Abstract
18-hydroxycortisol (18-OHF) and 18-oxocortisol (18oxo-F) are derivatives of cortisol found in Primary Aldosteronism but whose origin and regulation in normal subjects is uncertain. 18-OHF can be synthesised by zona fasciculata 11-β hydroxylase; 18-oxoF can only be produced by zona glomerulosa aldosterone synthase (AS). Stably transfected cell lines expressing either CYP11B1 (11β-hydroxylase) or CYP11B2 (AS) were incubated with cortisol and other substrates over a range of concentrations. Both enzymes could synthesise 18-OHF from cortisol but only AS could synthesise 18-oxoF. AS was more efficient than 11β-hydroxylase at 18-hydroxylation. The apparent Km of AS for cortisol was estimated to be 2.6μM. In 5 patients with adrenal insufficiency maintained on hydrocortisone, urinary free cortisol and cortisone levels were high; 18-oxoF was detectable in all patients and 18-hydroxycortisol in 3. It is likely that the 18-oxygenated steroids were synthesised from circulating cortisol, either in the zona glomerulosa or at extra-adrenal sites. In 8 male volunteers, dexamethasone treatment decreased urinary excretion rates of free cortisol, cortisone, 18-OHF and 18-oxoFl, confirming dependence of 18-oxygenated steroid levels on cortisol availability. In both groups, hydrocortisone administration resulted in detectable levels of 18-OHF and raised levels of 18-oxoF. There was close correlation between 18-oxoF and cortisol excretion during hydrocortisone administration in normal subjects (r=0.86, p<0.001).
These data show, for the first time, that 18-OHF and 18oxoF can be synthesised from circulating cortisol. The close correlation between 18-oxoF and cortisol suggests that 18-oxoF is normally produced by the action of aldosterone synthase utilising circulating cortisol as a substrate. Although 18OHF can be synthesized using circulating cortisol as substrate, our data suggest this is normally produced in the zona fasciculata by 11β-hydroxylase from locally available cortisol.
INTRODUCTION
There are two principal active end products of corticosteroidogenesis in man. Aldosterone from the zona glomerulosa (ZG) is formed from 11-deoxycorticosterone (DOC) by aldosterone synthase (AS) and cortisol from the zona fasciculata (ZF) is formed from 11-deoxycortisol by 11β-hydroxylase. This latter enzyme also converts DOC to corticosterone. Importantly, 11β-hydroxylase is probably also the source of many circulating 18-hydroxysteroids, for example, 18-OH-DOC, 18-hydroxycorticosterone and 18-hydroxyprogesterone, in normal subjects.
In 1982, Chu & Ulick isolated a novel compound from the urine of patients with primary hyperaldosteronism, which they identified as the 20,18-hemiketal form of 11β, 17α, 18,21-tetrahydroxy-4-pregnene-3, 20-dione (18-hydroxycortisol, ≡ 17α-hydroxyaldosterone) (1). In 1983, they isolated another C-18-oxygenated steroid after incubating cortisol with bullfrog interrenal gland; this was identified as 11β, 17α, 21-trihydroxy-3, 20-diketo-4-pregnene-18-al (18-oxo-cortisol) (2). Subsequently, Lifton and Pascoe coined the term ‘hybrid corticosteroids’ for these compounds with both ZG (i.e. 18-oxygenation) and ZF (i.e. cortisol-like, hydroxylation at C-17) characteristics (3–5). High plasma concentrations in patients with aldosterone-producing tumours (Conn’s syndrome) was attributed to abnormal access of aldosterone synthase to cortisol (6,7). AS is abnormally expressed in the ZF of patients with glucocorticoid-remediable aldosteronism (GRA); they also have high levels of these compounds, presumably also due to high local cortisol levels (3).
However, both compounds have now been identified in normal subjects (8,9). In the normal human adrenal cortex, 18-hydroxycortisol may be produced in the ZF from cortisol by the action of 11β-hydroxylase (10). In vitro studies suggest that 18-hydroxycortisol originates from the action of 11β-hydroxylase. Apparent control of its levels by ACTH is further evidence of this (6,11). However, 18-oxo-cortisol synthesis requires the additional ‘18-oxidation’ step, the capacity for which is restricted to the normal ZG. Cortisol is not made in this zone and, since blood flow is centripetal, it is not likely to perfuse from the ZF. What, therefore, is the origin of 18-oxo-cortisol in the normal circulation? One possibility is that cortisol enters the adrenal cortex in the peripheral circulation and is converted during passage through the zona glomerulosa. Although the Km for the action of aldosterone synthase on cortisol is not known, plasma cortisol is largely bound to CBG and the concentration of free cortisol is low.
In the following study, we investigated the origins of 18-hydroxycortisol and 18-oxocortisol. We evaluated the potential of 11β-hydroxylase and AS to metabolise cortisol in vitro and obtained an estimate of Km for the latter reaction. We then tested the effect of increasing cortisol levels in vivo on 18-hydroxycortisol and 18-oxocortisol excretion rates.
METHODS
Cell Culture and Transfection
V79 Chinese hamster lung cells stably transfected with either CYP11B1 (11β-hydroxylase) or CYP11B2 (aldosterone synthase) were a gift from Dr. Rita Bernhardt (Universitat des Saarlandes, Saarbrucken, Germany). They were grown to confluence in medium (DMEM supplemented with L-glutamine, 5% foetal calf serum, penicillin G, streptomycin and amphotericin B) The medium was removed and the cells washed in phosphate-buffered saline followed by trypsin-EDTA solution and incubated at room temperature for 2 minutes. The cells were isolated by centrifugation, re-suspended in incubation medium and aliquots transferred to 6-well plates. These were incubated at 37°C for 24h before use. CYP11B1-transfected cells were incubated with cortisol or 11-deoxycortisol. CYP11B2-transfected cells were incubated with cortisol, DOC or 18-hydroxycortisol. Incubation was for 24h at 37°C. Medium was then removed and stored at −20°C. Where necessary, protein was measured by the Biorad procedure.
Patients and volunteers
Local ethical committee approval for the study was obtained from the North Glasgow Hospitals University NHS Trust.
Patients
24-Hour urine collections were obtained from 6 patients with primary adrenocortical insufficiency. Five had autoimmune adrenal failure (Addison’s disease) and one had undergone bilateral adrenalectomy for phaeochromocytoma. Five of the subjects were taking hydrocortisone (15 – 30mg/day) as replacement and one was on dexamethasone (0.75mg/day).
Volunteers
Eight healthy male volunteers were recruited by advertisement. They were aged between 27 – 34 years and their body mass index (BMI) was between 20 – 25 kg/m2. None had a history of hypertension (i.e. above the normal limits for age), excessive alcohol intake (>21 U per week) or obesity [BMI >33 kg/m2]. The study was subdivided into three stages, one month apart. In stage one, 24-hour urine collections were obtained without treatment. In stage two, 24-hour urine collections were obtained after treatment with dexamethasone only (1mg bd for three days). In stage three, 24-hour urine collections were obtained after treatment with dexamethasone (1mg bd for three days) and hydrocortisone (20mg bd for one day only). A period of one month separated each phase.
Steroid analysis
Urinary steroid excretion rates were measured by gas chromatography-mass spectrometry using the methods of Shackleton (12) and Palermo et al (13) with minor modifications. Results were compared by Student’s paired t.
RESULTS
In vitro cell incubations
CYP11B1-transfected cells converted 11-deoxycortisol to cortisol (c42%) in a dose-dependent manner (figure 1) Cortisol was converted to 18-hydroxycortisol (c0.5%) but not to 18-oxo-cortisol (figure 2). Small quantities of 18-OH-11-deoxycortisol, identified by mass spectrum only (results not shown), were also found. It was not possible to measure conversion rate to this steroid because no reference standard was available.
Figure 1.
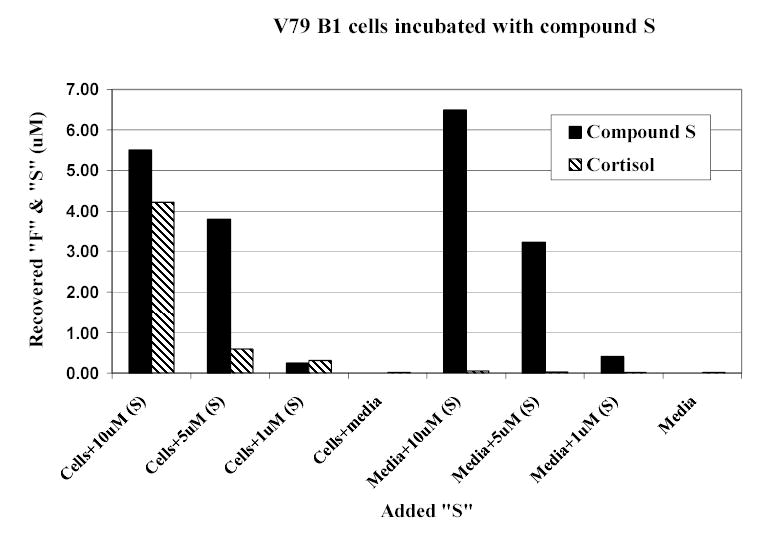
Cortisol (F) production by CYP11B1-transfected V79 Chinese hamster lung cells incubated for 24h with 11-deoxycortisol (S).
Figure 2.
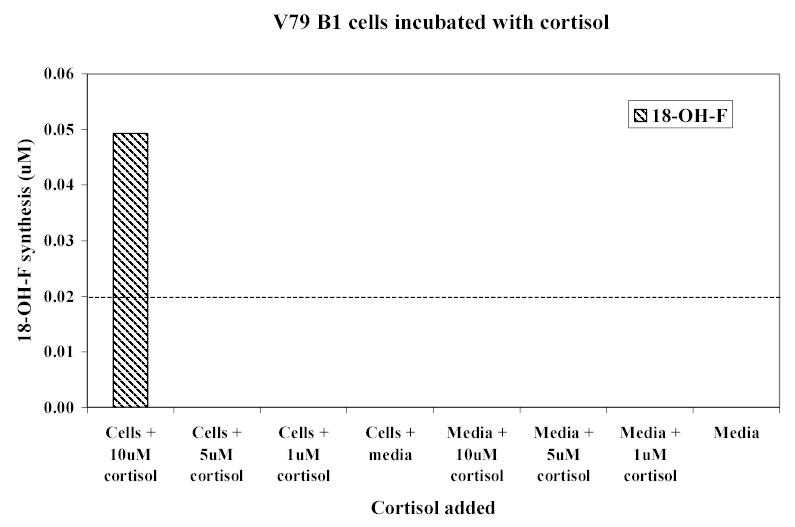
18-Hydroxycortisol (18-hydroxF) production by CYP11B1-transfected Chinese hamster lung cells incubated for 24h with cortisol.
CYP11B2-transfected cells converted DOC to corticosterone (c 1.8%), 18-hydroxycorticosterone (c 1.7%), and aldosterone (c 3%), again in a dose-dependent manner (figure 3). Cortisol was converted to 18-hydroxycortisol (c4.3%) and to 18-oxo-cortisol (c1.1%) (figure 4).
Figure 3.
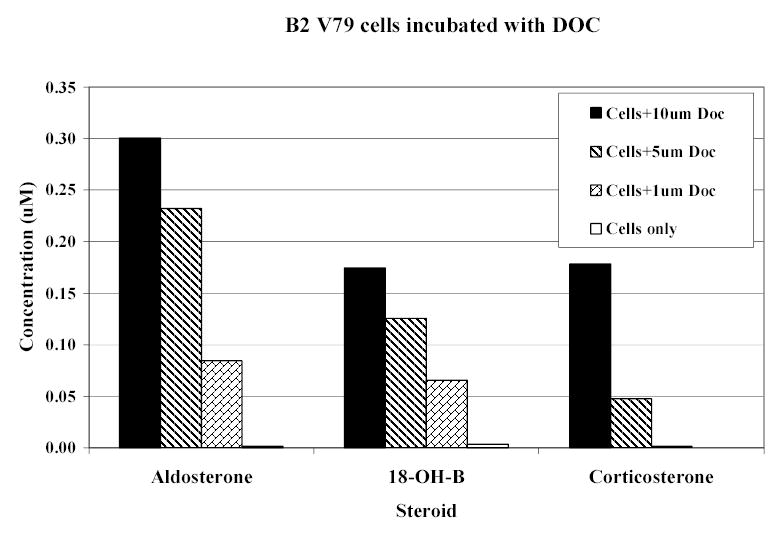
Corticosteroid production by CYP11B2-transfected V79 Chinese hamster lung cells incubated for 24h with 11-deoxycorticosterone (DOC). (18-OH-B: 18-hydroxycorticosterone.)
Figure 4.
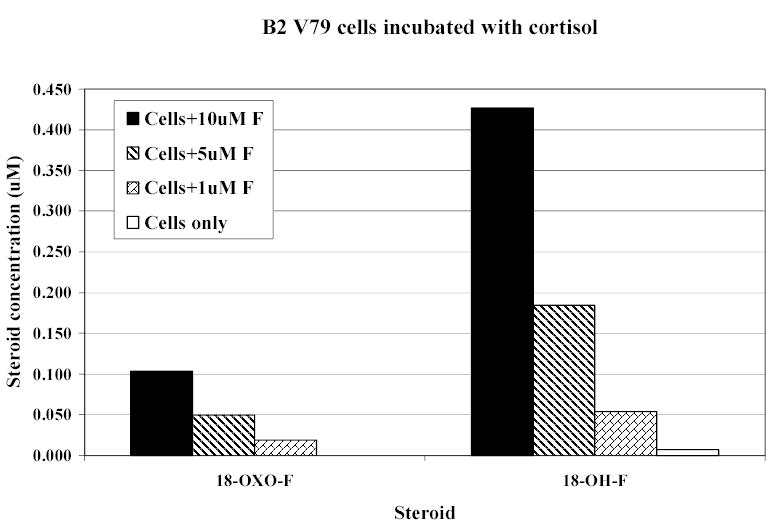
18-Oxocortisol (18-oxo-F) and 18-hydroxycortisol (18-OH-F) production by CYP11B2-transfected Chinese hamster lung cells incubated for 24h with cortisol (F).
Ratios of steroids synthesised by CYP11B1- and CYP11B2-transfected cells to their substrates are presented in figure 5. 11β-Hydroxylase converted 11-deoxycortisol into cortisol with high efficiency but efficiency to convert cortisol to 18-hydroxycortisol was much lower. AS converted DOC into aldosterone less efficiently than 11β-hydroxylase converted 11-deoxycortisol into cortisol. The ability of AS to convert cortisol to 18-hydroxycortisol was higher than that of 11β-hydroxylase.
Figure 5.
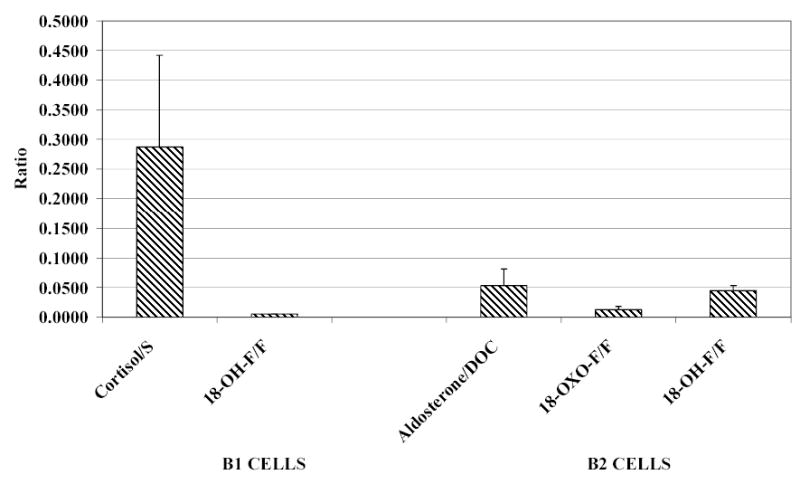
Precursor: product ratios of steroid synthesis by CYP11B1- and CYP11B2- transfected Chinese hamster lung cells. (cortisol: F; 11-deoxycortisol: S.)
The apparent Km of AS for cortisol (see discussion) was estimated as 2.6μM. No steroids apart from 18-hydroxycortisol were detected when CYP11B2-transfected cells were incubated with 18-hydroxycortisol.
Patients
Cortisol and cortisone were found in high concentration in the urine of the 5 patients taking hydrocortisone replacement therapy (figure 6). Low concentrations of 18-hydroxycortisol, at the limit of detection, were also found in the urine of three patients who were taking hydrocortisone. 18-Oxo-cortisol was also found in all patients who were taking cortisol. No endogenous steroids were detected in the patient receiving dexamethasone.
Figure 6.
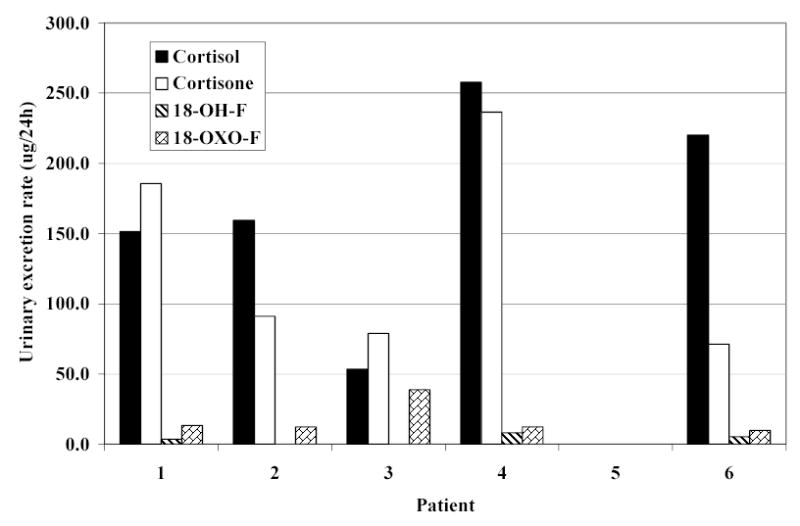
Urinary steroid excretion rates in 6 patients with adrenal insufficiency. (18-hydroxycortisol: 18-OH-F; 18-oxocortisol: 18-oxo-F.)
Volunteers
Average 24-hour urinary excretion rates of cortisol, cortisone, 18-hydroxycortisol and 18-oxo-cortisol are shown in figure 7. Comparisons between urinary excretion rates of these steroids are presented in table 1 and table 2. Urinary excretion rates of cortisol, cortisone, and 18-oxo-cortisol were significantly decreased in volunteers who were receiving dexamethasone compared with no treatment. They were significantly increased when the volunteers were taking cortisol and dexamethasone together compared with dexamethasone alone. Using the sum of cortisol and cortisone excretion rates as an index of cortisol ‘status’, 18-oxo-cortisol excretion rate correlated closely with this (r=0.86, p<0.01) while that of 18-hydroxycortisol did not (r=0.12; p>0.05).
Figure 7.
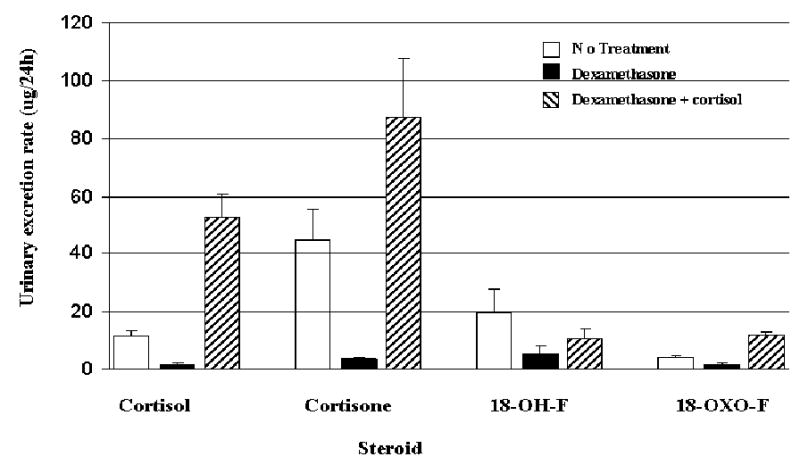
Urinary steroid excretion rates in 8 healthy male volunteers; the effects of dexamethasone or dexamethasone + cortisol on basal rates (mean ± SEM).(18-hydroxycortisol: 18-)H-F; 18-oxocortisol: 18-oxo-F.)
Table 1.
Urinary steroid metabolites excretion rates (μg/24h) in 8 normal male subjects on no treatment and dexamethasone plus cortisol. The results were compared by 2-sample Student’s t test [mean (±SEM)].
| STEROID | No Treatment | Dexamethasone + Cortisol | P |
|---|---|---|---|
| Cortisol | 11.1 ± 2.5 | 52.6 ± 8.2 | 0.001 |
| Cortisone | 44.7 ± 10.8 | 87.3 ± 20.2 | 0.09 |
| 18-OH-F | 19.4 ± 8.5 | 10.9 ± 3.14 | 0.37 |
| 18-Oxo-F | 4.04 ± 0.8 | 11.6 ± 1.4 | 0.0007 |
Table 2.
Urinary steroid metabolites excretion rates (μg/24h) in 8 normal male subjects on dexamethasone and dexamethasone plus cortisol treatment. The results were compared by 2-sample Student s t test [mean (±SEM)].
| STEROID | Dexamethasone | Dexamethasone + Cortisol | P |
|---|---|---|---|
| Cortisol | 1.3 ± 0.7 | 52.6 ± 8.2 | 0.0004 |
| Cortisone | 3.4 ± 0.7 | 87.3 ±20.2 | 0.004 |
| 18-OH-F | 5.3 ± 2.7 | 10.9 ± 3.1 | 0.2 |
| 18-Oxo-F | 1.8 ± 0.4 | 11.6 ± 1.4 | 0.0001 |
DISCUSSION
The cell incubation experiments confirm that 11β-hydroxylase is capable of converting cortisol to 18-hydroxycortisol but not to 18-oxo-cortisol. AS was capable of both conversions. All conversions were dose-dependent. The substrate conversion rates provide useful information on individual enzyme reactions. In CYP11B1-transfected cells, 11β-hydroxylation by 11β-hydroxylase is clearly more efficient than 18-hydroxylation. Cozza et al (14) incubated slices of rat adrenals with 11-deoxycortisol. Both ZG and ZF were able to produce 18-hydroxy-11-deoxycortisol. Mulatero et al (15) made a tentative identification of this compound in transfected COS-1 cells. In the current study, when CYP11B1-transfected cells were incubated with 11-deoxycortisol, they were also able to produce 18-hydroxy-11-deoxycortisol, confirming the ubiquitous ability of 11β-hydroxylase to 18-hydroxylate steroids. In CYP11B2-transfected cells, 18-hydroxylation by AS is more efficient than in the CYP11B1-transfected cells. This may be related to the orientation of the substrate molecule at the catalytic site; for AS, this is perhaps more likely to favour interaction with the angular methyl group. Mulatero et al (15) studied this problem in some detail and demonstrated that amino acid residues 288 and 320 are crucial to 18-hydroxylation and subsequent oxidation. That AS was unable to use 18-hydroxycortisol as a precursor for 18-oxo-cortisol in the current study may again be due to difficulties in accessing the catalytic site. The overall activity of CYP11B1-transfected cells (11β-hydroxylase) in converting 11-deoxycortisol into cortisol was higher than the activity of CYP11B2-transfected cells (AS) in converting DOC into aldosterone. This may reflect functional differences between the two enzymes; the AS reaction is more complex and released intermediates (18-hydroxycorticosterone and corticosterone) were not taken into account in calculating efficiency. In addition, transfection efficiency of the two enzymes in vitro may have been different.
In the patients with primary adrenal insufficiency, traces of 18-oxo-cortisol and 18-hydroxycortisol were found in the urine in those on hydrocortisone treatment but none was detectable in the patient on dexamethasone treatment. Since endogenous adrenal cortisol production is absent, circulating cortisol from replacement therapy must have been the substrate for the production of these steroids. In the one adrenalectomised patient, it is likely that they were synthesised from cortisol at extra-adrenal sites (for review see 16); in the other four patients, a proportion might also have arisen from perfusion by plasma of residual functional zona glomerulosa tissue.
In the volunteers, suppressing the adrenal source of cortisol by dexamethasone reduced 18-hydroxycortisol and 18-oxo-cortisol excretion rates. Subsequent administration of hydrocortisone to the volunteers simultaneously with dexamethasone increased excretion rates of both compounds. In this hydrocortisone + dexamethasone phase, 18-oxo-cortisol excretion rate exceeded control (untreated) levels but that of 18-hydroxycortisol did not achieve control levels. The cortisol status achieved, of which the sum of cortisol and cortisone excretion rates is a convenient index, was higher during cortisol + dexamethasone than during the control (untreated) period. This suggests that, in this extreme situation, the zona glomerulosa used extra-adrenal cortisol to make 18-oxo-cortisol and 18-hydroxycortisol. 11β-Hydroxylase production of 18-hydroxycortisol is probably less important here because extra-adrenal cortisol is less accessible to the ZF than to the ZG. Moreover, 11β-hydroxylase is less efficient than AS at 18-hydroxylation (see above).
Although the conversion efficiency of cortisol to 18-hydroxycortisol was low in the transfected cells in vitro, the estimate of Km suggests that, in vivo, high plasma levels of cortisol could contribute to the maintenance of normal levels of 18-hydroxycortisol. Two reservations must however be emphasised. Firstly, true apparent Km of AS for cortisol cannot be calculated accurately because of the complex nature of the reaction and because no pure enzyme protein is available. The estimate given here is an approximation. Secondly, in plasma most cortisol is specifically bound to corticosteroid-binding globulin and only a small fraction is free; availability to AS (and 11β-hydroxylase) will depend on competing affinities of enzyme and plasma protein.
In human subjects, AS is probably the only source of 18-oxo-cortisol. Its excretion rate was significantly increased by exogenous cortisol treatment, suggesting that cortisol reperfusing the ZG is a significant source of this steroid. However, AS and 11β-hydroxylase are expressed in organs other than the adrenal glands (17–20) albeit at much lower rates; extra-adrenal production cannot be ruled out. The close correlation between urinary cortisol metabolites and 18-oxocortisol levels in the volunteer study supports the conclusion that conversion of circulating cortisol is a major source of this steroid. Other studies also support the dependence of 18-oxo- and 18-hydroxy-cortisol on cortisol availability. The plasma levels of both compounds correlate with that of cortisol and not aldosterone (9) and, although the renin-angiotensin system may have some influence (21), the major control factor is ACTH (21,22). Dexamethasone suppressed the levels of both compounds in the current study and in others (23).
In normal subjects, cortisol for 18-hydroxycortisol or 18-oxocortisol synthesis may derive either by reperfusion from the circulation or from local tissue production. Cortisol is produced in the ZF but AS is essential at least to 18-oxocortisol synthesis. Direct penetration of ZF-synthesised cortisol to the ZG is unlikely because the blood supply of the adrenal gland is centripetal. There are, however, plausible alternative explanations. A transitional anatomical zone was identified between the ZF and ZG in rats (24,25) but not so far humans. In the human adrenal gland, ZG tissue may penetrate the ZF along the surfaces of small blood vessels, providing a potentially extensive interface for sufficient for cortisol to enter ZG cells. Finally, aldosterone might be converted to 18-oxo-cortisol by 17α-hydroxylase in the ZF. Early experiments (14) showed that 18-hydroxycortisol and 18-oxo-cortisol are produced in beef adrenal outer slices. The authors suggested that AS and 17α-hydroxylase coexist in these outer slices, allowing synthesis of these two steroids, and inferred that 17α-hydroxylase could convert aldosterone into 18-oxo-cortisol and 18-hydroxycorticosterone into 18-hydroxycortisol. Interpretation is difficult because in vitro incubation of mixed cell slices destroys the strict compartmentalision of enzymes and substrates maintained in vivo. There is as yet no similar evidence in human tissue.
Thus, cortisol can be converted to 18-hydroxycortisol in both the ZF and ZG by 11α-hydroxylase and AS respectively. Since cortisol synthesis is restricted to the ZF in vivo, it is likely that most 18-hydroxycortisol in normal subjects is produced by 11α-hydroxylase. 18-Oxo-cortisol is a unique product of AS. The source of cortisol in normal subjects may be circulating cortisol or ZF cortisol diffusing across the interphase between ZG and ZF.
Acknowledgments
ED/RF/JMCC are supported by a Programme Grant (G9317119) from the Medical Research Council. MF is supported by a Clinical Training Fellowship from the Wellcome Trust.
References
- 1.Chu MD, Ulick S. Isolation and identification of 18-hydroxycortisol from the urine of patients with primary aldosteronism. J Biol Chem. 1982;257:2218–2224. [PubMed] [Google Scholar]
- 2.Ulick S, Chu MD, Land M. Biosynthesis of 18-oxocortisol by aldosterone-producing adrenal tissue. J Biol Chem. 1983;258:5498–5502. [PubMed] [Google Scholar]
- 3.Lifton RP, Dluhy RG, Powers M, Rich GM, Gutkin M, Fallo F, Gill JR, Jr, Feld L, Ganguly A, Laidlaw JC, Murnaghan DJ, Kaufman C, Stockigt JR, Ulick S, Lalouel JM. Hereditary hypertension caused by chimaeric gene duplications and ectopic expression of aldosterone synthase. Nat Genet. 1992;2:66–74. doi: 10.1038/ng0992-66. [DOI] [PubMed] [Google Scholar]
- 4.Lifton RP, Dluhy RG, Powers M, Rich G, Cook S, Ulick S, Lalouel JM. A chimaeric 11 beta-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature. 1992;355:262–265. doi: 10.1038/355262a0. [DOI] [PubMed] [Google Scholar]
- 5.Pascoe L, Curnow KM, Slutsker L, Connell J, Speiser P, New M, White P. Glucocorticoid-suppressible hyperaldosteronism results from hybrid genes created by unequal crossovers between CYP11B1 and CYP11B2. Proc Natl Acad Sci USA. 1992;89:8327–8331. doi: 10.1073/pnas.89.17.8327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fraser R, Connell JM, Budd PS, Corrie JE, Kenyon CJ. The origin and significance of 18-hydroxycortisol: studies in hyperaldosteronism and in bovine adrenocortical cells in vitro. J Steroid Biochem Mol Biol. 1991;39:839–850. doi: 10.1016/0960-0760(91)90034-3. 163. [DOI] [PubMed] [Google Scholar]
- 7.Ulick S, Chu MD. Hypersecretion of a new corticosteroid, 18-hydroxycortisol, in two types of adrenocortcal hypertension. Clin Exp Hypertens A. 1982;4:1771–1777. doi: 10.3109/10641968209061640. [DOI] [PubMed] [Google Scholar]
- 8.Shackleton CHL, Honour JW. Identification and measurement of 18-hydroxycorticosterone metabolites by gas chromatography-mas spectrometry. J Steroid Bichem. 1977;28:89–94. doi: 10.1016/0022-4731(77)90051-6. [DOI] [PubMed] [Google Scholar]
- 9.Yamakita N, Gomez-Sanchez CE, Mune T, Morita H, Yoshida H, Miyazaki S, Yasuda K. Simultaneous measurement of plasma 18-oxocortisol and 18-hydroxycortisol levels in normal man. Eur J Endocrinol. 1994;131:74–79. doi: 10.1530/eje.0.1310074. [DOI] [PubMed] [Google Scholar]
- 10.Ohta M, Fujii S, Miura R, Nonaka Y, Okamoto M. Bovine adrenal cytochrome P450(11 beta)-mediated conversion of 11-deoxycortisol to 18- and 19-hydroxy derivatives; structural analysis by IH-NMR. Journal of Steroid Biochemistry & Molecular Biology. 1991;39(6):911–20. doi: 10.1016/0960-0760(91)90349-a. [DOI] [PubMed] [Google Scholar]
- 11.Gomez-Sanchez CE, Clore JN, Estep HL, Watlington CO. Effect of chronic adrenocorticotropin stimulation on the excretion of 18-hydroxycortisol and 18-oxocortisol. J Clin Endocrinol Metab. 1988;67:322–326. doi: 10.1210/jcem-67-2-322. [DOI] [PubMed] [Google Scholar]
- 12.Shackleton CHL. Mass spectrometry in the diagnosis of steroid-related disorders and in hypertension research. J Steroid Biochem Mol Biol. 1983;45:127–140. doi: 10.1016/0960-0760(93)90132-g. [DOI] [PubMed] [Google Scholar]
- 13.Palermo M, Gomez-Sanchez CE, Roitman E, Shackleton CHL. Quantitation of cortisol and related 3-oxo-4-ene steroids in urine using gas chromatography/mass spectrometry with stable isotope-labelled internal standards. Steroids. 1996;61:583–589. doi: 10.1016/s0039-128x(96)00118-9. [DOI] [PubMed] [Google Scholar]
- 14.Cozza EN, Chavarri MR, Foecking MF, Gomez-Sanchez CE. Synthesis of 18-hydroxycortisol and 18-oxocortisol in bovine adrenal zona glomerulosa mitochondria. Proc Soc Exp Biol Med. 1993;203:317–322. doi: 10.3181/00379727-203-43605. [DOI] [PubMed] [Google Scholar]
- 15.Mulatero P, Curnow KM, Aupetit Faisant B, Foekling M, Gomez-Sanchez C, Veglio F, Jeunemaitre X, Corvol P, Pascoe L. Recombinant CYP11B genes encode enzymes that can catalyze conversion of 11-deoxycortisol to cortisol, 18-hydroxycortisol and 18-oxocortisol. J Clin Endocrinol Metab. 1998;83:3996–4001. doi: 10.1210/jcem.83.11.5237. [DOI] [PubMed] [Google Scholar]
- 16.MacKenzie SM, Fraser R, Connell JMC, Davies E. Local renin-angiotensin systems and their interactions with extra-adrenal corticosteroid production. J Renin-Angiotensin-Aldosterone System. 2002;3:214–221. doi: 10.3317/jraas.2002.043. [DOI] [PubMed] [Google Scholar]
- 17.Gomez-Sanchez CE, Zhou MY, Cozza EN, Morita H, Foecking MF, Gomez-Sanchez EP. Aldosterone synthesis in the rat brain. Endocrinology. 1997;138:3369–3373. doi: 10.1210/endo.138.8.5326. [DOI] [PubMed] [Google Scholar]
- 18.Hatakeyama H, Miyamori I, Fujita T, Takeda Y, Takeda R, Yamamoto H. Vascular aldosterone. Biosynthesis and a link to angiotensin II-induced hypertrophy of vascular smooth muscle cells. J Biol Chem. 1994;269:24316–24320. 27465. [PubMed] [Google Scholar]
- 19.MacKenzie SM, Clark CJ, Ingram MC, Lai M, Seckl J, Gomez-Sanchez CE, Fraser R, Connell JM, Davies E. Corticosteroid production in fetal rat hippocampal neurons. Endocr Res. 2000;26:531–535. doi: 10.3109/07435800009048566. 465484. [DOI] [PubMed] [Google Scholar]
- 20.MacKenzie SM, Clark CJ, Fraser R, Gomez-Sanchez CE, Connell JM, Davies E. Expression of 11beta-hydroxylase and aldosterone synthase genes in the rat brain. J Mol Endocrinol. 2000;24:321–328. doi: 10.1677/jme.0.0240321. 484485. [DOI] [PubMed] [Google Scholar]
- 21.Miyamori I, Takeda Y, Takasaki H, Itoh Y, Iki K, Takeda R. Determination of urinary 18-hydroxycortisol in the diagnosis of primary aldosteronism. J Endocrinol Invest. 1992;15:19–24. doi: 10.1007/BF03348648. [DOI] [PubMed] [Google Scholar]
- 22.Yamakita N, Gomez-Sanchez CE, Mune T, Yoshida H, Miyazaki S, Yasuda K, Nakai T. J Steroid Biochem Mol Biol. Regulation of 18-oxocortisol and 18-hydroxycortisol by the renin-angiotensin system and ACTH in man;49:395–399. doi: 10.1016/0960-0760(93)90230-t. [DOI] [PubMed] [Google Scholar]
- 23.Gomez-Sanchez CE, Upcavage RJ, Zager PG, Foecking MF, Holland OB, Ganguly A. Urinary 18-hydroxycortisol and its relationship to the excretion of other adrenal steroids. J Clin Endocrinol Metab. 1987;65:310–314. doi: 10.1210/jcem-65-2-310. [DOI] [PubMed] [Google Scholar]
- 24.Shigematsu K, Kawa K, Tsuchiyama H. Functional morphology of the adrenocortical glomerula zone by incomplete ligation of bilateral ureters of rats. An experimental model for secondary aldosteronism. Acta Pathol Jpn. 1985;35:1435–1444. doi: 10.1111/j.1440-1827.1985.tb01440.x. [DOI] [PubMed] [Google Scholar]
- 25.Gomez-Sanchez CE. 18-Hydroxycortisol and 18-oxocortisol; steroids from the adrenal zone. Endocr Res. 1985;10:609–615. doi: 10.1080/07435808409036519. [DOI] [PubMed] [Google Scholar]


