Abstract
Background
Mild perioperative hypothermia produces morbid cardiac outcomes that may result from sympathetically induced hypertension. However, volatile anesthetics produce vasodilatation that may reduce the haemodynamic response to hypothermia. We tested the hypothesis that the volatile anesthetics isoflurane and desflurane blunt the normal cold-induced hypertensive response.
Methods
We analyzed prospective data from three analogous studies: 1) ten volunteers given desflurane (2.6 Volume %) maintained in left-lateral position, 2) nine volunteers without anesthesia or anesthetized with various doses of desflurane, and 3) eight volunteers given various concentrations of isoflurane. Mean skin temperature was reduced to 31°C, which decreased core body temperature and triggered thermoregulatory vasoconstriction. Mean arterial pressures were determined before and after hypothermia provoked intense thermoregulatory vasoconstriction.
Results
The hemodynamic responses to thermoregulatory vasoconstriction were similar without anesthesia and at all concentrations of desflurane and isoflurane. On average, mean arterial pressure increased 14 (SD=5) mmHg with and without anesthesia.
Conclusion
We conclude that thermoregulatory vasoconstriction significantly increases arterial pressure with or without isoflurane or desflurane anesthesia.
Keywords: Anesthesia, Arterial pressure, Hypothermia, Thermoregulation, Vasoconstriction
Surface cooling increases arterial pressure and decreases heart rate in unanesthetized subjects (1). Hypertension is associated with earlier onset of angina in patients with coronary artery disease during cold stress (1,2). Consistent with these observations, hypertension is worse in the winter (3) and the risk of myocardial infarction increases (4).
Cardiac morbidity is the leading cause of otherwise unexpected perioperative death (5). Mild hypothermia triples the risk of morbid postoperative myocardial events (6). A likely mechanism is cold-induced sympathetic activation and consequent hypertension (7). Cold-induced hypertension has been demonstrated in young, unanesthetized volunteers (8). However, volatile anesthetics are potent peripheral and central vasodilators (9,10) that potentially counteract cold-induced vasoconstriction and the resulting increase of arterial pressure. Volatile anesthetics may thus minimize the hemodynamic consequence of thermoregulatory vasoconstriction during anesthesia.
Interestingly, the effects of cold-induced vasoconstriction on arterial pressure during volatile anesthesia remain poorly characterized. We therefore compared the effects of cold-induced vasoconstriction on arterial pressure in unanesthetized volunteers with the effects in volunteers given various concentrations of isoflurane and desflurane. Specifically, we tested the hypothesis that the volatile anesthetics isoflurane and desflurane blunt the normal blood pressure response to cold.
Methods
We analyzed data from three prospective studies to determine the effect of cold-induced vasoconstriction on arterial pressure with and without volatile anesthesia. All data were recorded prospectively and treatments within each study were randomly assigned. Each followed a similar study protocol. Data for this manuscript were extracted from the original case report forms of all three studies; the blood pressure measurements we present here were not previously analyzed or reported.
With IRB approval and written informed consent, we studied healthy male volunteers. None was obese, taking medication, or had a history of thyroid disease, dysautonomia, or Raynaud’s syndrome. The studies started in the morning so that responses were triggered at similar times each day to minimize circadian thermoregulatory fluctuations (11). Volunteers were minimally clothed during the protocol; ambient temperature was maintained at 22–23°C. An intravenous catheter was inserted in the left forearm for fluid administration. Anesthesia was induced by administration of propofol (3–5 mg·kg−1) and maintained with a volatile anesthetic.
In the first study (12), general anesthesia was maintained with 2.8 volume % desflurane in ten volunteers. The volunteers were subsequently turned to the left-lateral position. Padding was positioned under the rib cage to avoid compression of the brachial artery and consequent flow restriction to the dependent arm. These patients were paralyzed and mechanically ventilated with a PEEP of zero. In the second study (13), nine volunteers were each evaluated on three different days: 1) no anesthesia (control), 2) a target end-tidal desflurane concentration of 3.5% and, 3) a target end-tidal desflurane concentration of 5.6%. These patients breathed spontaneously. In the third study (14), eight volunteers were each evaluated on four days: 1) a target end-tidal isoflurane concentration of 0.6%, 2) a target end-tidal isoflurane concentration of 0.7%, 3) no anesthesia (control) and a target end-tidal concentration of 0.8%, and 4) a target end-tidal concentration of 1.0%. These patients also breathed spontaneously.
On each study day, thermoregulatory vasoconstriction was induced by reducing mean-skin temperature to 31°C with a circulating-water mattress (Cincinnati Sub-Zero, Cincinnati, OH) and forced-air cooler (Augustine Medical, Inc., Eden Prairie, MN). Throughout the protocol, arms were protected from active warming and cooling to avoid locally mediated vasomotion (15).
Measurements
Heart rate and arterial pressure were determined oscillometrically (Modulus CD, Ohmeda Inc., Salt Lake City, UT) at 5-minute intervals. End-tidal volatile anesthetic concentrations were measured using a Rascal anesthetic monitor (Ohmeda Inc. Salt Lake City, UT). End tidal PCO2 was maintained between 35 and 40 mmHg in all three studies (12–14).
Vascular tone was estimated using Laser Doppler flowmetry with an integrating multiprobe (Periflux 3, Perimed Inc., Piscataway, NJ, “wide-band” setting) positioned on the fourth finger of the right hand (16). Laser Doppler flowmetry evaluates both capillary and arteriovenous shunt flows, although the one we used is most sensitive to capillary flow. Vascular tone was also evaluated on the right second finger using the perfusion index, a measure that is derived from absorption of two infrared wavelengths. All measures of flow were recorded at 1-minute intervals.
In each case, core body temperature was measured at 1-minute intervals from the tympanic membrane (Tyco-Mallinckrodt Anesthesiology Products, Inc., St. Louis, MO). Mean skin-surface temperature was calculated from measurements at 15 area-weighted sites (17). All temperatures were displayed at 1-second intervals, and recorded at 1-minute intervals from thermocouples connected to calibrated Iso-Thermex thermometers having an accuracy of 0.1°C and a precision of 0.01°C (Columbus Instruments, Corp., Columbus, OH).
Our primary measurement for peripheral vasodilatation and cold-induced vasoconstriction was the gradient between the skin-surface temperature on the fingertip and the skin-surface temperature of the forearm on the exposed arm. There is a strong correlation between the skin-temperature gradient and laser Doppler flowmetry, volume plethysmography, and the perfusion index (18).
Data Analysis
Because both skin and core temperatures contribute to control of thermoregulatory responses (19), we arithmetically compensated for actual skin temperature using a previously described equation (20). Beta was set to 0.2 with a designated mean-skin temperature of 34°C.
We expressed thermoregulatory vasoconstriction in terms of the forearm-minus-fingertip skin temperature gradient (18). Volunteers with gradients exceeding +2°C were considered vasoconstricted; those with gradients less than −2°C were considered vasodilated.
All arterial pressure, heart rate, laser Doppler, and perfusion index data were averaged within each volunteer, and then averaged among the volunteers treated similarly. All values during vasodilatation were similarly averaged within each volunteer, and then averaged among the volunteers.
Morphometric characteristics of the patients in the three studies were compared with one-way analysis of variances. Arterial pressure, heart rate, perfusion index, and laser Doppler flowmetry values at each anesthetic concentration were compared between the vasodilatation and during vasoconstriction phases using two-tailed, paired t tests. Differences in arterial pressures, heart rates, perfusion index, and laser Doppler flowmetry values between the vasodilatation and vasoconstriction phases were compared between the control group and the anesthesia group at various concentrations of the volatile anesthetics used and between the control group and the data of patients receiving various concentrations of anesthetics together using two-tailed, unpaired t tests with Bonferroni correction. Results are presented as means (SDs); P < 0.05 was considered statistically significant.
Results
The demographic and morphometric characteristics of the volunteers in the three studies were comparable (Table 1). The control group shown listed in Tables 1 and 2 consisted of unanesthetized volunteers from the second and third studies. We report a complete set of data for 10 volunteers on the control day. That explains the difference between the total numbers in Tables 1 and 2.
Table 1.
Demographic and Morphometric Factors, and Ambient and Mean Skin Temperature.
| Control (no anesthesia, Studies 2 & 3) | Study 1 Desflurane –Lateral Position (2.8 Vol %) | Study 2 Desflurane (3.5& 5.6 Vol %) | Study 2 Isoflurane(0.55, 0.7, 0.85, & 1.0 Vol %) | |
|---|---|---|---|---|
| Number of Study Subjects | 10 | 10 | 9 | 8 |
| Weight (kg) | 67 (8) | 79 (11) | 70 (6) | 67 (11) |
| Height (cm) | 172 (7) | 176 (8) | 173 (3) | 172 (9) |
| Ambient Temperature (°C) | 22.4 (0.7) | 22.2 (0.5) | 22.1 (0.2) | 22.1 (0.4) |
| Mean Skin Temperature (°C) | 31.5 (0.6) | 31.0 (0.1) | 31.0 (0.4) | 31.4 (0.3) |
Data are presented as means (SDs). There were no statistically significant differences between the study groups.
The control group consists of volunteers from Studies 2 and 3 who completed the study-day without anesthesia.
Table 2.
The Effects of Vasoconstriction on Arterial Pressure, Heart Rate, and Finger Flow.
| Vasodilatation | Vasoconstriction | Difference | P | |
|---|---|---|---|---|
| Mean Arterial Pressure (mmHg) | ||||
| Control (no anesthesia, Studies 2 & 3) | 81 (5) | 95 (5) | 14 (5) | < 0.001 |
| Study 1 (Desflurane – Lateral Position) | ||||
| 2.8 Vol % Dependent Arm | 80 (20) | 96 (23) | 16 (14) | 0.005 |
| 2.8 Vol % Upper Arm | 62 (18) | 86 (20) | 24 (13) | < 0.001 |
| Study 2 (Desflurane) | ||||
| 3.5 Vol % | 74 (8) | 84 (5) | 14 (9) | 0.038 |
| 5.6 Vol % | 73 (4) | 90(13) | 17(14) | 0.008 |
| Study 3 (Isoflurane) | ||||
| 0.55 Vol % | 71(4) | 85(14) | 14(15) | 0.038 |
| 0.70 Vol % | 73(5) | 80(3) | 7(5) | 0.007 |
| 0.85 Vol % | 72(3) | 86(11) | 14(8) | 0.002 |
| 1.0 Vol % | 76(10) | 85(7) | 9(8) | 0.007 |
| Heart Rate (beats/min) | ||||
| Control (no anesthesia, Studies 2 & 3) | 61(9) | 55(7) | −6(4) | 0.007 |
| All Anesthesia Groups | 63(2) | 59(4) | −4(3) | 0.255 |
| Perfusion Index | ||||
| Control (no anesthesia, Studies 2 & 3) | 1.7 (0.7) | 0.5 (0.3) | 1.1(2) | 0.001 |
| All Anesthesia Groups | 2.6 (0.5) | 0.4 (0.1) | 2.0 (0.5) | < 0.001 |
| Laser Doppler | ||||
| Control (no anesthesia, Studies 2 & 3) | 143 (42) | 34(17) | 106 (52) | <0.001 |
| All Anesthesia Groups | 185(14) | 28(6) | 161(14) | < 0.001 |
We expressed thermoregulatory vasoconstriction in terms of the forearm-minus-fingertip skin temperature gradient (18). Volunteers with gradients exceeding +2°C were considered vasoconstricted; those with gradients less than −2°C were considered vasodilated.
No statistically significant differences were found between control and anesthesia groups for mean arterial pressure, heart rate, perfusion index, or laser doppler. Data are presented as means (SDs).
When the forearm-minus-fingertip skin temperature gradient was less than −2°C, the perfusion index averaged 2.6 (0.5) units and the laser Doppler averaged 185 (14) units in the anesthetized subjects. In contrast, when the forearm-minus-fingertip skin temperature gradient was greater than +2°C, the perfusion index averaged 0.4 (0.1) units and the laser Doppler averaged 28 (6) units in the same anesthetized subjects. These differences indicated a transition from nearly complete vasodilatation to nearly complete vasoconstriction. Results with and without anesthesia were comparable.
In the first study, mean arterial pressure in the dependent arm was significantly greater (80 (20) mmHg) than in the upper arm (62 (17) mmHg, P = 0.003). After vasoconstriction, arterial pressure increased significantly in both arms, by 16 (14) mmHg in the dependent arm and by 24 (31) mmHg in the upper arm (P < 0.005). Heart rate changes were not significant. All these measurements were made during general anesthesia.
In the second and third studies, thermoregulatory vasoconstriction increased mean arterial pressure significantly during each anesthetic and at every concentration. Averaged across all groups, vasoconstriction significantly increased mean arterial pressure from 74 (6) mmHg to 87 (5) mmHg. Mean arterial pressure thus increased 14 (5) mmHg, which was virtually identical to the increase without anesthesia (Control, Table 2). The increases in systolic and diastolic arterial pressures were also similar. Heart rate changes in the anesthetized groups were not statistically significant (Fig. 1). In contrast, the control group had a significant decrease in heart rate (Table 2).
Fig. 1.
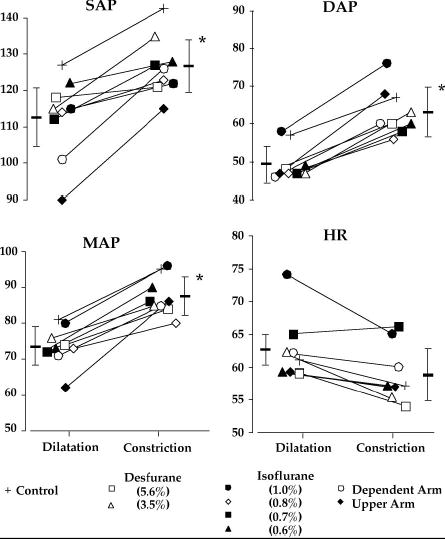
Systolic arterial pressure (SAP), diastolic arterial pressure (DAP), mean arterial pressure (MAP), and heart rate (HR) before and after vasoconstriction. Each symbol represents one group of study subjects. Data are represented as means of the individual studies, with the average and standard deviations for the entire study population also shown. Asterisks (*) identify statistically significant differences from vasodilatation (P < 0.001). See Table 2 for statistical analysis and detailed information.
Throughout all studies, the threshold for vasoconstriction occurred at core temperatures that are typical in unwarmed surgical patients (Table 3).
Table 3.
Core Temperatures and Calculated Vasoconstriction Thresholds (at a Mean Skin Temperature of 34°C).
| Core Temperature (°C) | Vasoconstriction Threshold (°C) | |
|---|---|---|
| Control (no anesthesia, Studies 2 & 3) | 36.9 (0.4) | 37.0 (0.3) |
| Study 1 (Desflurane – Lateral Position) | ||
| 2.6 Vol % | 36.3 (0.3) | 35.6 (0.3) |
| Study 2 (Desflurane) | ||
| 3.5 Vol % | 35.8 (0.4) | 35.3 (0.5) |
| 5.6 Vol % | 34.6 (1.2) | 33.5 (1.7) |
| Study 3 (Isoflurane) | ||
| 0.55 Vol % | 36.4 (0.2) | 36.3 (0.3) |
| 0.70 Vol % | 35.9 (0.5) | 35.7 (0.7) |
| 0.85 Vol % | 35.1 (0.7) | 34.5 (0.9) |
| 1.0 Vol % | 33.6 (0.8) | 32.5 (1.2) |
Within each study, all thresholds differ significantly. Results are presented as means (SDs).
Discussion
Exposure to cold increases circulating concentrations of epinephrine, norepinephrine, and cortisol in both unanesthetized (21,22) and anesthetized subjects (6). An increase in plasma concentrations of norepinephrine and epinephrine contributes to cold-induced myocardial ischaemia (23,24). Cardiac events remain the leading cause of perioperative death (5), and even mild hypothermia triples the risk of a morbid postoperative cardiac outcome (25). Interestingly, shivering per se does not appear to be the cause. Instead, adverse cardiac outcomes are likely to result from cold-induced hypertension, combined with a concomitant three-fold increase in plasma norepinephrine concentrations.
Hypothermia triggers thermoregulatory vasoconstriction, and it is well established that vasoconstriction increases mean arterial pressure in unanesthetized subjects (1,3) and in the postoperative period (25). Our purpose was thus to determine the extent to which volatile anesthetics impair the hypertensive response to thermoregulatory vasoconstriction. In our first study, arterial pressures in the upper and dependent arms differed by 22 mmHg, an amount similar to previous reports (26). Mean arterial pressure increased significantly more in the upper than dependent arm, perhaps because hydrostatic forces counteracted the central vasoconstriction signals more in the dependent arm. However, vasoconstriction substantially increased mean arterial pressure in each arm.
The second and third studies revealed almost identical increases in arterial pressure in volunteers given various concentrations of isoflurane and desflurane. No dose-dependence was detected, and the results were comparable with each anesthetic. Interestingly, the increase in mean arterial pressure during anesthesia (14 [5] mmHg) was virtually identical to that observed without anesthesia (14 [5] mmHg). We thus conclude that the hypertensive response to thermoregulatory vasoconstriction remains well preserved during volatile anesthesia.
The core temperatures that triggered vasoconstriction were typical for unwarmed surgical patients. The use of currently available warming devices to maintain normothermia can prevent intraoperative thermoregulatory vasoconstriction.
Bradycardia in the control subjects was probably mainly a reflex reaction to cold-induced hypertension. However, there is also a parasympathetic control of heart rate that is independent of arterial pressure and stroke volume (27). For example, there is normally a linear relationship between heart rate and skin temperature between 35°C and 23°C that is mediated by the vagus nerve (28). However, it is well established that volatile anesthetics blunt vagal reflexes that normally protect unanesthetized humans (29,30). Thus, in our anesthetized subjects, the bradycardia to cold-induced hypertension may have been attenuated by the anesthesia.
We present a retrospective analysis of data that were prospectively collected for other purposes. However, the studies were all conducted over a relatively brief period, in the same laboratory, and used virtually identical methodology. Furthermore, our primary outcome (arterial pressure) is an objective measure that was surely recorded accurately and is not subjected to investigator bias. It thus seems unlikely that our results would differ were the entire study repeated prospectively. However, a prospective design would have allowed us to evaluate plasma catecholamine concentrations and record other measurements that may have helped identify the specific mechanism by which mild hypothermia triggers the observed substantial and clinically important increase in arterial pressure.
Arterial pressure was measured oscillometrically on the arm rather than at the radial artery. However, Hynson and co-workers have shown that radial arterial systolic arterial pressure is exaggerated by thermoregulatory vasoconstriction. Oscillometric arterial pressure thus more reliably reflects central arterial pressure (31). Participants in our first study were paralyzed during anesthesia and mechanically ventilated (PEEP = zero); in contrast, the others breathed spontaneously. However, neither the use of muscle relaxants per se nor the type of ventilation is likely to alter the thermoregulatory thresholds for vasoconstriction or steady-state arterial pressure.
A limitation of these studies was that cardiac output was not measured during the experimental procedure. However, since heart rate did not differ significantly in the vasodilated and vasoconstricted states, an increase in vascular resistance seems the most likely mechanism for the increase in arterial pressure.
In conclusion, cold-induced vasoconstriction significantly increased systolic, mean, and diastolic arterial pressures in both unanesthetized and anesthetized subjects. The increases were comparable with and without anesthesia and were independent of anesthetic dose. We, therefore, failed to confirm our hypothesis that volatile anesthesia at the concentrations used in these studies reduces the hemodynamic consequence of thermoregulatory vasoconstriction. Hypothermia-induced increases in intraoperative arterial pressure may contribute to perioperative myocardial morbidity. The best way to avoid thermoregulatory vasoconstriction is simply to maintain normothermia.
Acknowledgments
Supported by NIH Grants GM 58273 and GM 061655 (Bethesda, MD), the Joseph Drown Foundation (Los Angeles, CA), and the Commonwealth of Kentucky Research Challenge Trust Fund (Louisville, KY). Tyco-Mallinckrodt, Inc. (St. Louis, MO) donated the thermocouples we used. Dr. Sessler has a personal financial interest in Radiant Medical, Inc. We appreciate the assistance of Nancy Alsip, Ph.D.
References
- 1.Emmett JD. A review of heart rate and blood pressure responses in the cold in healthy subjects and coronary artery disease patients. J Cardiopulm Rehabil. 1995;15:19–24. doi: 10.1097/00008483-199501000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Juneau M, Johnstone M, Dempsey E, Waters DD. Exercise-induced myocardial ischemia in a cold environment. Effect of antianginal medications. Circulation. 1989;79:1015–1020. doi: 10.1161/01.cir.79.5.1015. [DOI] [PubMed] [Google Scholar]
- 3.Brennan PJ, Greenberg G, Miall WE, Thompson SG. Seasonal variation in arterial blood pressure. Br Med J (Clin Res Ed) 1982;285:919–923. doi: 10.1136/bmj.285.6346.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bainton D, Moore F, Sweetnam P. Temperature and deaths from ischaemic heart disease. Br J Prev Soc Med. 1977;31:49–53. doi: 10.1136/jech.31.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mangano DT. Perioperative cardiac morbidity. Anesthesiology. 1990;72:153–184. doi: 10.1097/00000542-199001000-00025. [DOI] [PubMed] [Google Scholar]
- 6.Frank SM, Fleisher LA, Breslow MJ, Higgins MS, Olson KF, Kelly S, Beattie C. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events: A randomized clinical trial. JAMA. 1997;277:1127–1134. [PubMed] [Google Scholar]
- 7.Frank SM, Beattie C, Christopherson R, Norris EJ, Perler BA, Williams GM, Gottlieb SO. Unintentional hypothermia is associated with postoperative myocardial ischemia. Anesthesiology. 1993;78:468–476. doi: 10.1097/00000542-199303000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Frank SM, Higgins MS, Fleisher LA, Sitzmann JV, Raff H, Breslow MJ. Adrenergic, respiratory, and cardiovascular effects of core cooling in humans. Am J Physiol. 1997;272:R557–R562. doi: 10.1152/ajpregu.1997.272.2.R557. [DOI] [PubMed] [Google Scholar]
- 9.Schwinn DA, McIntyre RW, Reves JG. Isoflurane-induced vasodilation: Role of the alpha-adrenergic nervous system. Anesth Analg. 1990;71:451–459. doi: 10.1213/00000539-199011000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Sessler DI, McGuire J, Moayeri A, Hynson J. Isoflurane-induced vasodilation minimally increases cutaneous heat loss. Anesthesiology. 1991;74:226–232. doi: 10.1097/00000542-199102000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Tayefeh F, Plattner O, Sessler DI, Ikeda T, Marder D. Circadian changes in the sweating-to-vasoconstriction interthreshold range. Pflügers Arch. 1998;435:402–406. doi: 10.1007/s004240050530. [DOI] [PubMed] [Google Scholar]
- 12.Greif R, Laciny S, Rajek A, Doufas AG, Sessler DI. The threshold and gain of thermoregulatory vasoconstriction differs during anesthesia in the dependent and upper arms in the lateral position. Anesth Analg. 2002;94:1019–1022. doi: 10.1097/00000539-200204000-00046. [DOI] [PubMed] [Google Scholar]
- 13.Annadata RS, Sessler DI, Tayefeh F, Kurz A, Dechert M. Desflurane slightly increases the sweating threshold, but produces marked, non-linear decreases in the vasoconstriction and shivering thresholds. Anesthesiology. 1995;83:1205–1211. doi: 10.1097/00000542-199512000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Xiong J, Kurz A, Sessler DI, Plattner O, Christensen R, Dechert M, Ikeda T. Isoflurane produces marked and non-linear decreases in the vasoconstriction and shivering thresholds. Anesthesiology. 1996;85:240–245. doi: 10.1097/00000542-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Hales JRS, Fawcett AA, Bennett JW, Needham AD. Thermal control of blood flow through capillaries and arteriovenous anastomoses in skin of sheep. Pflügers Arch. 1978;378:55–63. doi: 10.1007/BF00581958. [DOI] [PubMed] [Google Scholar]
- 16.Sessler DI, Olofsson CI, Rubinstein EH. The thermoregulatory threshold in humans during nitrous oxide-fentanyl anesthesia. Anesthesiology. 1988;69:357–364. doi: 10.1097/00000542-198809000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Sessler DI, Schroeder M. Heat loss in humans covered with cotton hospital blankets. Anesth Analg. 1993;77:73–77. doi: 10.1213/00000539-199307000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Rubinstein EH, Sessler DI. Skin-surface temperature gradients correlate with fingertip blood flow in humans. Anesthesiology. 1990;73:541–545. [PubMed] [Google Scholar]
- 19.Cheng C, Matsukawa T, Sessler DI, Kurz A, Merrifield B, Lin H, Olofsson P. Increasing mean skin temperature linearly reduces the core-temperature thresholds for vasoconstriction and shivering in humans. Anesthesiology. 1995;82:1160–1168. doi: 10.1097/00000542-199505000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Matsukawa T, Kurz A, Sessler DI, Bjorksten AR, Merrifield B, Cheng C. Propofol linearly reduces the vasoconstriction and shivering thresholds. Anesthesiology. 1995;82:1169–1180. doi: 10.1097/00000542-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Lamke LO, Lennquist S, Liljedahl SO, Wedin B. The influence of cold stress on catecholamine excretion and oxygen uptake of normal persons. Scand J Clin Lab Invest. 1972;30:57–62. doi: 10.3109/00365517209081090. [DOI] [PubMed] [Google Scholar]
- 22.Wilkerson JE, Raven PB, Bolduan NW, Horvath SM. Adaptations in man's adrenal function in response to acute cold stress. J Appl Physiol. 1974;36:183–189. doi: 10.1152/jappl.1974.36.2.183. [DOI] [PubMed] [Google Scholar]
- 23.Heusch G. Alpha-adrenergic mechanisms in myocardial ischemia. Circulation. 1990;81:1–13. doi: 10.1161/01.cir.81.1.1. [DOI] [PubMed] [Google Scholar]
- 24.Berkenboom GM, Abramowicz M, Vandermoten P, Degre SG. Role of alpha-adrenergic coronary tone in exercise-induced angina pectoris. Am J Cardiol. 1986;57:195–198. doi: 10.1016/0002-9149(86)90889-1. [DOI] [PubMed] [Google Scholar]
- 25.Frank SM, Higgins MS, Breslow MJ, Fleisher LA, Gorman RB, Sitzmann JV, Raff H, Beattie C. The catecholamine, cortisol, and hemodynamic responses to mild perioperative hypothermia. Anesthesiology. 1995;82:83–93. doi: 10.1097/00000542-199501000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Eggers GWN, Mo C, deGroot WJ, Tanner CR, Leonhard JJ. Hemodynamic changes associated with various surgical positions. JAMA. 1963;185:81–85. [Google Scholar]
- 27.Raven PB, Wilkerson JE, Horvath SM, Bolduan NW. Thermal, metabolic, and cardiovascular responses to various degrees of cold stress. Can J Physiol Pharmacol. 1975;53:293–298. doi: 10.1139/y75-041. [DOI] [PubMed] [Google Scholar]
- 28.LeBlanc J, Dulac S, Cote J, Girard B. Autonomic nervous system and adaptation to cold in man. J Appl Physiol. 1975;39:181–186. doi: 10.1152/jappl.1975.39.2.181. [DOI] [PubMed] [Google Scholar]
- 29.Ebert TJ, Harkin CP, Muzi M. Cardiovascular responses to sevoflurane: a review. Anesth Analg. 1995;81:S11–22. doi: 10.1097/00000539-199512001-00003. [DOI] [PubMed] [Google Scholar]
- 30.Carter JA, Clarke TN, Prys-Roberts C, Spelina KR. Restoration of baroreflex control of heart rate during recovery from anaesthesia. Br J Anaesth. 1986;58:415–421. doi: 10.1093/bja/58.4.415. [DOI] [PubMed] [Google Scholar]
- 31.Hynson JM, Sessler DI, Moayeri A, Katz JA. Thermoregulatory and anesthetic-induced alterations in the differences between femoral, radial, and oscillometric blood pressure measurements. Anesthesiology. 1994;81:1411–1421. doi: 10.1097/00000542-199412000-00016. [DOI] [PubMed] [Google Scholar]


