Abstract
Lipid droplet-associated proteins play an important role in adipocyte triglyceride (TG) metabolism. Here, we show that trans-10,cis-12 conjugated linoleic acid (CLA), but not cis-9,trans-11 CLA, increased lipolysis and altered human adipocyte lipid droplet morphology. Before this change in morphology, there was a rapid trans-10,cis-12 CLA-induced increase in the accumulation of perilipin A in the cytosol, followed by the disappearance of perilipin A protein. In contrast, protein levels of adipose differentiation-related protein (ADRP) were increased in cultures treated with trans-10,cis-12 CLA. Immunostaining revealed that ADRP localized to the surface of small lipid droplets, displacing perilipin. Intriguingly, trans-10,cis-12 CLA increased ADRP protein expression to a much greater extent than ADRP mRNA without affecting stability, suggesting translational control of ADRP. To this end, we found that trans-10,cis-12 CLA increased activation of the mammalian target of rapamycin/p70 S6 ribosomal protein kinase/S6 ribosomal protein (mTOR/p70S6K/S6) pathway. Collectively, these data demonstrate that the trans-10,cis-12 CLA-mediated reduction of human adipocyte TG content is associated with the differential localization and expression of lipid droplet-associated proteins. This process involves both the translational control of ADRP through the activation of mTOR/p70S6K/S6 signaling and transcriptional control of perilipin A.
Supplementary key words: conjugated linoleic acid, perilipin, adipose differentiation-related protein, triglycerides, mammalian target of rapamycin, mitogen-activated protein kinase kinase/extracellular signal-related kinase signaling
Abbreviations: ADRP, adipose differentiation-related protein; CLA, conjugated linoleic acid; ERK, extracellular signal-related kinase; GPCR, G protein-coupled receptor; HSL, hormone-sensitive lipase; MEK, mitogen-activated protein kinase kinase; mTOR, mammalian target of rapamycin; PKA, protein kinase A; p70S6K, p70 S6 ribosomal protein kinase; S6, S6 ribosomal protein; SV, stromal vascular; TG, triglyceride; TNF-α, tumor necrosis factor-α; UTR, untranslated region; 5′-TOP, 5′-terminal oligopyrimidine
Conjugated linoleic acid (CLA) refers to a group of dienoic derivatives of linoleic acid. The two primary isomers of CLA found in ruminant meats and milk products and commercial preparations are cis-9,trans-11 CLA and trans-10, cis-12 CLA. CLA isomers have potential anticancer (1, 2) and antiobesity properties (reviewed in 3, 4). Concerning the isomer specificity of CLA and obesity, numerous animal studies have demonstrated that trans-10,cis-12 CLA prevents the development of adiposity (5–9). Similarly, we have shown that trans-10,cis-12 CLA, but not cis-9,trans-11 CLA, inhibits the differentiation of human preadipocytes into adipocytes and reduces the triglyceride (TG) content of mature or newly differentiated human adipocytes (10–12). However, the molecular mechanism(s) and physiological consequences of CLA supplementation are unclear, especially in humans.
Recently, we reported that trans-10,cis-12 CLA treatment of cultures of human stromal vascular (SV) cells containing newly differentiated adipocytes caused delipidation by activating mitogen-activated protein kinase kinase/extra-cellular signal-related kinase (MEK/ERK) signaling (12). This relatively rapid activation of MEK/ERK signaling by trans-10,cis-12 CLA was followed by a decrease in the mRNA levels of peroxisome proliferator-activated receptor γ and many of its downstream adipogenic target genes, including perilipin, glucose transporter 4, adipocyte-specific fatty acid binding protein, lipoprotein lipase, and adiponectin. These CLA-mediated alterations were accompanied by decreased glucose and fatty acid uptake, leading to decreased cellular TG content (e.g., delipidation) of the cultures, and increased the number of cells containing small lipid droplets.
Given the important role of lipid droplet-associated proteins such as perilipin and adipose differentiation-related protein (ADRP) in facilitating lipid deposition or hydrolysis of fatty acids from lipid droplets, we hypothesized that these proteins were intimately involved in CLA’s delipidation of adipocytes. The perilipins, found exclusively at the outer surface of lipid storage droplets in adipocytes and steroidogenic cells, provide a protective protein coat on the lipid droplet surface that shields stored TG against the basal (i.e., nonhormonally stimulated) lipolytic actions of cellular lipases and serves as a cofactor for catecholamine-induce lipolysis (reviewed in 13). Increased expression of perilipin increases TG storage (14, 15), whereas tumor necrosis factor-α (TNF-α) stimulates lipolysis partly by terminating perilipin gene expression, leading to decreased TG storage (15). Perilipin ablation reduces fat mass, increases basal lipolysis, and alters lipid droplet morphology, including reducing adipocyte size (16, 17). Protein kinase A (PKA)-mediated phosphorylation of hormone-sensitive lipase (HSL) and perilipin promotes HSL movement to the lipid droplet and perilipin movement away from the lipid droplet, thereby promoting lipolysis by providing HSL access to TG stores otherwise protected by unphosphorylated perilipin (18, 19). In contrast to perilipin, ADRP is found on the surface of small lipid droplets during early adipocyte differentiation but not on large lipid droplets in mature adipocytes (20). ADRP is thought to play an important role in fatty acid flux in differentiating preadipocytes and many other cell types (21) by increasing fatty acid uptake kinetics (22) and concentrating unesterified fatty acids to the lipid droplet surface (23).
Based on our data demonstrating that trans-10,cis-12 CLA causes delipidation and alters lipid droplet morphology (12), and the dynamic role that perilipin and ADRP play in lipid metabolism, we examined the extent to which CLA altered perilipin and ADRP gene and protein expression and localization in primary cultures of SV cells containing newly differentiated adipocytes. Here, we demonstrate for the first time that trans-10,cis-12 CLA-mediated changes in lipid droplet morphology are associated with increased lipolysis and displacement of perilipin with ADRP on the surface of lipid droplets in human adipocytes. In contrast to the previously described MEK/ERK signaling-mediated reduction of perilipin A mRNA level (12), trans-10,cis-12 CLA-mediated induction of ADRP protein expression is mediated by a marked activation of the translational control mammalian target of rapamycin (mTOR) pathway.
MATERIALS AND METHODS
Materials
All cell culture ware and scintillation cocktail (ScintiSafe) were purchased from Fisher Scientific (Norcross, GA). [1-14C]oleic acid and Western Lighting Plus Chemiluminescence Substrate were purchased from Perkin-Elmer Life Science (Boston, MA). Gene-specific primers for real-time quantitative PCR and NUPAGE pre-cast gels and buffers for SDS-PAGE were purchased from Invitrogen (Carlsbad, CA). FBS was purchased from Cambrex/BioWhittaker (Walkersville, MD). Isomers of CLA (+98% pure) were purchased from Matreya (Pleasant Gap, PA). ADRP monoclonal antibody was purchased from Research Dignostics, Inc. (Flanders, NJ). Perilipin and HSL antibodies were generous gifts from Dr. C. Londos and Dr. F. Kraemer, respectively. Antibodies of caveolin-1 and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rhodamine red and fluorescein isothiocyanate-conjugated IgG were purchased from Jackson Immunoresearch (West Grove, GA). Rapamycin, pertussis toxin, calphostin C, and protein phosphatase 2, a c-SRC kinase inhibitor, were purchased from Calbiochem (La Jolla, CA). Total and phospho-specific antibodies used to measure translational control and U0126 and LY-294002 were obtained from Cell Signaling Technology (Beverly, MA). All other chemicals and reagents were purchased from Sigma Chemical Co. (St. Louis, MO), unless stated otherwise.
Cell cultures of human SV cultures containing newly differentiated adipocytes
Abdominal adipose tissue was obtained from females between 20 and 50 years of age with a body mass index ≤ 30 during lipo-suction or elective surgery with consent from the Institutional Review Board at the University of North Carolina at Greensboro. SV cells were isolated and cultured as defined previously (11, 12) or purchased from Zen Bio, Inc. (Research Triangle Park, NC). Under these isolation and culturing conditions, ~50–70% of the cells differentiated into adipocytes. Experimental treatment of cultures of SV cells containing newly differentiated adipocytes began on day 12–15 of differentiation.
Preparation of fatty acid
Both isomers of CLA were complexed to fatty acid-free (>98%) BSA at a 4:1 molar ratio using 1 mM BSA stocks as described previously (11).
Lipolysis ([14C]oleic acid release)
Cultures were seeded at 4 × 104 cells/cm2 on 48-well cell culture plates and allowed to differentiate for 12 days as described in the cell culture protocol. The lipolysis experiments were conducted based on Guan et al. (24) with minor modifications. Before preloading the cultures with [14C]oleic acid, cultures were serum-starved in DMEM-F12 Ham for 12 h. Then, 20 μl of HBSS containing 6.25 nmol of [14C]oleic acid (specific activity, 50 mCi/mmol) was added to the cultures for an additional 12 h. Approximately 90% of [14C]oleic acid was sequestered by the cultures during this incubation. The medium was then removed and the cultures were washed four times, resulting in a background radioactivity of <1,000 dpm. Each well was treated with 250 μl of fresh DMEM containing either 30 μM cis-9,trans-11 CLA or trans-10,cis-12 CLA, BSA vehicle, or 10 μM isoproterenol in the presence of 40 μM phloretin (a fatty acid-reuptake inhibitor). After 3 h of treatment, 200 μl of medium was collected from each well and delivered to the liquid scintillation counting vial to measure [14C]oleic acid release to the medium, as described previously (12). The amount of cellular protein per well did not differ among treatments; thus, data are expressed on a per well basis.
Preparation of cytosolic fractions and immunoblotting
Cytosolic fractions were prepared according to Clifford et al. (18). Cells were lysed in ice-cold 50 mM Tris-HCl buffer, pH 7.4, containing 225 mM sucrose, 1 mM EDTA, 1 mM bensamidine, 1 μg/ml pepstatin, 1 μg/ml leupeptin, 1 μg/ml antipain, and 50 mM NaF. After lysis, cells remained on ice for 15 min to allow the floating fat cake to solidify. The lysate was then vortexed vigorously for 1 min and centrifuged at 13,000 g at 4°C for 15 min. The cytosolic fraction was aspirated from below the solidified fat cake. Total cell lysate preparation, protein determination, and immunoblotting procedures were as described previously (12).
Immunofluorescence microscopy and phase-contrast images
Cells were cultured on cover slips for immunofluorescence microscopy and stained as described previously (12). For perilipin immunostaining, cells were fixed with 3.7% paraformaldehyde in PBS for 20 min, then cover slips were blocked and quenched in PBS containing 0.1% saponin, 10 mM glycine, and 1.25 mg/ml rabbit IgG for 1 h. Immunodetection was carried out by incubating cells with a 1:400 dilution of goat anti-perilipin antiserum for 6 h followed by three washes with PBS. Cover slips were then incubated with a 1:200 dilution of rhodamine red-conjugated rabbit anti-goat IgG for 1 h (Fig. 1). For perilipin and ADRP double staining, cover slips were blocked again with PBS containing 1.25 mg/ml goat IgG and incubated with a 1:50 dilution of mouse anti-ADRP for 12 h followed by fluorescein isothiocyanate-conjugated secondary antibody (1:100 dilution of FITC goat anti-mouse IgG). After adequate washing with PBS, fluorescent images were captured with a SPOT digital camera mounted on an Olympus BX60 fluorescence microscope. Phase-contrast images were captured using a SPOT digital camera mounted on an Olympus IX60 microscope.
Fig. 1.

Trans-10,cis-12 conjugated linoleic acid (CLA) acutely increases basal lipolysis and perilipin accumulation in the cytosol. A: Cultures of stromal vascular (SV) cells containing newly differentiated adipocyte were treated for 3 h with a BSA vehicle (BSA), 30 μM cis-9,trans-11 CLA (9,11), or 30 μM trans-10, cis-12 CLA (10,12) in the absence (−) or presence (+) of 10 μM isoproterenol. Lipolysis, expressed as the amount of [14C]oleic acid released into conditioned medium after treatment, was determined by scintillation counting. Data are expressed as a percentage of vehicle control (BSA, –isoproterenol) level. Values (means ± SEM; n = 16) not sharing a common superscript differ significantly (P < 0.05). B: Cultures were treated for 0, 3, 6, 12, 24, or 48 h with either BSA (B) or 30 μM trans-10,cis-12 CLA, and total cell extracts were immunoblotted for perilipin. C: Cultures were treated for 0, 3, 6, 12, or 24 h with 30 μM trans-10,cis-12 CLA, and cytosolic fractions were isolated and immunoblotted for perilipin. TRT, treatment. D: To determine isomer specificity of CLA, cultures were treated for 12 h with BSA, 30 μM trans-10,cis-12 CLA, or 30 μM cis-9,trans-11 CLA, and cytosolic fractions were isolated and immunoblotted for perilipin. A 30 min, 10 μM isoproterenol (ISO) treatment was used as a positive control for perilipin movement to cytosol upon its phosphorylation. E: Cultures grown on cover slips were pretreated for 72 h with either BSA or 30 μM trans-10,cis-12 CLA or for 12 h with 10 μM forskolin as a positive control. Cells were incubated with goat anti-perilipin antibody followed by rhodamine-conjugated anti-goat IgG. Localization of perilipin was visualized by immunofluorescence microscopy. Data shown in all three panels are representative of three to four independent experiments.
RNA isolation and real-time quantitative PCR
Total RNA extraction
Total RNA was isolated from the cultures using Tri Reagent (Molecular Research Center, Inc., Cincinnati, OH) according to the manufacturer’s protocol. RNA was extracted with phenol/1-bromo-3-chloropropane and precipitated with ethanol, dried, and resuspended in water. Contaminating genomic DNA was removed by treatment with DNase (DNA-free; Ambion).
Real-time quantitative PCR
First-strand cDNA synthesis and real-time quantitative PCR were carried out using the ABI PRISM 7700 Sequence Detection System (Applied Biosystems) as described previously (11). Primer sets for perilipin and TATA binding protein have been described (12). Primer sets for HSL (accession number NM_005357) were 5′-AAGTGGGCGCAAGTCCC-3′ (sense) and 5′-GCGCATCGGCTCTGCTAT-3′ (antisense), and those for ADRP (accession number NM_001122) were 5′-GCTGAGCACATTGAGTCACGTAC-3′ (sense) and 5′-CTGAGTCAGGTTGCGGGC-3′ (antisense).
Statistical analysis
Lipolysis data are expressed as means ± SEM of 16 independent observations from four different human subjects. Data were analyzed using one-way ANOVA followed by paired Student’s t-tests for multiple comparisons. Differences were considered significant at P < 0.05. All analyses were performed using JMP IN version 4.04 software (SAS Institute, Cary, NC).
RESULTS
Trans-10,cis-12 CLA acutely increases lipolysis
To determine the isomer-specific influence of CLA on lipolysis, [14C]oleic acid was preloaded into SV cells containing newly differentiated human adipocytes, allowing esterification of radiolabeled oleic acid into TG. The release of [14C]oleic acid to the medium was measured after 3 h of treatment with either 30 μM trans-10,cis-12 CLA or cis-9,trans-11 CLA or with BSA vehicle in the presence or absence of 10 μM isoproterenol, a β-adrenergic receptor agonist. As shown in Fig. 1A, basal (e.g., in the absence of isoproterenol) [14C]oleic acid release was ~70% higher in trans-10,cis-12 CLA-treated cultures than in the BSA controls. In contrast, in the presence of isoproterenol, lipolysis was not significantly affected by either CLA isomer.
Trans-10,cis-12 CLA acutely increases cytosolic accumulation of perilipin
Lipolysis is regulated by the activity and location of lipid lipases (i.e., HSL) and perilipin, with the activity and localization of these proteins controlled by phosphorylation via PKA (18). During basal lipolysis, perilipin surrounds lipid droplets, serving as a functional barrier to lipase access to neutral lipid substrates (25). Agents that increase intracellular cAMP levels, such as isoproterenol or forskolin, promote perilipin movement from the surface of lipid droplets to the cytosol by PKA-mediated phosphorylation of HSL and perilipin, leading to increased lipolysis. To determine the extent to which trans-10,cis-12 CLA-induced lipolysis was attributable to perilipin movement from the lipid droplet to the cytosol, we examined perilipin protein levels and changes in localization in the cultures. As seen in Fig. 1B, trans-10,cis-12 CLA increased the levels of perilipin in total cell extracts after 12 h of treatment. A subsequent time course study demonstrated that perilipin appeared in the cytosolic fractions as early as 3 h and peaked at 12 h after treatment with trans-10,cis-12 CLA (Fig. 1C). Cultures treated for 12 h with 30 μM trans-10,cis-12 CLA or for 30 min with 10 μM isoproterenol had appreciable amounts of perilipin in the cytosolic fractions (Fig. 1D). In contrast, perilipin was not detected in cytosolic fractions of cultures treated with either BSA or cis-9,trans-11 CLA.
Supporting our immunoblotting data, a considerable amount of perilipin was found in the cytosol of cultures treated for 12 h with either 30 μM trans-10,cis-12 CLA or 10 μM forskolin (Fig. 1E). CLA supplementation did not induce HSL movement from the cytosol to lipid droplets, as measured by cytosolic fractionation followed by immunoblotting or immunostaining (data not shown). Collectively, these data demonstrate that trans-10,cis-12 CLA acutely stimulates basal lipolysis in human adipocytes, in part by inducing perilipin movement to the cytosol, thereby exposing TG to lipid hydrolases. However, our data suggest that HSL may not be the primary hydrolase/lipase that promotes CLA-induced lipolysis.
Trans-10,cis-12 CLA chronically alters lipid droplet morphology and the expression and location of lipid droplet-associated proteins
To investigate the isomer-specific regulation of adipocyte morphology and lipid droplet-associated protein expression by CLA, cultures were treated with 30 μM cis-9,trans-11 CLA or trans-10,cis-12 CLA or with vehicle for 2–8 days, at which time changes in morphology and the expression and localization of perilipin and ADRP were measured. As seen in Fig. 2, cultures treated with BSA and cis-9,trans-11 CLA for 7 days contained few, but relatively large, lipid droplets within each adipocyte. In contrast, adipocytes in cultures treated with trans-10,cis-12 CLA had more, but smaller, lipid droplets compared with cultures supplemented with either BSA or cis-9,trans-11 CLA. The morphology of cells treated with trans-10,cis-12 CLA resembled that of a multilocular differentiating preadipocyte rather than a more unilocular adipocyte. In support of these data, we previously demonstrated that treatment of cultures for 7 days with trans-10,cis-12 CLA, but not cis-9,trans-11 CLA, significantly reduced the TG content of the cultures (12).
Fig. 2.
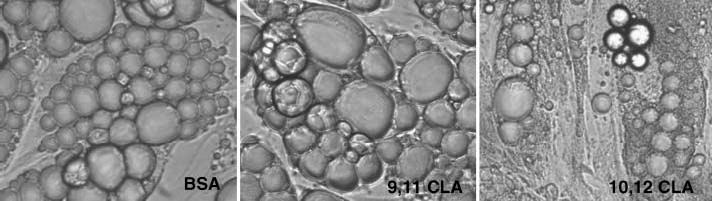
Trans-10,cis-12 CLA alters lipid droplet morphology. Cultures of SV cells containing newly differentiated adipocytes were treated for 7 days with BSA, 30 μM trans-10,cis-12 CLA (10,12 CLA), or 30 μM cis-9,trans-11 CLA (9,11 CLA). Phase-contrast images (100×) were taken to investigate isomer-specific effects of CLA on changes in lipid droplet morphology. Data shown are representative of three independent experiments.
Consistent with these observations, cultures treated with trans-10,cis-12 CLA for 4–8 days had much higher protein levels of ADRP and lower levels of perilipin in total cell extracts compared with cultures supplemented with BSA or cis-9,trans-11 CLA (Fig. 3). Levels of HSL protein decreased as the duration of trans-10,cis-12 CLA treatment increased.
Fig. 3.
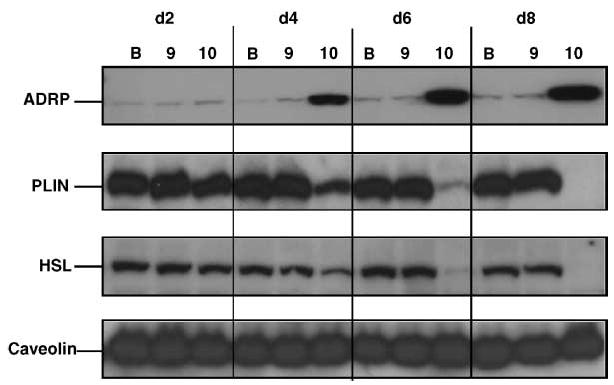
Trans-10,cis-12 CLA changes protein expression of lipid droplet-associated proteins in a time-dependent manner. Cultures of SV cells containing newly differentiated adipocytes were treated for 2, 4, 6, or 8 days (d2 to d8) with BSA (B), 30 μM trans-10,cis-12 CLA (10), or 30 μM cis-9,trans-11 CLA (9). Total cell extracts were immunoblotted for adipose differentiation-related protein (ADRP), perilipin (PLIN), hormone-sensitive lipase (HSL), and caveolin-1. Data shown are representative of three independent experiments.
It is generally accepted that ADRP associates with smaller neutral lipid droplets abundant in preadipocytes, whereas perilipin locates on the surface of larger lipid droplets of mature adipocytes (13). To determine whether the newly formed small lipid droplets occurring in trans-10,cis-12 CLA-treated cultures are covered with ADRP protein, immunostaining was conducted using an ADRP-targeting antibody. As seen in Fig. 4A (100× magnification), almost all lipid droplets were surrounded by ADRP protein in cultures treated for 7 days with trans-10,cis-12 CLA, whereas BSA controls had only background staining for ADRP. Strikingly, trans-10,cis-12 CLA treatment resulted in the accumulation of hundreds of distinct cytosolic lipid droplets within a single cell (Fig. 4A), whereas control cells had many fewer, but relatively larger, cytoplasmic droplets. To determine isomer-specific effects of CLA on the subcellular localization of lipid droplet-coating proteins, cultures were treated with 30 μM cis-9,trans-11 CLA or trans-10,cis-12 CLA or with BSA vehicle for 7 days, and then immunostaining with perilipin and ADRP was carried out. As seen in Fig. 4B (40× magnification), perilipin, but not ADRP, was abundantly expressed around the lipid droplets in the BSA and cis-9,trans-11 CLA-treated cultures. In contrast, ADRP, but not perilipin, was abundantly expressed around the lipid droplets in cultures treated with trans-10,cis-12 CLA (Fig. 4B). Taken together, these data suggest that trans-10,cis-12 CLA alters lipid droplet morphology from the perilipin-coated, large lipid droplets normally found in mature adipocytes to ADRP-coated, small lipid droplets that resemble differentiating preadipocytes.
Fig. 4.

Trans-10,cis-12 CLA alters perilipin and ADRP localization. Cultures of SV cells containing newly differentiated adipocytes grown on cover slips were treated for 7 days with BSA (B), 30 μM trans-10,cis-12 CLA (10,12 CLA), or 30 μM cis-9,trans-11 CLA (9,11 CLA). A: An ADRP immunofluorescence image was captured (100× magnification) in cultures treated with BSA vehicle or trans-10,cis-12 CLA. Results shown are representative of four separate experiments. B: Localization of perilipin and ADRP as detected by double immunostaining with anti-perilipin antibody/rhodamine-conjugated anti-goat (red) and subsequent anti-ADRP antibody/FITC-conjugated anti-mouse (green). Fluorescent images were captured at the same spot in each column (40× magnification).
Trans-10,cis-12 CLA differentially alters the expression of ADRP and perilipin
We previously demonstrated in cultures of SV cells containing newly differentiated adipocytes that trans-10,cis-12 CLA rapidly decreases perilipin gene expression before suppressing mRNA levels for peroxisome proliferator-activated receptor γ (12), a master regulator of adipocyte-specific genes, including perilipin (26). This trans-10,cis-12 CLA-mediated reduction of perilipin gene expression can be attenuated by pretreatment with the MEK inhibitor U0126 (12), implicating MEK/ERK signaling in CLA’s ability to control perilipin gene expression. To determine the degree to which the trans-10,cis-12 CLA-mediated changes in lipid droplet morphology were associated with changes in gene and protein expression for lipid droplet-associated proteins, we treated cultures for 3 days with 30 μM cis-9, trans-11 CLA or trans-10,cis-12 CLA or with BSA vehicle and compared the expression patterns for ADRP, perilipin, and HSL. The extent to which trans-10,cis-12 CLA reduced the protein expression of perilipin and HSL (Fig. 5A) was equivalent to its attenuation of gene expression (Fig. 5B). In contrast, ADRP protein expression (Fig. 5A) was markedly increased, whereas ADRP mRNA levels (Fig. 5B) were increased by only ~50% in cultures treated with trans-10,cis-12 CLA compared with cultures treated with cis-9,trans-11 CLA and BSA vehicle. Furthermore, neither ADRP mRNA nor protein stability appeared to be influenced by trans-10,cis-12 CLA treatment for up to 24 h in the presence of 5 μg/ml actinomycin D or 10 μM cycloheximide, respectively (data not shown). This differential effect of CLA on mRNA and protein levels of adipocyte-specific genes was accompanied by a robust increase in phospho-ERK (Fig. 5A), suggesting a role of MEK-ERK signaling. Collectively, these data suggest that the trans-10, cis-12 CLA-mediated increase of ADRP protein expression is primarily regulated by a specific mechanism that increases ADRP protein synthesis.
Fig. 5.
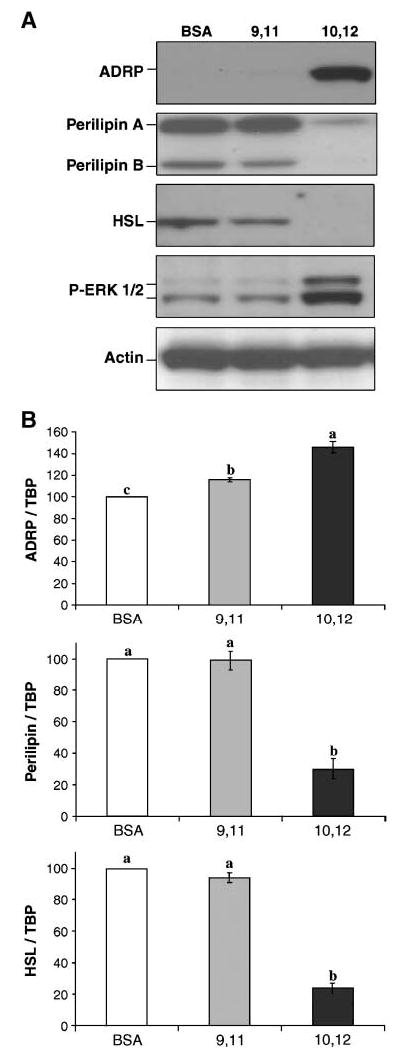
Trans-10,cis-12 CLA differentially affects gene and protein expression of lipid droplet-associated proteins. Cultures of SV cells containing newly differentiated adipocytes were treated for 3 days with BSA (B), 30 μM trans-10,cis-12 CLA (10,12), or 30 μM cis-9, trans-11 CLA (9,11). Cells were harvested on day 3 based on the data in Fig. 3, indicating that between days 2 and 4, ADRP increased and perilipin and HSL proteins decreased. A: Total cell extracts were immunoblotted for ADRP, perilipin, and HSL. Actin was used as a loading control for the Western blots. Results shown are representative of three separate experiments using cells isolated from different human subjects each time. Extracellular signal-related kinase (ERK) phosphorylation was measured using a phospho-specific antibody for ERK. B: Total RNA was harvested using Tri Reagent, and mRNA expression levels of ADRP, perilipin, and HSL were analyzed using real-time quantitative PCR. TATA binding protein (TBP) was used as a control for real-time quantitative PCR. Values (means ± SEM; n = 3 for ADRP, n = 2 for perilipin and HSL) not sharing a common superscript differ significantly (P < 0.05).
Trans-10,cis-12 CLA activates the mTOR/p70 S6 ribosomal protein kinase/S6 ribosomal protein pathway
We examined signaling pathways regulating translation given the relative greater increase in the levels of ADRP protein compared with ADRP mRNA in cultures treated with trans-10,cis-12 CLA (Fig. 5A, B). Mammalian cells possess an important nutrient-sensing pathway that controls protein synthesis at the level of translation (reviewed in 27, 28). A central player in this pathway is mTOR, which is activated by growth factors, amino acids, and mitogenic signals via a mechanism that is not yet fully understood. We examined the time-dependent effects of CLA on the phosphorylation status of key proteins known to regulate mTOR-dependent translation. As shown in Fig. 6A, cultures treated with trans-10,cis-12 CLA exhibited transiently activated S6 ribosomal protein (S6), a downstream target of mTOR, between 3 and 12 h compared with BSA controls. Furthermore, immunostaining using antibodies targeting to phospho-S6 (p-S6) demonstrated that both 20% FBS (positive control) and 30 μM trans-10,cis-12 CLA treatment for 30 min and 3 h, respectively, increased the phosphorylation of S6 compared with BSA controls (Fig. 6B).
Fig. 6.

Trans-10,cis-12 CLA induces mammalian target of rapamycin (mTOR) signaling. A: Cultures of SV cells containing newly differentiated adipocytes were treated for 0, 1, 3, 6, 12, 24, 48, or 72 h with either a BSA vehicle (BSA) or 30 μM trans-10,cis-12 CLA (10,12 CLA). Total cell extracts were immunoblotted using phospho-specific or non-phospho-specific antibodies targeting the S6 ribosomal protein (S6). B: Cultures grown on cover slips were treated for 3 h with either a BSA vehicle or 30 μM trans-10,cis-12 CLA. A 30 min 20% FBS treatment was used as a positive control. After treatment, p-S6 was visualized by immunostaining using rabbit anti-pS6 and FITC-conjugated anti-rabbit IgG. C: Cultures were treated for 3 h with a BSA vehicle, 30 μM cis-9,trans-11 CLA (9,11), or 30 μM trans-10,cis-12 CLA (10,12). A 10 min human insulin (100 nM) treatment or 30 min tumor necrosis factor-α (TNF-α; 100 ng/ml) treatment was used as a positive control. Total cell extracts were immunoblotted using phospho-specific antibodies targeting protein kinase B (Akt), mTOR, p70 S6 ribosomal protein kinase (p70S6K), S6, eukaryotic initiation factor 4E (eIF4E), or MNK1. Results shown are representative of two independent experiments.
To determine whether this early activation of the mTOR pathway was isomer-specific, cultures were treated for 3 h with either 30 μM cis-9,trans-11 CLA or trans-10,cis-12 CLA. In addition, a 30 min treatment was performed with two known potent activators of mTOR signaling, insulin (100 nM) and TNF-α (100 ng/ml). As seen in Fig. 6C, phosphorylated Akt was found only in insulin-treated cultures, whereas the levels of phosphorylated mTOR, p70 S6 ribosomal protein kinase (p70S6K), and S6 were markedly higher in cultures treated with trans-10,cis-12 CLA, insulin, and TNF-α than in cultures treated with cis-9,trans-11 CLA or BSA vehicle. Similarly, phosphorylation of MNK1, an ERK-activated protein that phosphorylates 40S ribosomal protein independent of mTOR, as well as phosphorylation of eukaryotic initiation factor 4E, a protein possessing RNA helicase activity, were also increased in cultures treated with trans-10,cis-12 CLA and TNF-α. In contrast, neither cis-9,trans-11 CLA, BSA vehicle, nor insulin increased Mnk1 or eukaryotic initiation factor 4E phosphorylation.
To determine the critical signaling steps involved in the trans-10,cis-12 CLA-induced mTOR signaling pathway, we used several inhibitors to block upstream regulators of p70S6K and S6 (Fig. 7). Activation of p70S6K and S6 kinase 2 can be specifically blocked by the immunosuppressant rapamycin, a bacterial macrolide, without affecting kinases involved in mitogenic responses, resulting in attenuation of the translational activation of 5′-terminal oligopyrimidine (5′-TOP) mRNA (29). In support of our concept that trans-10,cis-12 CLA-induced translational activation occurs via mTOR, phosphorylation of p70S6K and S6 by 3 h of treatment with trans-10,cis-12 CLA was blocked by pretreatment with rapamycin (Fig. 7). In addition, most inhibitors, including the MEK/ERK inhibitor U0126, the G protein-coupled receptor (GPCR) Gi/o coupling inhibitor pertussis toxin, the phosphatidylinositol 3-kinase inhibitor LY-294002, the protein kinase C inhibitor calphostin C, and the c-SRC kinase protein inhibitor protein phosphatase 2, blocked or attenuated CLA’s induction of phosphorylation of p70S6K and S6.
Fig. 7.
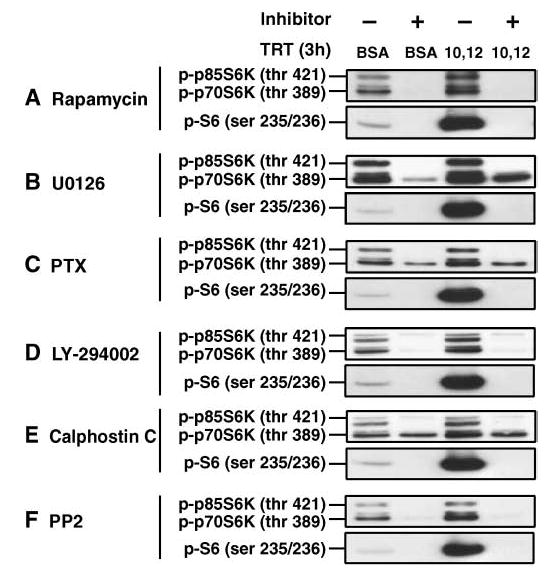
Pharmacological inhibitors block or attenuate trans-10,cis-12 CLA activation of p70S6K and S6. Cultures of SV cells containing newly differentiated adipocytes were pretreated for 1 h with (+) or without (−) the following inhibitors and then treated (TRT) for an additional 3 h with either a BSA vehicle (BSA) or 30 μM trans-10,cis-12 CLA (10,12): 100 nM rapamycin (A), an inhibitor of mTOR; 10 μM U0126 (B), an inhibitor of mitogen-activated protein kinase kinase (MEK) phosphorylation; 100 ng/ml pertussis toxin (PTX; C), an inhibitor of G-coupled protein receptor activation of Gi and Rac1/cdc42; 100 μM LY-294002 (D), an inhibitor of phosphatidylinositol 3-kinase and mTOR; 100 nM calphostin C (E), an inhibitor of protein kinase C; or 10 μM protein phosphatase 2 (PP2; F), an inhibitor of SRC kinase and p70S6K. Total cell extracts were then immunoblotted with phospho-specific antibodies targeting p70S6K, p85S6K, or S6. Results shown are representative of two independent experiments.
Taken together, these data suggest that 1) CAL-mediated activation of the motor signaling pathway is isomer-specific, and 2) its regulation is controlled by multiple factors that could potentially affect 40S ribosomal protein activation of 5′-TOP mRNA translation, including MEK/ERK, protein kinase C, protein kinase B, and GPCR Gi protein. These data indicate a potential role of the mTOR pathway as a signal integrator of CLA’s TG-lowering actions and a potential mechanism by which trans-10,cis-12 CLA increases ADRP protein levels.
Rapamycin blocks CLA’s increase in ADRP protein expression
Based on the isomer-specific activation of S6 by CLA, we hypothesized that translational induction of ADRP by trans-10,cis-12 CLA is associated with activation of the mTOR pathway. To test this hypothesis, we treated cultures for 24 h with either 30 μM trans-10,cis-12 CLA or BSA in the presence or absence of the mTOR-specific inhibitor rapamycin. As hypothesized, CLA’s induction of ADRP protein was blocked by pretreatment with rapamycin (Fig. 8). In con trast, neither perilipin nor caveolin-1 expression was affected by rapamycin. It was also notable that 24 h treatment with trans-10,cis-12 CLA modestly attenuated perilipin-A and perilipin-B gene expression even in the presence of rapamycin, suggesting that a rapamycin-sensitive pathway may not be necessary for a reduction of perilipin expression by trans-10,cis-12 CLA (Fig. 8). In fact, our data support the notion that the trans-10,cis-12 CLA-mediated reduction of perilipin expression is mediated by a more chronic activation of cytokine-induced MEK/ERK signaling (Fig. 5A), which we have described previously (12). Collectively, these data suggest that the trans-10,cis-12 CLA-mediated increase in the levels of the small lipid droplet-associated protein ADRP is partly attributable to a rapamycin-sensitive increase in ADRP protein synthesis.
Fig. 8.
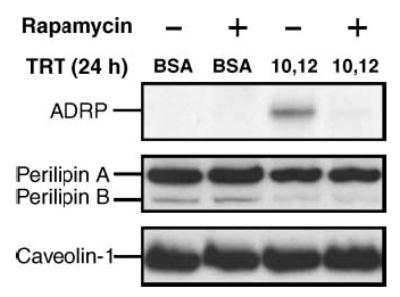
Trans-10,cis-12 CLA-induced ADRP levels are blocked by rapamycin. Cultures of SV cells containing newly differentiated adipocytes were pretreated for 1 h with (+) or without (−) 100 nM rapamycin and then treated (TRT) for an additional 24 h with either a BSA vehicle (BSA) or 30 μM trans-10,cis-12 CLA (10,12). Total cell extracts were then immunoblotted with antibodies targeting ADRP, perilipins A and B, and caveolin-1. Results shown are representative of three independent experiments.
DISCUSSION
Feeding mixed CLA isomers or trans-10,cis-12 CLA to humans decreases body fat (30–32), and supplementing cultures of human adipocytes with trans-10,cis-12 CLA reduces cell size and TG content (12). However, little is known about the mechanism by which CLA controls the flux of neutral lipids in and out of adipocyte lipid droplets. To our knowledge, the data presented here are the first to demonstrate that trans-10,cis-12 CLA alters adipocyte lipid droplet morphology from perilipin-associated large lipid droplets to ADRP-associated small lipid droplets. This process involves the movement of perilipin to the cytosol, presumably by posttranslational regulation (e.g., perilipin phosphorylation), and differential regulation of lipid droplet-associated protein (ADRP, perilipin, and HSL) expression, as shown in Fig. 9. Furthermore, our data demonstrate for the first time that trans-10,cis-12 CLA induces the mTOR pathway, which could be responsible, in part, for the robust increase in the levels of ADRP protein. Alternatively, the CLA-mediated increase in perilipin movement away from large lipid droplets may provide greater access for ADRP binding on the lipid droplet surface, thereby increasing its stability. Taken together, we propose in our working model (Fig. 9) that trans-10,cis-12 CLA increases basal lipolysis and reduces lipid droplet TG content by 1) promoting perilipin dispersion to the cytosol, 2) downregulating perilipin gene expression, and 3) increasing the levels of ADRP protein bound to lipid droplets facilitated by migration of perilipin away from the large lipid droplets and/or by increased ADRP translation induced by the mTOR pathway.
Fig. 9.
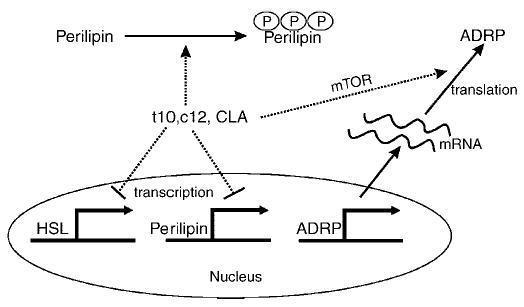
Model of trans-10,cis-12 CLA-mediated changes of morphology in adipocytes: transcriptional, translational, and posttranslational regulation of lipid droplet-associated proteins. Under normal, non-lipolytic conditions, perilipin is expressed exclusively in mature adipocytes, serving as a barrier to protect lipid droplets from hydrolysis by lipases such as HSL. Chronic supplementation of trans-10,cis-12 CLA (t10,c12 CLA) changes adipocyte morphology by promoting the development of numerous small lipid droplets coated with ADRP. Trans-10,cis-12 CLA induces perilipin phosphorylation, which drives perilipin movement from the surface of lipid droplets to the cytosol and thus increases the susceptibility to hydrolysis by lipase(s), resulting in increased basal lipolysis. As a physiological consequence of trans-10,cis-12 CLA supplementation, ADRP replaces perilipin as a lipid droplet-coating protein by CLA’s combined action on the transcriptional repression of perilipin and HSL and the translational activation of ADRP in a mTOR pathway-dependent manner.
We demonstrated that CLA acutely stimulates basal lipolysis in cultures of human adipocytes, as previously shown in murine adipocytes (3, 5, 6). However, it is not clear whether this CLA-induced lipolysis is mediated by HSL activation via classic cAMP and PKA signaling, because we did not observe an increase in isoproterenol-stimulated lipolysis in the CLA-treated cultures. Although we detected perilipin dispersion to cytosol within 3 h of treatment with trans-10,cis-12 CLA, we failed to observe HSL phosphorylation or HSL translocation to lipid droplets in CLA-treated cultures. One possible explanation for these observations is that CLA may stimulate lipolysis via a HSL-independent mechanism. In support of this possibility, growing evidence suggests that HSL is not the only lipase capable of TG hydrolysis in adipocytes (33, 34). The fact that trans-10,cis-12 CLA stimulates basal, but not isoproterenol-sensitive, lipolysis in our model (Fig. 1A) supports the likelihood that trans-10,cis-12 CLA may increase basal lipolysis by a mechanism that is distinct from the classic cAMP-driven, PKA-mediated phosphorylation of HSL and perilipin. However, this notion requires rigorous testing.
Alternatively, CLA may stimulate HSL activation without concomitant translocation to the lipid droplet. In support of this concept, it was suggested recently that perilipin redistribution to the cytosol is not necessarily accompanied by HSL translocation to the lipid droplet surface (reviewed in 35). It has also been shown in 3T3-L1 adipocytes that HSL can be phosphorylated via the ERK pathway (36). In agreement with these data, we previously reported that trans-10,cis-12 CLA induced ERK activation (12), supporting the idea that the CLA-mediated increase in lipolysis may occur via an ERK-dependent activation of HSL or other candidate lipase, such as adipose tissue TG lipase (37) or desnutrin (38).
Interestingly, trans-10,cis-12 CLA acutely (e.g., 3–12 h) induced perilipin accumulation in the cytosol (Fig. 1C, D) but chronically (e.g., >24 h) decreased the levels of perilipin mRNA and protein (Figs. 1B–D, 3, 5). Zhang et al. (39) demonstrated in human adipocytes that TNF-α increased lipolysis and perilipin phosphorylation by an ERK-dependent pathway that increased PKA activity in a cAMP-dependent manner. In agreement with these data, we found that trans-10,cis-12 CLA increased ERK phosphorylation in an isomer-specific manner (Fig. 5A). These data provide further support for CLA-induced ERK activation as a possible cause of perilipin movement to the cytosol via phosphorylation. Furthermore, we found that treatment with interleukin-6 or interleukin-8, which increases MEK/ERK signaling (12), increased cytosolic perilipin (our unpublished data), suggesting that CLA-induced adipokines may play an important role in TG efflux from lipid droplets. In contrast, the protein level of caveolin-1 (Fig. 3), which plays a pivotal role in lipid droplet biogenesis and cholesterol metabolism (40), was not affected by trans-10,cis-12 CLA.
ADRP, or its human analog adipophilin, is a membrane-bound protein associated with lipid accumulation in diverse cell types (21, 41). ADRP is thought to act as a “shuttling protein” for lipids, particularly long-chain fatty acids (22), from the plasma membrane to the lipid droplet. Long-chain fatty acids induce ADRP gene transcription (13, 42). ADRP is highly upregulated during early stages of 3T3-L1 preadipocyte differentiation (43) but is not expressed at appreciable levels in mature adipocytes (20). In perilipin ablation studies, a compensatory ADRP coat is found on existing lipid droplets (16).
In our cell model, CLA induced the appearance of small lipid droplets and ADRP expression in both the adipocyte portion and the SV portion of the cultures, based on the immunostaining shown in Fig. 4. Interestingly, Gatlin et al. (44) have reported the development of intramuscular microscopic lipid droplets in CLA-fed animals, which may have been caused by increased ADRP expression. Given the fact that ADRP and perilipin share a competitive and exclusive relationship on the surface of lipid droplets, a CLA-mediated reduction in lipid droplet size and perilipin protein may increase ADRP binding to the lipid droplet surface, thereby increasing ADRP stability. However, cycloheximide treatment did not appear to differentially affect ADRP abundance in cultures treated with trans-10,cis-12 CLA (data not shown), suggesting that CLA does not increase ADRP stability.
Alternatively, trans-10,cis-12 CLA may increase ADRP translation by activating several protein kinase cascades, including the mTOR and ERK pathways, that converge to increase translation efficiency. This hypothesis is based on the following: 1) data in Fig. 5A and our previous data (12) demonstrating that trans-10,cis-12 CLA induces MEK/ERK signaling; 2) data in Fig. 6 showing that trans-10,cis-12 CLA increases the phosphorylation of mTOR, p70S6K, and S6; and 3) data in Fig. 7 demonstrating that trans-10,cis-12 CLA activation of p-p70S6K or p-S6 was inhibited or attenuated, respectively, by the MEK inhibitor U0126 (Fig. 7B) and other inhibitors of the mTOR pathway (Fig. 7A, C–F). Both mTOR and ERK can converge to activate p70S6K/S6 signaling, which is known to regulate the translation of a specific type of mRNA called 5′-TOP mRNA (28). There are three important criteria for determining whether a transcript is under translational control of the mTOR pathway (45). First, translation of 5′-TOP mRNA is rapamycin-sensitive (Fig. 7A). Second, cis-acting elements in the 5′-untranslated region (UTR) form extensive secondary structures prohibiting the access of translation machinery, which include an uninterrupted guanidine-cytosine-rich region of 4–14 pyrimidines with a cytosine cap site or a long 5′-UTR possessing multiple hairpin structures (46). Third, 5′-TOP mRNAs are sequestered in translationally inactive messenger ribonucleoprotein particles. Redistribution of 5′-TOP mRNA from messenger ribonucleoprotein to large polysomes (40–60S) is obligatory for efficient translation upon growth stimuli, including mitogens, hormones, growth factors, and branched chain amino acids such as leucine (27).
Using these three criteria, we speculate that ADRP might be a 5′-TOP mRNA based on the following evidence. First, pretreatment of rapamycin efficiently inhibits CLA-induced ADRP protein expression (Fig. 8). Second, even though human ADRP mRNA lacks the conventional structural 5′-TOP moiety, it has 178 nucleotides in the 5′-UTR region, which is rich in guanidine-cytosine content (68%), and contains two independent oligopyrimidine stretches of 6 and 11 bases. This would potentially allow for at least 17 thermodynamically stable RNA secondary structures (ΔG ≤ −70 to −80 kcal/mol) containing complicated hairpins, as we predicted using a computer simulation model (RNA Structure, version 4.1; data not shown). Third, Brasaemle et al. (20) suggested that ADRP mRNA may be recruited to polysomes for protein synthesis rather than to the endoplasmic reticulum, based on a discrepancy between mRNA and protein expression levels in differentiated 3T3-L1 adipocytes. Fourth, leptin, a peptide that senses the lipid status of adipocytes and is a 5′-TOP mRNA (47), is also increased by trans-10,cis-12 CLA treatment (12). Based on these observations, we hypothesize that trans-10,cis-12 CLA increases ADRP protein levels by initiating the translation of ADRP mRNA via activation of the mTOR/p70S6K/S6 signaling pathways that recruit ADRP mRNA into translational 40S ribosomal machinery.
In summary, these data demonstrate that acute treatment (within 3 h) of the cultures with trans-10,cis-12 CLA increases basal lipolysis and the appearance of perilipin in the cytosolic fraction. Chronic treatment (≥48 h) of the cultures with trans-10,cis-12 CLA decreases perilipin and HSL gene and protein expression and enhances ADRP protein expression and abundance on the lipid droplet. The robust increase in ADRP protein levels by trans-10,cis-12 CLA was accompanied by phosphorylation of ERK and acute activation of the mTOR pathway, including activation of p70S6K and its downstream target S6, which was abolished by coincubation of the CLA-treated cultures with specific inhibitors of the mTOR/p70S6K/S6 pathway. Collectively, these data suggest that trans-10,cis-12 CLA promotes adipocyte delipidation, in part by promoting lipolysis, which may result from ERK-dependent alterations in perilipin and ADRP gene expression, protein activation, and localization. Furthermore, the MEDIATED-mediated increase in ADEPT protein may be attributable to enhanced ADEPT translation induced by the activation of mTOR/p70S6K/S6 signaling.
Acknowledgments
The authors are very grateful to GlaxoSmith for supplying the BRL49653 compound. The authors also thank F. Kraemer and C. Londos for the HSL and perilipin antibodies, respectively, O. Fabiyi for her wonderful technical assistance, and S. Mandrup for her critical review of this work. This work was supported by grants from the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases/Office of Dietary Supplements Grant DK-63070) and the North Carolina Agriculture Research Service (Grant 06520) to M.M.
References
- 1.Ha Y, Grimm N, Pariza M. Anticarcinogens from fried ground beef: heat-altered derivatives of linoleic acid. Carcinogenesis. 1987;8:1881–1887. doi: 10.1093/carcin/8.12.1881. [DOI] [PubMed] [Google Scholar]
- 2.Belury M. Inhibition of carcinogenesis by conjugated linoleic acid: potential mechanisms of action. J Nutr. 2002;132:2995–2998. doi: 10.1093/jn/131.10.2995. [DOI] [PubMed] [Google Scholar]
- 3.Evans M, Brown JM, McIntosh M. Isomer-specific effects of conjugated linoleic acid (CLA) on adiposity and lipid metabolism. J Nutr Biochem. 2002;13:508–516. doi: 10.1016/s0955-2863(02)00211-5. [DOI] [PubMed] [Google Scholar]
- 4.Brown JM, McIntosh M. Conjugated linoleic acid in humans: regulation of adiposity and insulin sensitivity. J Nutr. 2003;133:3041–3046. doi: 10.1093/jn/133.10.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park Y, Albright K, Liu W, Storkson J, Cook M, Pariza M. Effect of conjugated linoleic acid on body composition in mice. Lipids. 1997;32:853–858. doi: 10.1007/s11745-997-0109-x. [DOI] [PubMed] [Google Scholar]
- 6.Park Y, Storkson J, Albright K, Liu W, Cook M, Pariza M. Evidence that the trans-10,cis-12 isomer of conjugated linoleic acid induces body composition changes in mice. Lipids. 1999;34:235–241. doi: 10.1007/s11745-999-0358-8. [DOI] [PubMed] [Google Scholar]
- 7.Park Y, Albright K, Storkson J, Liu W, Pariza M. Changes in body composition in mice during feeding and withdrawal of conjugated linoleic acid. Lipids. 1999;33:243–248. doi: 10.1007/s11745-999-0359-7. [DOI] [PubMed] [Google Scholar]
- 8.Delany J, Blohm F, Truett A, Scimeca J, West D. Conjugated linoleic acid rapidly reduces body fat content in mice without affecting energy intake. Am J Physiol. 1999;276:R1172–R1179. doi: 10.1152/ajpregu.1999.276.4.R1172. [DOI] [PubMed] [Google Scholar]
- 9.Ostrowska E, Muralitharian M, Cross R, Bauman D, Dunshea F. Dietary conjugated linoleic acid increases lean tissue and decreases body fat deposition in growing pigs. J Nutr. 1999;129:2037–2042. doi: 10.1093/jn/129.11.2037. [DOI] [PubMed] [Google Scholar]
- 10.Brown JM, Halvorsen YD, Lea-Currie R, Geigerman C, McIntosh M. Trans-10, cis-12, but not cis-9, trans-11, conjugated linoleic acid attenuates lipogenesis in primary cultures of stromal vascular cells isolated from human adipose tissue. J Nutr. 2001;131:2316–2321. doi: 10.1093/jn/131.9.2316. [DOI] [PubMed] [Google Scholar]
- 11.Brown JM, Sandberg-Boysen M, Jensen S, Morrison R, Storkson J, Lea-Currie R, Pariza M, Mandrup S, McIntosh M. Isomer-specific regulation of metabolism and PPARγ signaling by CLA in human preadipocytes. J Lipid Res. 2003;44:1287–1300. doi: 10.1194/jlr.M300001-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown JM, Boysen MS, Chung S, Fabiyi O, Morrison R, Mandrup S, McIntosh M. Conjugated linoleic acid induces human adipocyte delipidation: autocrine/paracrine regulation of MEK/ERK signaling by adipocytokines. J Biol Chem. 2004;279:26735–26747. doi: 10.1074/jbc.M401766200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landos C, Brasaemle D, Schultz C, Segrest J, Kimmel A. Perilipins, ADRP, and other proteins that associate with intracellular lipid droplets in animal cells. Cell Dev Biol. 1999;10:51–58. doi: 10.1006/scdb.1998.0275. [DOI] [PubMed] [Google Scholar]
- 14.Brasaemle D, Levin D, Adler-Wailes D, Londos C. The lipolytic stimulation of 3T3-L1 adipocytes promotes the translocation of hormone sensitive lipase to the surface of lipid storage droplets. Biochim Biophys Acta. 2000;1483:251–262. doi: 10.1016/s1388-1981(99)00179-1. [DOI] [PubMed] [Google Scholar]
- 15.Souza S, de Vargas L, Yamamoto M, Lien P, Franciosa M, Moss L, Greenberg A. Overexpression of perilipin A and B blocks the ability of tumor necrosis factor alpha to increase lipolysis in 3T3-L1 adipocytes. J Biol Chem. 1998;273:24665–24669. doi: 10.1074/jbc.273.38.24665. [DOI] [PubMed] [Google Scholar]
- 16.Tansey J, Sztalryd C, Gruia-Grav J, Roush D, Gavrilova J, Reitman M, Deng CX, Li C, Kimmel A, Londos C. Perilipin ablation results in a lean mouse with aberrant lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc Natl Acad Sci USA. 2001;98:6494–6499. doi: 10.1073/pnas.101042998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez-Botas J, Anderson J, Tessier D, Lapillonne A, Chang B, Quast M, Gorenstein D, Chen K, Chan L. Absence of perilipin results in leanness and reverses obesity in Lepr (db/db) mice. Nat Genet. 2000;26:474–479. doi: 10.1038/82630. [DOI] [PubMed] [Google Scholar]
- 18.Clifford G, Londos C, Kraemer F, Vernon R. Translocation of hormone-sensitive lipase and perilipin upon lipolytic stimulation of rat adipocytes. J Biol Chem. 2000;275:5011–5015. doi: 10.1074/jbc.275.7.5011. [DOI] [PubMed] [Google Scholar]
- 19.Sztalryd C, Xu G, Dorward H, Tansey J, Contreras J, Kimmel A, Landos C. Perilipin A is essential for the translocation of hormone sensitive lipase during lipolytic activation. J Cell Biol. 2003;161:1093–1103. doi: 10.1083/jcb.200210169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brasaemle D, Barber T, Wolins N, Serrero G, Blanchette-Mackie E, Londos C. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J Lipid Res. 1997;38:2249–2262. [PubMed] [Google Scholar]
- 21.Heid H, Moll R, Schwetlick I, Rackwitz H, Keenan T. Adipophilin is a specific marker of lipid accumulation in diverse cell types and diseases. Cell Tissue Res. 1998;294:309–321. doi: 10.1007/s004410051181. [DOI] [PubMed] [Google Scholar]
- 22.Gao J, Serrero G. Adipose differentiation related protein (ADRP) expressed in transfected COS-7 cells selectively stimulates long chain fatty acid uptake. J Biol Chem. 1999;274:16825–16830. doi: 10.1074/jbc.274.24.16825. [DOI] [PubMed] [Google Scholar]
- 23.Serrero G, Frolov F, Schroeder K, Tanaka L, Gelhaar L. Adipose differentiation related protein: expression, purification of recombinant protein in Escherichia coli and characterization of its fatty acid binding properties. Biochim Biophys Acta. 2000;1488:245–254. doi: 10.1016/s1388-1981(00)00128-1. [DOI] [PubMed] [Google Scholar]
- 24.Guan H, Li Y, Jensen M, Newfard C, Steppan C, Lazar M. A futile metabolic cycle activated in adipocytes by antidiabetic agents. Nat Med. 2002;8:1122–1128. doi: 10.1038/nm780. [DOI] [PubMed] [Google Scholar]
- 25.Brasaemle D, Rubin B, Harten I, Gruia-Guy J. Perilipin A increases triacylglycerol storage by decreasing the rate of triacylglycerol hydrolysis. J Biol Chem. 2000;275:38486–38493. doi: 10.1074/jbc.M007322200. [DOI] [PubMed] [Google Scholar]
- 26.Dalen K, Schoonjans K, Ulven S, Weedon-Fekjaer M, Bentzen T, Koutnikova A, Auwerx J, Nebb H. Adipose tissue expression of lipid droplet-associating proteins S3–12 and perilipin is controlled by peroxisome proliferator-activated receptor-γ. Diabetes. 2004;53:1243–1252. doi: 10.2337/diabetes.53.5.1243. [DOI] [PubMed] [Google Scholar]
- 27.Tokunaga C, Yoshino K, Yonezawa K. mTOR integrates amino acid- and energy-sensing pathways. Biochem Biophys Res Commun. 2004;313:443–446. doi: 10.1016/j.bbrc.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 28.Hay N, Sonnenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 29.Terada N, Patel H, Takase K, Kohno K, Nairn A, Pearson R, Thomas G. Rapamycin selectively inhibits translation of mRNAs encoding elongation factors and ribosomal proteins. Proc Natl Acad Sci USA. 1994;91:11477–11481. doi: 10.1073/pnas.91.24.11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thom E, Wadstein J, Gudmundsen O. Conjugated linoleic acid reduces body fat in healthy exercising humans. J Int Med Res. 2001;29:392–396. doi: 10.1177/147323000102900503. [DOI] [PubMed] [Google Scholar]
- 31.Blankson H, Stakkestad J, Fagertun H, Thom E, Wadstein J, Gudmundsen O. Conjugated linoleic acid reduces body fat mass in overweight and obese humans. J Nutr. 2000;130:2943–2948. doi: 10.1093/jn/130.12.2943. [DOI] [PubMed] [Google Scholar]
- 32.Riserus U, Arner P, Brismar K, Vessby B. Treatment with dietary trans10cis12 conjugated linoleic acid causes isomer-specific insulin resistance in obese men with the metabolic syndrome. Diabetes Care. 2002;25:1516–1521. doi: 10.2337/diacare.25.9.1516. [DOI] [PubMed] [Google Scholar]
- 33.Okazaki H, Osuga J, Tamura Y, Yahagi N, Tomita S, Shionoiri F, Iizuka Y, Ohashi K, Harada K, Kimura S, Gotoda T, Shimano H, Nobuhiro Y, Ishibashi S. Lipolysis in the absence of hormone-sensitive lipase. Diabetes. 2002;51:3368–3375. doi: 10.2337/diabetes.51.12.3368. [DOI] [PubMed] [Google Scholar]
- 34.Soni K, Lehner R, Metalnikov R, O’Donnell P, Semache M, Gao W, Ashman K, Pshezhetsky A, Mitchell G. Carboxylesterase 3 (EC 3.1.1.1) is a major adipocyte lipase. J Biol Chem. 2004;279:40683–40689. doi: 10.1074/jbc.M400541200. [DOI] [PubMed] [Google Scholar]
- 35.Holm C. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem Soc Trans. 2003;31:1120–1124. doi: 10.1042/bst0311120. [DOI] [PubMed] [Google Scholar]
- 36.Greenberg A, Shen W, Muliro K, Patel S, Souza S, Roth R, Kraemer F. Stimulation of lipolysis and hormone-sensitive lipase via the extracellular signal-regulated kinase pathway. J Bio Chem. 2001;276:45456–45461. doi: 10.1074/jbc.M104436200. [DOI] [PubMed] [Google Scholar]
- 37.Zimmerman R, Strauss J, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Esienhaber F, Hermetter A, Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 38.Villena J, Roy S, Sarkadi-Nagy E, Kim K, Sul HS. Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids. J Biol Chem. 2004;279:47066–47075. doi: 10.1074/jbc.M403855200. [DOI] [PubMed] [Google Scholar]
- 39.Zhang H, Halbleib M, Ahmad F, Manganiello V, Greensberg A. Tumor necrosis factor alpha stimulates lipolysis in differentiated human adipocytes through activation of extracellular signal-related kinase and elevation of intracellular cAMP. Diabetes. 2002;51:2929–2935. doi: 10.2337/diabetes.51.10.2929. [DOI] [PubMed] [Google Scholar]
- 40.Cohen A, Razani B, Schubert W, Williams T, Wang X, Iyengar P, Brasaemle D, Scherer P, Lisanti M. Role of caveolin-1 in the modulation of lipolysis and lipid droplet formation. Diabetes. 2004;53:1261–1270. doi: 10.2337/diabetes.53.5.1261. [DOI] [PubMed] [Google Scholar]
- 41.Imamura M, Inoguchi T, Ikuyama S, Taniguchi S, Kobayashi K, Nakashima N, Nawata H. ADRP stimulates lipid accumulation and lipid droplet formation in murine fibroblasts. Am J Physiol Endocrinol Metab. 2002;283:E775–E783. doi: 10.1152/ajpendo.00040.2002. [DOI] [PubMed] [Google Scholar]
- 42.Gao J, Ye H, Serrero G. Stimulation of adipose differentiation related protein (ADRP) expression in adipocyte precursors by long-chain fatty acids. J Cell Physiol. 2000;182:297–302. doi: 10.1002/(SICI)1097-4652(200002)182:2<297::AID-JCP19>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 43.Jiang H, Serrero G. Isolation and characterization of a full-length cDNA coding for an adipose differentiation-related protein. Proc Natl Acad Sci USA. 1992;89:7856–7860. doi: 10.1073/pnas.89.17.7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gatlin L, See M, Larick D, Lin X, Odle J. Conjugated linoleic acid in combination with supplemental dietary fat alters pork fat quality. J Nutr. 2002;132:3105–3112. doi: 10.1093/jn/131.10.3105. [DOI] [PubMed] [Google Scholar]
- 45.Gingras A, Raught B, Soneburg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001;15:807–826. doi: 10.1101/gad.887201. [DOI] [PubMed] [Google Scholar]
- 46.Tuxworth W, Saghir A, Spruill L, Menick D, McDermott P. Regulation of protein synthesis by eIF4E of secondary structure in the 5′-untranslated region of mRNA. Biochem J. 2004;378:73–82. doi: 10.1042/BJ20031027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roh C, Han J, Tzatsos A, Kandror K. Nutrient-sensing mTOR-mediated pathway regulates leptin production in isolated rat adipocytes. Am J Physiol Endocrinol Metab. 2003;284:E322–E330. doi: 10.1152/ajpendo.00230.2002. [DOI] [PubMed] [Google Scholar]


