Ketamine patients than in the other two groups at 10, 15, and 20 minutes after beginning infusion of the treatment drug. However, sedation scores never reached 3 in any patients (Fig. 2).
Fig. 1.
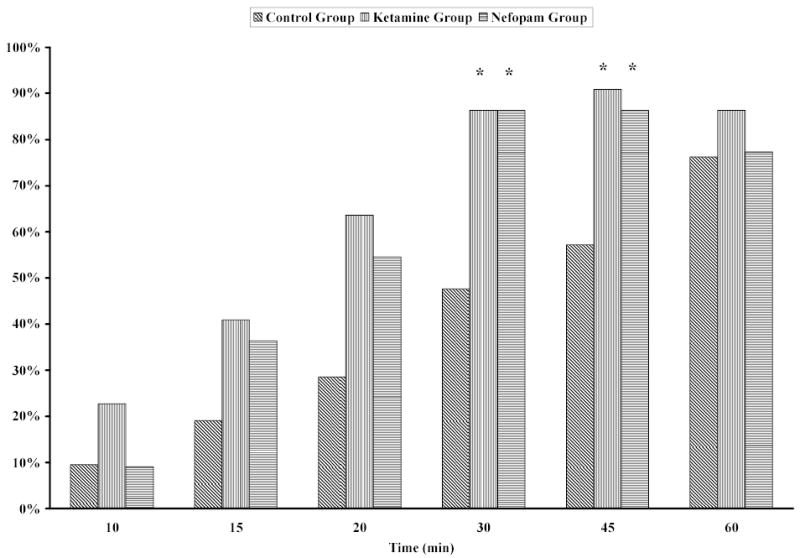
Percentage of patients with little or no pain (VRS pain score < 2) after beginning infusion of saline, ketamine, or nefopam. Pain scores were significantly less in Nefopam and Ketamine groups than in the Control group after 30 and 45 elapsed minutes (*P < 0.01). There were no significant differences between the Ketamine and Nefopam groups at any times.
Fig. 2.
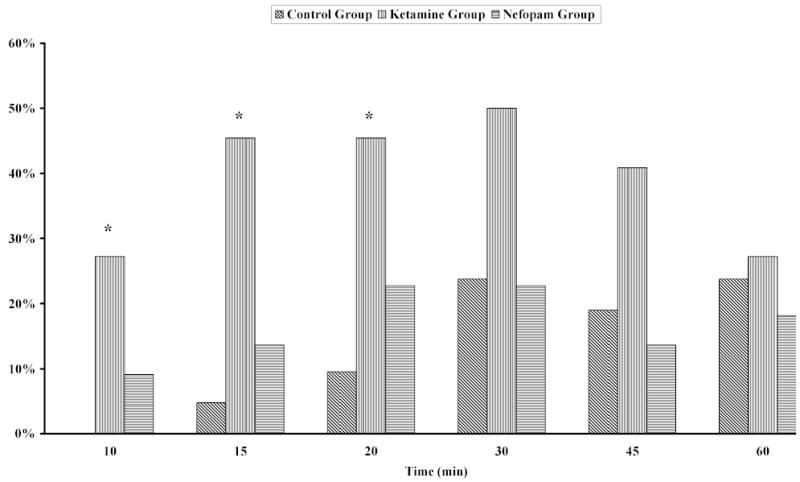
Percentage of patients with a sedation score > 1 after the start of the treatment infusion. Sedation scores were significantly greater in the patients given ketamine than in the patients given either nefopam or saline at 10, 15, and 20 elapsed minutes. *P < 0.05 compared to the Nefopam and Control groups.
Discussion
The present study demonstrates that in patients with pain only partly alleviated by morphine, intravenous administration of nefopam or small-dose ketamine improves the effects of subsequent morphine titration. Specifically, 40% less subsequent morphine was necessary to obtain adequate pain relief in patients given either nefopam or ketamine. Furthermore, morphine titration had to be discontinued in four patients of the Control group whereas morphine proved successful in all patients in each of the treatment groups.
More of our patients were resistant to morphine than reported in a recent study (12) in which only 22% of patients were resistant to 2-mg boluses of morphine given every 4–5 min for 25 to 30 minutes. Moreover, the mean titration in the control group used larger morphine doses than in other previous reports (18). These differences presumably result because we included only major surgeries in our study as compared with other studies (12,18). Our results are nonetheless consistent with Weinbroum’s study (12) in that resistant patients who were subsequently given ketamine required only one third of the morphine that patients not treated with morphine required. Analgesia from small-dose ketamine is thought to result from its action as a noncompetitive N-methyl-d-aspartate (NMDA) receptor antagonist (3). Opioids not only exert antinociceptive effect, they also activate central NMDA processes, resulting in hyperalgesia and acute opioid tolerance (19). NMDA receptor antagonists, such as small-dose ketamine, have been demonstrated able to increase the analgesic effect of opioids, likely by limiting these NMDA-mediated facilitating processes (10,11).
The exact mechanism of action of nefopam remains unknown. However, it is a centrally acting antinociceptive compound (20,21) with supra-spinal and spinal sites of action (22) and does not bind to opiate receptors (23). It inhibits monoamines reuptake (24), modulates descending serotoninergic pain (25) and may also interact with a dopaminergic pathway (26). Moreover, nefopam shows pre-emptive analgesic effects in a rat model of neuropathy (27), which involves the activation of NMDA receptors (28). However, it appears that nefopam modulates glutaminergic transmission presynaptically via inhibition of voltage-sensitive sodium channels rather than via a NMDA receptor antagonism like ketamine (29).
Nefopam was as effective as ketamine in producing a morphine-sparing effect in resistant patients. These results are consistent with other postoperative studies (16,17). Nefopam has been shown to produce a morphine-sparing effect of between 30 and 50%, with variable improvement in pain scores (16,17). Moreover, nefopam reduces thermal hyperalgesia induced by incision of the plantaris muscle of one hind-paw of rats (30). Also as shown previously with ketamine, nefopam produces an anti-hyperalgesic effect.
Both ketamine and nefopam comparably reduced the amount of morphine required to produce adequate analgesia. The dose of nefopam (20 mg) was relatively large and is a dose that by itself is clearly analgesic. It is impossible to determine from our study design the relative analgesic and anti-hyperalgesic contributions of nefopam to the observed reduction in morphine requirement. In contrast, 10 mg of ketamine is an extremely small dose that by itself produces trivial analgesic — but nonetheless exerts a powerful anti-hyperalgesic effects (4).
The most frequent side effects were sedation in the Ketamine group and tachycardia and profuse sweating in the Nefopam group. This finding is consistent with previous observations (17,23). Mimoz, et al (17) reported a lower incidence of tachycardia with a continuous infusion of nefopam over 30 minutes (compared with 12 minutes in our patients); however, the proportion of patients with profuse sweating was similar. Reduced morphine consumption in the two treatment groups did not decrease the incidence of nausea as might be expected (although our study has little power to evaluate the incidence of nausea). However, the incidence of PONV is often not reduced by morphine-sparing strategies (31,32). Furthermore, nefopam is reportedly emetic (23), a finding that is consistent with the relatively high incidence of PONV observed in the nefopam patients.
Despite sedation in the Ketamine group, all patients of this group were easily arousal and able to evaluate their pain level with VRS pain score. It is thus unlikely that accurate evaluation of pain was precluded by sedation. Because ketamine is readily available and is not associated with side effects other than moderate sedation in the doses we used, it may be preferable to nefopam as a co-analgesic treatment for patients resistant to opioids alone. We also note that nefopam is not even available in all European countries, much less in the United States, whereas ketamine is readily available world wide.
Different types of surgery were included, which may constitute a limitation of our study. However, most of our patients (86%) underwent abdominal surgery (colectomy by laparotomy) and no differences were observed in the distribution of surgical procedures among the three treatment groups.
In summary, ketamine 10 mg and nefopam 20 mg comparably potentiate opioid analgesia, each reducing opioid need by approximately 40%. Ketamine administration was associated with sedation, whereas nefopam produced tachycardia and sweating. However, none of the observed side effects was serious. Either drug can thus be used to potentiate opioid analgesia, although ketamine may be preferable and is more readily available.
Footnotes
Supported by NIH Grant GM 061655 (Bethesda, MD), the Gheens Foundation (Louisville, KY), the Joseph Drown Foundation (Los Angeles, CA), and the Commonwealth of Kentucky Research Challenge Trust Fund (Louisville, KY).
References
- 1.Paqueron X, Lumbroso A, Mergoni P, et al. Is morphine-induced sedation synonymous with analgesia during intravenous morphine titration? Br J Anaesth. 2002;89:697–701. [PubMed] [Google Scholar]
- 2.Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993;77:1048–56. doi: 10.1213/00000539-199311000-00030. [DOI] [PubMed] [Google Scholar]
- 3.Kohrs R, Durieux ME. Ketamine: teaching an old drug new tricks. Anesth Analg. 1998;87:1186–93. doi: 10.1097/00000539-199811000-00039. [DOI] [PubMed] [Google Scholar]
- 4.Schmid RL, Sandler AN, Katz J. Use and efficacy of low-dose ketamine in the management of acute postoperative pain: a review of current techniques and outcomes. Pain. 1999;82:111–25. doi: 10.1016/S0304-3959(99)00044-5. [DOI] [PubMed] [Google Scholar]
- 5.Menigaux C, Fletcher D, Dupont X, et al. The benefits of intraoperative small-dose ketamine on postoperative pain after anterior cruciate ligament repair. Anesth Analg. 2000;90:129–35. doi: 10.1097/00000539-200001000-00029. [DOI] [PubMed] [Google Scholar]
- 6.Menigaux C, Guignard B, Fletcher D, et al. Intraoperative small-dose ketamine enhances analgesia after outpatient knee arthroscopy. Anesth Analg. 2001;93:606–12. doi: 10.1097/00000539-200109000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez P, Saavedra G, Hernandez A, et al. Synergistic antinociceptive effects of ketamine and morphine in the orofacial capsaicin test in the rat. Anesthesiology. 2003;99:969–75. doi: 10.1097/00000542-200310000-00033. [DOI] [PubMed] [Google Scholar]
- 8.Bossard AE, Guirimand F, Fletcher D, et al. Interaction of a combination of morphine and ketamine on the nociceptive flexion reflex in human volunteers. Pain. 2002;98:47–57. doi: 10.1016/s0304-3959(01)00472-9. [DOI] [PubMed] [Google Scholar]
- 9.Sethna NF, Liu M, Gracely R, et al. Analgesic and cognitive effects of intravenous ketamine-alfentanil combinations versus either drug alone after intradermal capsaicin in normal subjects. Anesth Analg. 1998;86:1250–6. doi: 10.1097/00000539-199806000-00022. [DOI] [PubMed] [Google Scholar]
- 10.Kissin I, Bright CA, Bradley EL., Jr The effect of ketamine on opioid-induced acute tolerance: can it explain reduction of opioid consumption with ketamine-opioid analgesic combinations? Anesth Analg. 2000;91:1483–8. doi: 10.1097/00000539-200012000-00035. [DOI] [PubMed] [Google Scholar]
- 11.Angst MS, Koppert W, Pahl I, et al. Short-term infusion of the o-opioid agonist remifentanil in humans causes hyperalgesia during withdrawal. Pain. 2003;106:49–57. doi: 10.1016/s0304-3959(03)00276-8. [DOI] [PubMed] [Google Scholar]
- 12.Weinbroum AA. A single small dose of postoperative ketamine provides rapid and sustained improvement in morphine analgesia in the presence of morphine-resistant pain. Anesth Analg. 2003;96:789–95. doi: 10.1213/01.ANE.0000048088.17761.B4. [DOI] [PubMed] [Google Scholar]
- 13.Guirimand F, Dupont X, Bouhassira D, et al. Nefopam strongly depresses the nociceptive flexion (R(III)) reflex in humans. Pain. 1999;80:399–404. doi: 10.1016/s0304-3959(98)00238-3. [DOI] [PubMed] [Google Scholar]
- 14.Sunshine A, Laska E. Nefopam and morphine in man. Clin Pharmacol Ther. 1975;18:530–4. doi: 10.1002/cpt1975185part1530. [DOI] [PubMed] [Google Scholar]
- 15.Mok MS, Lippmann M, Steen SN. Comparison of intravenous nefopam versus morphine for the relief of postoperative pain. Clin Pharmacol Ther. 1979;25:237–8. [Google Scholar]
- 16.McLintock TT, Kenny GN, Howie JC, et al. Assessment of the analgesic efficacy of nefopam hydrochloride after upper abdominal surgery: a study using patient controlled analgesia. Br J Surg. 1988;75:779–81. doi: 10.1002/bjs.1800750818. [DOI] [PubMed] [Google Scholar]
- 17.Mimoz O, Incagnoli P, Josse C, et al. Analgesic efficacy and safety of nefopam vs. propacetamol following hepatic resection. Anaesthesia. 2001;56:520–5. doi: 10.1046/j.1365-2044.2001.01980.x. [DOI] [PubMed] [Google Scholar]
- 18.Aubrun F, Langeron O, Qesnel C, et al. Relationships between measurement of pain using visual analog score and morphine requirements during postoperative intravenous morphine titration. Anesthesiology. 2003;98:1415–21. doi: 10.1097/00000542-200306000-00017. [DOI] [PubMed] [Google Scholar]
- 19.Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: a current view of their possible interactions. Pain. 1995;62:259–74. doi: 10.1016/0304-3959(95)00073-2. [DOI] [PubMed] [Google Scholar]
- 20.Piercey MF, Schroeder LA. Spinal and Supraspinal sites for morphine and nefopam analgesia in the mouse. Eur J Pharmacol. 1981;74:135–40. doi: 10.1016/0014-2999(81)90523-9. [DOI] [PubMed] [Google Scholar]
- 21.Tresnak-Rustad NJ, Wood ME. In vitro biochemical effects of nefopam hydrochloride, a new analgesic agent. Biochem Pharmacol. 1981;30:2847–50. doi: 10.1016/0006-2952(81)90424-x. [DOI] [PubMed] [Google Scholar]
- 22.Berge OG, Fasmer OB, Hole K, et al. Analgesic effects of nefopam at spinal and supraspinal sites. Br J Pharmacol. 1986;89:639P. [Google Scholar]
- 23.Heel RC, Brogden RN, Pakes GE, et al. Nefopam: a review of its pharmacological properties and therapeutic efficacy. Drugs. 1980;19:249–67. doi: 10.2165/00003495-198019040-00001. [DOI] [PubMed] [Google Scholar]
- 24.Fuller RW, Snoddy HD. Evaluation of nefopam as a monoamine uptake inhibitor in vivo in mice. Neuropharmacology. 1993;32:995–9. doi: 10.1016/0028-3908(93)90064-a. [DOI] [PubMed] [Google Scholar]
- 25.Hunskaar S, Fasmer OB, Broch OJ, Hole K. Involvement of central serotonergic pathways in nefopam-induced antinociception. Eur J Pharmacol. 1987;138:77–82. doi: 10.1016/0014-2999(87)90339-6. [DOI] [PubMed] [Google Scholar]
- 26.Esposito E, Romandini S, Merlo-Pich E, et al. Evidence of the involvement of dopamine in the analgesic effect of nefopam. Eur J Pharmacol. 1986;128:157–64. doi: 10.1016/0014-2999(86)90762-4. [DOI] [PubMed] [Google Scholar]
- 27.Biella GE, Groppetti A, Novelli A, et al. Neuronal sensitization and its behavioral correlates in a rat model of neuropathy are prevented by a cyclic analog of orphenadrine. J Neurotrauma. 2003;20:593–601. doi: 10.1089/089771503767168519. [DOI] [PubMed] [Google Scholar]
- 28.Kim YI, Na HS, Yoon YW, et al. NMDA receptors are important for both mechanical and thermal allodynia from peripheral nerve injury in rats. NeuroReport. 1997;8:2149–53. doi: 10.1097/00001756-199707070-00011. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez-Sanchez MT, Diaz-Trelles R, Groppetti A, et al. Nefopam, an analogue of orphenadrine, protects against both NMDA receptor-dependent and independent veratridine-induced neurotoxicity. Amino Acids. 2002;23:31–6. doi: 10.1007/s00726-001-0106-6. [DOI] [PubMed] [Google Scholar]
- 30.Girard P, Pansart Y, Coppe MC, Gillardin JM. Nefopam reduces thermal hypersensitivity in acute and postoperative pain models in the rat. Pharmacol Res. 2001;44:541–5. doi: 10.1006/phrs.2001.0886. [DOI] [PubMed] [Google Scholar]
- 31.Hyllested M, Jones S, Pedersen JL, Kehlet H. Comparative effect of paracetamol, NSAIDs or their combination in postoperative pain management: a qualitative review. Br J Anaesth. 2002;88:199–214. doi: 10.1093/bja/88.2.199. [DOI] [PubMed] [Google Scholar]
- 32.Aubrun F, Kalfon F, Mottet P, et al. Adjunctive analgesia with intravenous propacetamol does not reduce morphine-related adverse effects. Br J Anaesth. 2003;90:314–9. doi: 10.1093/bja/aeg076. [DOI] [PubMed] [Google Scholar]


