Genetic studies of eukaryotic transcription often yield mutants that are impaired in the regulation of chromatin remodeling. Yeast or fly mutants have been isolated that carry mutations in genes encoding the enzymatic activities that covalently modify histones through acetylation, methylation, and phosphorylation.1–4 Mutants of SWI/SNF and related complexes that use the energy of ATP hydrolysis to alter histone-DNA contacts have also been isolated.5–7 In most cases, genetics has produced a mutant of a chromatin modifier before its biochemical characterization. The physiological relevance of many biochemical discoveries were validated by preexisting mutants, rapidly advancing our understanding of chromatin structure and how it is reconfigured for transcription.
In principle, any protein that remodels the chromatin structure of a gene of interest can be genetically identified as long as mutants are viable and display a detectable phenotype. This approach has worked well for genes that are highly dependent on chromatin modifiers for transcription because the mutant phenotypes are robust. For example, the screens in yeast that identified genes encoding subunits of the SWI/SNF complex examined expression of the inducible genes HO and SUC2, which both require SWI/SNF for full activation.8
For some genes, multiple chromatin modifiers may work together to facilitate transcription, but no single modifier is absolutely required. An example of such a gene in yeast is PHO5, and although genetic screens have identified many regulators of its expression,9–11 none of these activate transcription by altering chromatin. Because most screens are not saturating, it is possible that chromatin modifiers were missed. However, a likely possibility is that the phenotypes of these mutants were too weak to be detected under the conditions of the screens.
In this chapter, we present two examples of genetic strategies designed to identify chromatin modifiers that regulate PHO5 transcription. In the first example, we describe a genetic selection recently performed to isolate mutants that cannot activate PHO5 expression.12 In the second example, we describe a screen that is currently in progress to identify chromatin modifiers by searching the collection of 4847 viable haploid yeast deletion strains for those that exhibit a kinetic delay in PHO5 activation (S. Huang and E. K. O’Shea, unpublished data). This screen is based on the observation that in strains with mutations in chromatin-modifying genes, including gcn5,13 arp8, and swi2 (E. S. Haswell and E. K. O’Shea, unpublished data), the steady-state levels of activated PHO5 transcription are similar to the wild-type level, but the time required to reach steady-state is longer than that for the wild-type strain. Although the screens described here focus on PHO5, their underlying principles can be applied to other genes.
Genetic Selection for PHO5 Uninducible Mutants
Transcription of PHO5, which encodes a secreted acid phosphatase is regulated in response to phosphate availability by the transcription factors Pho4 and Pho2.14 The activity of Pho4 is regulated through phosphorylation by the cyclin-CDK (cyclin-dependent kinase) complex Pho80-Pho85.15 When yeast cells are grown in phosphate-rich medium, Pho4 is phosphorylated and localized to the cytoplasm.16 In addition, four positioned nucleosomes reside over the PHO5 promoter, and PHO5 transcription is repressed.17 When cells are starved for phosphate, the CDK inhibitor Pho81 inactivates Pho80-Pho85,18 Pho4 is unphosphorylated and localized to the nucleus,16 the positioned nucleosomes are no longer detectable,17 and PHO5 transcription is induced. Remodeling of PHO5 chromatin structure requires Pho4 and Pho219 and is a prerequisite for transcriptional induction of PHO5.20 A Gcn5-containing histone acetyltransferase (HAT) complex and the SWI/SNF and INO80 complexes have been implicated in facilitating this process.12,13,21,22
We recently performed a genetic selection to identify candidate regulators of PHO5 expression by mutagenizing a pho80ts strain and looking for mutants that failed to induce PHO5 in high phosphate when raised to the nonpermissive temperature12 (see Fig. 1). In addition to replacing the endogenous PHO80 gene with a temperature sensitive pho80 allele in the starting strain, the promoter of the CAN1 gene was replaced with the PHO5 promoter. Cells expressing CAN1 die in the presence of canavanine, a toxic arginine analog, such that cells carrying the pPHO5-CAN1 fusion are sensitive to canavanine in conditions inducing PHO5 transcription. The pho80ts allele was used to induce PHO5 transcription in high phosphate media. Its gene product is functional and PHO5 transcription repressed at 25°, while it is nonfunctional and PHO5 transcription activated at 37°. In arginine dropout media containing 5–10 μg/ml canavanine, the starting strain grows at 25° and dies at 37°.
Fig. 1.
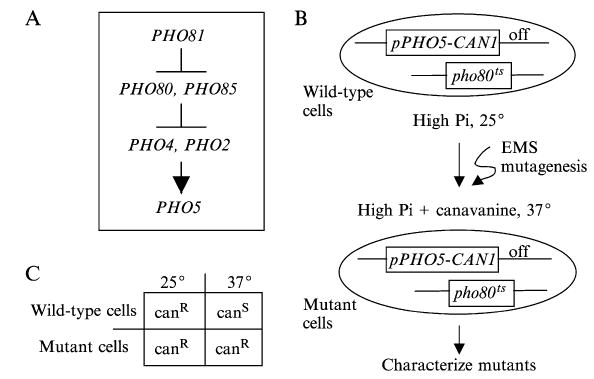
Genetic selection to identify PHO5 uninducible mutants. (A) A partial genetic view of PHO5 regulation. (B) Schematic of the selection strategy. The starting strain (K699 MATa pho80ts can1 :: pPHO5-CAN1 pho81Δ:: TRP1B ade2-1 trp1-1 leu2-3, 112 his3-11, 15 ura3-1) was treated with ethylmethane sulfonate (EMS) to approximately 50% viability, and plated on SD-arginine + 8 μg/ml canavanine medium (high phosphate [Pi]) at room temperature for 5 h. After recovery, the mutagenized cells were transferred to 37°. Cells unable to turn on pPHO5-CAN1 upon inactivation of the pho80ts allele grow at 37°. (C) Conditions under which the starting strain (wild-type cells) and mutant cells are either sensitive (canS) or resistant (canR) to canavanine.
The pho80ts allele was used to help identify factors involved in chromatin remodeling. It limited our search to genes acting downstream of PHO80. It also provided a sensitized background for the selection because the activation of PHO5 transcription is more dependent on chromatin modifiers. The activation of PHO5 transcription is decreased many fold in gcn5Δ pho80ts, snf6Δ pho80ts, and arp8Δpho80ts mutants compared to pho80ts cells when induced in high phosphate conditions by temperature shift,12 whereas it is activated to near wild-type levels in the gcn5, swi2, and arp8 mutants in PHO80 cells starved for phosphate13 (E. S. Haswell and E. K. O’Shea, unpublished data).
To isolate mutant strains defective in the activation of PHO5 transcription, the starting strain was treated with mutagen and plated for growth at 37° on high phosphate medium containing canavanine. We picked 964 canavanine- resistant mutants and analyzed these by secondary screens to eliminate undesirable classes of mutants. Mutations in the pPHO5-CAN1 fusion gene were detected by examining expression of the endogenous PHO5 gene by an acid phosphatase assay performed directly on cells on plates. We discarded 513 mutants that were able to induce expression of the endogenous PHO5 gene at 37°. Mutations in PHO4, PHO2, or dominant mutations were detected by mating to a pho2Δ pho4Δ strain. Of the remaining 451 mutants, 431 failed to complement the pho2Δ pho4Δ strain, and these were discarded. Mutations causing improper localization of Pho4 were detected by examining the subcellular localization of Pho4-GFP. We found three mutants that failed to localize Pho4-GFP to the nucleus at 37°. They comprised a single complementation group, and by screening a yeast genomic library for plasmids that complement the PHO5 uninducible phenotype, were determined to carry mutations in PSE1, the nuclear import receptor for Pho4.
Mutants surviving these secondary screens were thought to carry mutations in genes affecting PHO5 transcription downstream of PHO2 and PHO4. Of the remaining 17 mutants, only one displayed a defect in PHO5 mRNA induction as measured by northern analysis. Mutants not impaired in PHO5 mRNA induction were presumed to affect PHO5 expression downstream of transcription and were not examined further. By examining the structure of PHO5 promoter chromatin with a restriction enzyme accessibility assay, we found that the transcriptional defect in the remaining mutant resulted from a block in PHO5 chromatin remodeling. We subsequently determined that the mutant carried a mutation in ARG82/IPK2 by complementation analysis with the yeast genomic library and recovery of the mutant allele by gap repair.
Arg82 is a nuclear inositol polyphosphate kinase that functions in a pathway with Plc1 and Ipk1 to produce soluble inositol polyphosphates in the nucleus.23 Its identification in the genetic selection was crucial to revealing a connection between inositol polyphosphate production and chromatin remodeling. Given that we obtained only one allele of ARG82, the selection was not saturating. By analyzing more mutants, additional unexpected activities may be discovered to regulate chromatin remodeling and transcription at PHO5 and perhaps other genes.
Screening the Collection of 4847 Viable Haploid Yeast Deletion Strains for Those That Exhibit Impaired PHO5 Expression
The kinetics of PHO5 transcription are delayed in response to phosphate starvation in gcn5Δ, snf6Δ, and arp8Δ strains, but the steady-state levels of activated transcription are similar to the wild-type level13 (E. S. Haswell and E. K. O’Shea, unpublished data). In contrast, PHO5 transcription levels at steady state are significantly decreased in these mutants in the pho80ts background when induced by temperature shift in high phosphate medium.12 A simple interpretation of these observations is that an activity (or activities) is present in phosphate starvation conditions that functions redundantly with these chromatin modifiers to induce PHO5. Thus, it seems likely that there are factors participating in PHO5 transcription and chromatin remodeling that remain unknown.
To identify these, we are currently screening the collection of 4847 viable haploid yeast deletion strains for strains that exhibit a kinetic delay in PHO5 induction (S. Huang and E. K. O’Shea, unpublished data). Pho5 acid phosphatase activity is quantified as a function of time after cells are transferred to no phosphate medium. Each strain is grown in high phosphate medium to mid-log phase, then transferred to medium lacking phosphate. Aliquots of approximately equal cell numbers are removed after 0, 2, 4, and 6 h of growth in no phosphate medium and assayed for acid phosphatase activity. To expedite handling of the 4847 strains, all procedures are performed in 96-deep-well plates with the aid of a liquid handling robot.
Although the screen is still in progress, it is reassuring that we detect defects in Pho5 activity in strains carrying deletions in regulators of PHO5 expression. For example, the pho80Δ and pho85Δ strains display constitutively high levels of Pho5 activity. Furthermore, strains with deletions in subunits of the SAGA, SWI/SNF and INO80 complexes have less Pho5 activity at the early time points compared to the majority of the other deletion strains.
Several secondary screens will be performed for strains displaying a kinetic delay in Pho5 production. To determine that the PHO5 production phenotype is linked to the deletion, each mutant will be backcrossed to the wild-type strain and the phenotypes of progeny analyzed. Mutants whose phenotype is linked to the deletion will be transformed with a plasmid overexpressing a constitutively active allele of PHO81 and Pho5 activity examined. Mutants that remain impaired for PHO5 expression will carry mutations in genes functioning downstream of PHO81, and will be examined further. Pho4 localization, PHO5 mRNA production, and PHO5 chromatin remodeling will be examined as described earlier.
A strength of this screen is that it is saturating for nonessential genes. Another advantage is that the mutation within each strain is known. Thus, for strains passing the secondary screens, we may have immediate insight into how a particular gene functions to regulate PHO5 expression.
Summary
Novel discoveries result from genetic analyses of transcription and chromatin remodeling because these methods identify activities in an unbiased manner. By describing our genetic approaches to identify regulators of PHO5 transcription and chromatin remodeling, we hope to encourage others to apply similar strategies to their genes of interest.
Acknowledgments
We are grateful to members of the O’Shea laboratory for many interesting and helpful discussions. This work was supported by the National Institutes of Health grant GM51377. D. J. S. was funded by the Leukemia and Lymphoma Society as a Special Fellow.
References
- 1.Reuter G, Spierer P. Bioessays. 1992;14:605. doi: 10.1002/bies.950140907. [DOI] [PubMed] [Google Scholar]
- 2.Thon G, Klar AJ. Genetics. 1992;131:287. doi: 10.1093/genetics/131.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger SL, Pina B, Silverman N, Marcus GA, Agapite J, Reigier JL, Triezenberg SJ, Guarente L. Cell. 1992;70:251. doi: 10.1016/0092-8674(92)90100-q. [DOI] [PubMed] [Google Scholar]
- 4.Allshire RC, Javerzat JP, Redhead NJ, Cranston G. Cell. 1994;76:157. doi: 10.1016/0092-8674(94)90180-5. [DOI] [PubMed] [Google Scholar]
- 5.Stern M, Jensen R, Herskowitz I. J Mol Biol. 1984;178:853. doi: 10.1016/0022-2836(84)90315-2. [DOI] [PubMed] [Google Scholar]
- 6.Neigeborn L, Carlson M. Genetics. 1984;108:845. doi: 10.1093/genetics/108.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pirrotta V. Cell. 1998;93:333. doi: 10.1016/s0092-8674(00)81162-9. [DOI] [PubMed] [Google Scholar]
- 8.Winston F, Carlson M. Trends Genet. 1992;8:387. doi: 10.1016/0168-9525(92)90300-s. [DOI] [PubMed] [Google Scholar]
- 9.To EA, Ueda Y, Kakimoto SI, Oshima Y. J Bacteriol. 1973;113:727. doi: 10.1128/jb.113.2.727-738.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ueda Y, To EA, Oshima Y. J Bacteriol. 1975;122:911. doi: 10.1128/jb.122.3.911-922.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lau WW, Schneider KR, O’Shea EK. Genetics. 1998;150:1349. doi: 10.1093/genetics/150.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steger DJ, Haswell ES, Miller AL, Wente SR, O’Shea EK. Science. 2003;299:114. doi: 10.1126/science.1078062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbaric S, Walker J, Schmid A, Svejstrup JQ, Hörz W. EMBO J. 2001;20:4944. doi: 10.1093/emboj/20.17.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oshima Y. Genes Genet Syst. 1997;72:323. doi: 10.1266/ggs.72.323. [DOI] [PubMed] [Google Scholar]
- 15.Kaffman A, Herskowitz I, Tjian R, O’Shea EK. Science. 1994;263:1153. doi: 10.1126/science.8108735. [DOI] [PubMed] [Google Scholar]
- 16.Komeili A, O’Shea EK. Science. 1999;284:977. doi: 10.1126/science.284.5416.977. [DOI] [PubMed] [Google Scholar]
- 17.Almer A, Rudolph H, Hinnen A, Hörz W. EMBO J. 1986;5:2689. doi: 10.1002/j.1460-2075.1986.tb04552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider KR, Smith RL, O’Shea EK. Science. 1994;266:122. doi: 10.1126/science.7939631. [DOI] [PubMed] [Google Scholar]
- 19.Fascher KD, Schmitz J, Hörz W. EMBO J. 1990;9:2523. doi: 10.1002/j.1460-2075.1990.tb07432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmid A, Fasher KD, Hörz W. Cell. 1992;71:853. doi: 10.1016/0092-8674(92)90560-y. [DOI] [PubMed] [Google Scholar]
- 21.Vogelauer M, Wu J, Suka N, Grunstein M. Nature. 2000;408:495. doi: 10.1038/35044127. [DOI] [PubMed] [Google Scholar]
- 22.Shen X, Mizuguchi G, Hamiche A, Wu C. Nature. 2000;406:541. doi: 10.1038/35020123. [DOI] [PubMed] [Google Scholar]
- 23.Odom AR, Stahlberg A, Wente SR, York JD. Science. 2000;287:2026. doi: 10.1126/science.287.5460.2026. [DOI] [PubMed] [Google Scholar]


