Abstract
Background
Endothelial dysfunction signals the initiation and progression of atherosclerosis. Elevated LDL-cholesterol concentrations have been suggested to induce endothelial dysfunction, but direct in vivo evidence for the relation is still lacking.
Objective
We examined the hypothesis that a high-cholesterol, high-fat (HCHF) diet can directly cause endothelial dysfunction in vivo.
Design
We measured inflammatory and endothelial dysfunctional markers in circulating blood and directly in endothelial cells, which were collected by femoral artery biopsies, in 10 baboons before and after a 7-wk HCHF dietary challenge.
Results
We found that the HCHF diet induced a high inflammatory status, as indicated by increased concentrations of interleukin 6, tumor necrosis factor α (TNF-α), and monocyte chemoattractant protein 1. Although the concentrations of endothelial dysfunctional markers, such as soluble vascular cell adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule 1, were not increased by the HCHF diet, membrane-bound VCAM-1 and membrane-bound E-selectin on endothelial cells were highly increased after 7 wk of the HCHF diet (P < 0.01). In contrast, the concentrations of endothelial nitric oxide synthase in endothelial cells were significantly reduced by the 7-wk HCHF diet (P < 0.01). Furthermore, the dietary challenge attenuated endothelial cell responses to TNF-α, lipopolysaccharide, native LDL cholesterol, and oxidized LDL-cholesterol stimulation.
Conclusions
Our results show that an HCHF diet can directly induce inflammation and endothelial dysfunction. Prior in vivo exposure to an HCHF diet attenuates the in vitro responses of endothelial cells to atherogenic risk factors. This preconditioning phenomenon may have significant clinical relevance.
Keywords: Nonhuman primates, endothelium, inflammation, high-cholesterol, high-fat diet
INTRODUCTION
It is well known that not every individual with known atherogenic risk factors, such as hypercholesterolemia, develops atherosclerosis. Moreover, these known risk factors explain only ≈50% of the difference between healthy and clinically affected persons. Currently, most biochemical risk factors for coronary artery disease, such as lipid and inflammation markers, are assessed by measuring their concentrations in the blood. Except for an indirect Doppler measurement of brachial arterial blood flow, noninvasive tools are not available to directly measure changes in the arterial wall, which is where pathologic vascular changes occur (1). Yet the cells of the arterial wall determine its endogenous susceptibility to exogenous vascular disease risk factors. No studies have documented the interactions between circulating environmental risk factors for atherosclerosis and the arterial wall in living human subjects.
Endothelial cells (ECs) form the interior lining of the arterial wall and function as modulators of arterial wall function. ECs sense both circulating environmental factors and local arterial wall demands and accordingly adjust the expression and activity of molecules that affect vascular wall function. In arteries, ECs are the first line of defense against constant insults from circulating risk factors for atherosclerosis, which include reactive oxygen species, inflammatory and infectious agents, high LDL-cholesterol concentrations, and turbulent blood flow. Once the EC defense fails, excess LDL cholesterol infiltrates and accumulates within the subendothelial extracellular space. Circulating macrophages and leukocytes are then recruited to the arterial wall and initiate the following inflammatory process: macrophages become foam cells, vascular smooth muscle cells proliferate, and atheroma forms (2, 3). Failure of the EC defense is often characterized by dysfunctional changes, which include the overexpression of adhesion molecules such as intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin (4, 5); inflammatory markers, such as tumor necrosis factor (TNF) receptors, interleukin 6 (IL-6), and monocyte chemoattractant protein 1 (MCP-1); and some integrins that regulate endothelial permeability (6). Although ECs are important in atherogenesis, direct assessments of endothelial functional changes are generally prohibited because of the inability to directly access vascular ECs in human subjects. This limitation has hindered the discovery of risk factors that may cause early atherosclerotic changes, ie, endothelial dysfunction, and hence the opportunity for early diagnosis and treatment.
Baboons provide an ideal nonhuman primate model to explore the direct effect of circulating risk factors for atherosclerosis on endothelial dysfunction. In the present study, we used baboon femoral artery ECs (BFAECs), which were obtained with a femoral artery biopsy method after baboons were fed a high-cholesterol, high-fat (HCHF) diet, to explore the in vivo responses to the following risk factors for atherosclerosis: elevated LDL-cholesterol concentrations, oxidative stress caused by oxidized LDL (oxLDL) cholesterol, and inflammatory stimuli. Additionally, we examined whether preexposure to an HCHF diet in vivo modified the responses to these risk factors in the same ECs cultured in vitro.
MATERIALS AND METHODS
Study design
We designed the experiments to investigate the endothelial responses to atherogenic stimuli both in vivo and in vitro (Figure 1). For the in vivo experiments, baboons were challenged with the HCHF diet (Table 1), and the direct effects of the diet on ECs were measured in both circulating blood and BFAECs obtained from biopsies. Although the intent of the in vivo experiments was to identify functional changes in ECs after an atherogenic diet, such experiments cannot determine the specific components that are responsible for these changes. Therefore, we also designed an in vitro challenge experiment. For the in vitro experiment, BFAECs that were collected from the same baboons that were treated in vivo were cultured for ≤4 passages before they were exposed to TNF-α, lipopolysaccharide, and native LDL and oxLDL cholesterol, factors that simulate the inflammatory responses and increased serum cholesterol concentrations triggered in vivo by the HCHF diet.
FIGURE 1.
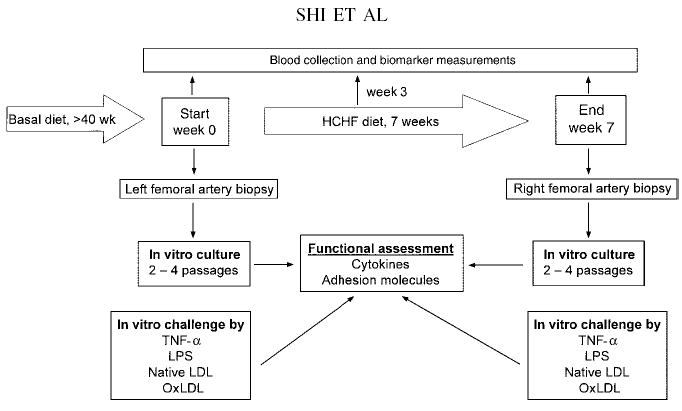
Flow chart of the high-fat, high-cholesterol (HCHF) dietary challenge. Baboon femoral artery biopsy samples were collected before (left femoral artery) and after (right femoral artery) the 7-wk HCHF diet challenge. During the 7-wk HCHF diet challenge, blood samples were collected from the baboons at weeks 0, 3, and 7. TNF-α, tumor necrosis factor α; LPS, lipopolysaccharide; oxLDL, oxidized LDL cholesterol.
TABLE 1.
Measured composition of the basal and high-cholesterol, high-fat (HCHF) diets
| Basal diet | HCHF diet | |
|---|---|---|
| Energy (kcal/g) | 3.1 | 3.8 |
| Carbohydrates (%) | 75 | 39 |
| Protein (%) | 18 | 20 |
| Fat (%) | 7 | 41 |
| Cholesterol content (mg/g) | 0.02 | 6.37 |
| Fat composition | ||
| Saturated fat (%) | 23.9 | 44.4 |
| Monounsaturated fat (%) | 25.2 | 40.7 |
| Polyunsaturated fat (%) | 50.9 | 14.9 |
| Polyunsaturated/saturated | 2.2 | 0.33 |
| Monounsaturated/polyunsaturated | 0.5 | 2.7 |
| Fatty acid composition (%) | ||
| Myristic acid (14:0) | 1.0 | 1.8 |
| Palmitic acid (16:0) | 17.0 | 24.8 |
| Palmitoleic acid (16:1) | 1.1 | 2.0 |
| Stearic acid (18:0) | 5.0 | 17.8 |
| Oleic acid (18:1) | 25.6 | 38.7 |
| Linoleic acid (18:2) | 46.7 | 13.9 |
| Linolenic acid (18:3) | 3.4 | 0.9 |
Baboons
We selected 10 adult baboons (6 males and 4 females, aged 7–15 y, mean age: 10.8 y) to be challenged by the HCHF diet, which increases blood cholesterol concentrations in most baboons. The baboons selected for this study were previously classified as high responders because their serum LDL-cholesterol concentrations increased after an HCHF diet (7, 8). The baboons had been fed a normal unpurified stock diet for monkeys (SWF Primate Diet; Harlan Teklad, Madison, WI) for >40 wk before the 7-wk HCHF dietary challenge. Both diets were fed ad libitium. To observe the changes in circulation and blood vessels after the challenge, serum samples were taken at the beginning of the dietary challenge (week 0) and again at weeks 3 and 7 of the challenge as shown in Figure 1. A biopsy of the left femoral artery was performed immediately before the HCHF diet was initiated (week 0). A biopsy of the right femoral artery was performed at the end of the 7-wk challenge. All baboons that were used in this experiment were maintained at the Southwest National Primate Research Center in San Antonio, TX. The study was approved by the Institutional Animal Care and Use Committee of the Southwest Foundation for Biomedical Research.
Biopsies
Femoral artery biopsy samples were taken from the baboons by experienced veterinarians. Each animal was immobilized with ketamine (10 mg/kg, intramuscular) and valium (5 mg, intravenous) and anesthetized with isofluorane (1.5% vol:vol, inhalation). The leg was prepared for aseptic surgery. A skin incision was made over the femoral region midway between the pubis and knee. One inch of the femoral artery was isolated and ligated, the branches were ligated, and the segment was removed by sterile methods. The muscles and skin were closed with simple continuous sutures. After the biopsy procedure, the baboons were returned to their normal living conditions. No long-term disability, with regard to mobility or use of legs, was observed in the baboons that were subjected to biopsy procedures.
Cell isolation, culture, and treatment
BFAECs were isolated from all 10 experimental baboons. ECs were harvested from biopsy samples ≤2 h after the vessels had been excised. The artery was placed in a large sterile dish, and the surface of the artery was wiped with 1% antibiotic-antimycotic solution (Gibco-BRL, Grand Island, NY) in phosphate-buffered saline (PBS). The artery was gently cannulated at one end with a short blunt needle and was flushed with PBS to remove any blood remaining inside the vessel. To obtain ECs, the vessel was injected with 0.1% collagenase and incubated at 37 °C for 20 min. After the completion of the collagen digestion, the vessel was massaged gently and flushed with culture medium. The released cells were collected by centrifugation at 300 × g for 10 min at 4 °C and resuspended in media. The cells were seeded immediately on 1.0% gelatin-coated culture plates. The endothelial growth medium was composed of F-12K medium supplemented with 20% fetal calf serum, 75 μg endothelial-derived growth factor/mL, 50 μg heparin/mL, 10 mmol HEPES/L, 2 mmol glutamine/L, and antibiotics (Cambrex, East Rutherford, NJ). Confluent cells were dislodged with a 0.05% trypsin and versene solution (Cambrex) and subcultured in a 3-fold dilution, ie, a 1:3 subculture.
BFAECs were seeded at a density of 5–10 × 104 cells/mL in endothelial growth medium in a 100-mm petri dish. The cells were allowed to grow to 70–90% confluence before the in vitro treatment. We added 10 ng TNF-α/mL or 1.0 μg lipopolysaccharide/mL (final concentrations, in endothelial growth media) for a 20-h treatment. For LDL-cholesterol treatments, we conditioned ECs for 20 h with endothelial basal medium. Then, 100 μg native LDL or oxLDL cholesterol/mL (final concentrations in endothelial basal medium) was added, and the treatment was continued for another 20 h. At the end of the indicated treatments, we collected culture supernatant fluid and cell lysates for the indicated measurements.
Preparation of native and oxidized LDL cholesterol
The LDL cholesterol that was used for the entire experiment was isolated from 3 healthy, randomly selected baboons. Their plasma samples were pooled before the LDL-cholesterol preparation. LDL cholesterol (density range: 1.019–1.063 g/mL) was isolated from baboon serum samples that contained 1 mg EDTA/mL by sequential ultracentrifugation at 105 000 × g for 24 h at 4 °C. A final concentration of 0.1 mmol antioxidant butylated hydroxytoluene/L was added to the serum that was used for obtaining native LDL cholesterol. For the oxidation of LDL cholesterol, we used a method from Ziouzenkova et al (9, 10) with modifications. The extent of oxidation was assessed by measuring the thiobarbituric acid–reactive substances content (ZeptoMetrix, Buffalo, NY) and the electrophoretic mobility of the oxLDL cholesterol. Native LDL cholesterol had a mean (±SEM) thiobarbituric acid–reactive substances content of 1.7 ± 0.1 nmol/mg protein, and oxLDL cholesterol had a mean (±SEM) thiobarbituric acid-reactive substances content of 18.2 ± 0.2 nmol/mg protein. Relative to the native LDL-cholesterol electrophoretic mobility ratio, oxLDL cholesterol had a mean (±SEM) electrophoretic mobility ratio of 2.8 ± 0.2. (For an example of the electrophoregram, see Figure 1 under “Supplemental data” in the current issue at www.ajcn.org.) We found only trace amounts of endotoxin in the lipoprotein preparations (<0.01 U endotoxin/mg LDL cholesterol) with a limulus assay (QCL1000; Whittaker Bioproducts Inc, Walkersville, MD). LDL cholesterol labeled with the fluorescent probe 1,1′-dioactadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (Dil-LDL cholesterol) was purchased from Biomedical Technologies (Stoughton, MA).
Preparation of cell lysates
We removed the growth medium from the culture dishes into a 15-mL centrifuge tube and collected the floating cells by centrifugation at 300 × g for 10 min at 4 °C; the collected cells were then mixed with the adherent cells for the analyses. The adherent cells were rinsed twice with cold PBS before 1.0-mL cell lysis buffer (Sigma-Aldrich, St Louis, MO) was added to each culture dish. The cells were then incubated for 15 min on ice in an orbital shaker before they were scraped and collected. The lysed floating cells were then mixed together with the lysed adherent cells. The lysed cells were centrifuged for 10 min at 12 000 × g at 4 °C to obtain a pellet containing cell debris. The protein-containing supernatant fluid was then recovered into chilled microcentrifuge tubes, and the samples were stored at −70 °C until analyzed.
Measurement of serum lipoprotein profiles and biomarkers
All serum variables were measured at weeks 0, 3, and 7 of the HCHF dietary challenge. The lipoprotein profiles included total cholesterol concentrations, which were measured as previously described (7). Total antioxidant status was measured as previously described (11). High-sensitivity C-reactive protein (CRP) concentrations were measured with the use of an assay kit from Kamiya Biomedical (Seattle, WA) with a latex particle-enhanced immunoturbidometric method. oxLDL-cholesterol concentrations were measured with an enzyme-linked immunosorbent assay (ELISA) kit from ALPCO Diagnostics (Windham, NH). Soluble ICAM-1 and soluble VCAM-1 concentrations were measured by ELISA methods (R&D Systems, Minneapolis, MN). Soluble TNF-α receptor I (p55) and TNF-α receptor II (p75) concentrations were measured by ELISA methods (BioSource, Camarillo, CA). The intra- and interassay CVs for TNF-α receptor I were 1.7% and 5.7%, respectively; the intra- and interassay CVs for TNF-α receptor II were 6.5% and 8.9%, respectively.
Proinflammatory cytokine concentrations in sera and cell culture supernatant fluid
We measured the concentrations of proinflammatory cytokines [granulocyte-macrophage colony stimulating factor (GM-CSF), IL-1β, IL-4, IL-6, IL-8, MCP-1, interferon γ(IFN-γ), and regulated on activation, normal T cell expressed and secreted (RANTES) factors] in serum and in cell culture supernatant fluid with the use of the Human Cytokine Lincoplex kit (LINCO Research Inc, St Louis, MO). The intraassay CVs for MCP-1, GM-CSF, IL-8, and RANTES were 11.1%, 7.8%, 6.3%, and 1.6%, respectively; interassay CVs for these biomarkers were 13.7%, 11.7%, 9.0%, and 13.3%, respectively.
Quantification of endothelial functional markers in cell lysates
We used antibody-based immunoassays to measure the concentrations of membrane-bound VCAM-1 (mVCAM-1) and membrane-bound E-selectin (mE-selectin) as well as TNF-α receptors I (p55) and II (p75). The measurements of cellular membrane-bound adhesion molecules (mVCAM-1 and mE-selectin) in cell lysates were performed with kits from R&D Systems. We measured endothelial nitric oxide synthase (eNOS) concentrations in cell lysates with the use of an ELISA kit (R&D Systems), as previously described (12).
Statistical analysis
Data are expressed as means ± SEMs. Paired Student’s t tests were used to compare the mean differences before and after HCHF dietary challenges. An analysis of variance (ANOVA) was applied for comparisons of ≥3 groups. Bonferroni-corrected 2-tailed P values < 0.05 in relation to the baseline or no-treatment values were regarded as statistically significant. We examined the distribution patterns of all measured variables. Most of the measured markers were normally distributed and were compared with the use of either parametric ANOVAs or Student’s t tests. Measurements that were skewed in distribution (which included concentrations of RANTES, IL-8, TNF-α, and IFN-γ) were log-transformed before the parametric comparisons; their original concentrations were presented for the purpose of cross-study comparison. We used a 2-factor ANOVA to evaluate the interactive effects of the in vivo dietary challenge and the in vitro risk factor treatment on cytokine release in cultured ECs. Each variable was measured in duplicate in 3 independent experiments, and the average of these values was used in the analyses. We used SPSS version 12.0 (SPSS Inc, Chicago, IL) for the statistical analyses.
RESULTS
Lipid profiles after the HCHF dietary challenge
As reported previously (11), the HCHF diet substantially changed the lipoprotein profile in the baboons. Total cholesterol concentrations were dramatically increased (85.6%; P < 0.0001) after 3 wk of the HCHF diet (Table 2). By the end of the 7-wk challenge, total cholesterol concentrations had increased 105% (P < 0.0001) compared with basal concentrations. Non-HDL-cholesterol concentrations increased more than did HDL-cholesterol concentrations (160% and 64% at 7 wk, respectively). Furthermore, we observed a significant increase in serum oxLDL-cholesterol concentrations at the 3rd week of the HCHF diet compared with basal concentrations (54.9%, P = 0.03), but the concentration increase at the 7th week of the dietary challenge (19.6%) was not significant (P = 0.11). No statistically significant changes in the concentration of triacylglycerols or the ratio of total cholesterol to HDL cholesterol were observed during the HCHF dietary challenge.
TABLE 2.
Concentrations of serum cholesterol and lipoprotein components at 0, 3, and 7 wk of the high-cholesterol, high-fat diet1
| 0 wk | 3 wk | 7 wk | P2 | |
|---|---|---|---|---|
| Total cholesterol (mg/dL) | 102.4 ± 6.5 | 190.1 ± 17.53 | 209.7 ± 21.73 | 0.0001 |
| HDL cholesterol (mg/dL) | 58.9 ± 6.5 | 94.1 ± 7.33 | 96.5 ± 8.13 | 0.001 |
| Non-HDL cholesterol (mg/dL) | 43.5 ± 4.9 | 96.0 ± 18.7 | 113.2 ± 26.84 | 0.015 |
| Triacylglycerol (mg/dL) | 47.1 ± 4.0 | 57.4 ± 6.2 | 63.0 ± 12.0 | 0.389 |
| Apolipoprotein B (mg/dL) | 31.0 ± 3.9 | 54.7 ± 8.4 | 50.2 ± 12.1 | 0.149 |
| oxLDL cholesterol (mU/mL) | 5.1 ± 0.7 | 7.9 ± 1.24 | 6.1 ± 1.6 | 0.03 |
| TC/HDL-C | 1.76 ± 0.09 | 2.17 ± 0.35 | 2.54 ± 0.63 | 0.434 |
All values are ; n = 10 baboons. oxLDL, oxidized LDL; TC/HDL-C, ratio of total cholesterol to HDL cholesterol.
ANOVA.
Significantly different from 0 wk (ANOVA with post hoc Bonferroni correction for between-group comparisons):
P < 0.001,
P < 0.05.
Effects of the HCHF diet on proinflammatory cytokines, total antioxidant status, CRP, and soluble cell adhesion molecules in sera
Our results showed that the following 4 patterns of proinflammatory changes occurred when baboons were fed the HCHF diet (Table 3): 1) MCP-1 concentrations significantly increased and remained high until the end of the 7-wk dietary challenge; 2) IL-6 and TNF-α concentrations were dramatically increased after 3 wk of the dietary challenge but decreased to below baseline concentrations by the end of the challenge; 3) soluble TNF-α receptor II concentrations were significantly lower than baseline concentrations after 7 wk of the dietary challenge but not after 3 wk; and 4) the total antioxidant status and the concentrations of IFN-γ, IL-1β, and CRP did not change significantly during the entire dietary challenge period. The concentrations of other endothelial dysfunctional markers, ie, soluble VCAM-1 and soluble ICAM-1, did not change significantly during the full 7-wk HCHF challenge.
TABLE 3.
Concentrations of proinflammatory cytokines in serum at 0, 3, and 7 wk of the high-cholesterol, high-fat diet1
| 0 wk | 3 wk | 7 wk | P2 | |
|---|---|---|---|---|
| TAS (mmol/L) | 1.38 ± 0.01 | 1.41 ± 0.02 | 1.40 ± 0.02 | 0.374 |
| CRP (mg/dL) | 0.30 ± 0.07 | 0.27 ± 0.04 | 0.28 ± 0.02 | 0.900 |
| RANTES (pg/mL) | 115.9 ± 39.1 | 235.7 ± 28.7 | 208.4 ± 55.8 | 0.134 |
| TNFRI (pg/mL) | 0.53 ± 0.02 | 0.49 ± 0.04 | 0.43 ± 0.04 | 0.170 |
| TNFRII (pg/mL) | 0.59 ± 0.09 | 0.51 ± 0.0.07 | 0.29 ± 0.073 | 0.029 |
| IL-1β (pg/mL) | <3.2 | <3.2 | <3.2 | NA |
| IL-6 (pg/mL) | 15.99 ± 4.0 | 48.3 ± 5.44 | 3.4 ± 4.53 | 0.0001 |
| IL-8 (pg/mL) | 131.6 ± 35.6 | 261.8 ± 41.8 | 287.7 ± 75.0 | 0.107 |
| GM-CSF (pg/mL) | 6.0 ± 1.8 | 5.4 ± 0.1 | 6.6 ± 0.3 | 0.947 |
| INF-γ (pg/mL) | 48.2 ± 12.5 | 41.3 ± 13.9 | 44.4 ± 17.6 | 0.946 |
| MCP-1 (pg/mL) | 31.5 ± 2.1 | 41.8 ± 4.63 | 52.5 ± 4.34 | 0.003 |
| TNF-α (pg/mL) | 8.0 ± 8.5 | 16.0 ± 3.53 | 1.2 ± 0.63 | 0.048 |
| sVCAM-1 (ng/mL) | 425.3 ± 2.8 | 425.8 ± 3.1 | 419.1 ± 3.2 | 0.246 |
| sICAM-1 (ng/mL) | 2.6 ± 0.8 | 2.5 ± 0.8 | 2.0 ± 0.7 | 0.840 |
All values are ; n = 10 baboons. TAS, total antioxidant status; CRP, C-reactive protein; RANTES, regulated on activation normal T cell expressed and secreted factor; TNFRI, tumor necrosis factor α receptor I; INFRII, TNF-α receptor II; IL, interleukin; GM-CSF, granulocyte macrophage colony-stimulating factor; INF-γ, interferon γ; MCP-1, monocyte chemoattractant protein 1; TNF-α, tumor necrosis factor α; sVCAM-1, soluble vascular cell adhesion molecule 1; sICAM-1 soluble intercellular cell adhesion molecule 1.
ANOVA.
Significantly different from 0 wk (ANOVA with post hoc Bonferroni correction for between-group comparisons):
P < 0.05,
P < 0.01.
Ex vivo endothelial dysfunctional changes induced by the HCHF diet
To test whether the HCHF diet caused changes in the arterial endothelium of the baboons that paralleled the significant inflammatory changes, we collected a sample of left femoral artery after the baboons were fed basal diets and before they began the HCHF diet challenge and collected a sample of right femoral artery at the end of the 7-wk HCHF dietary challenge. After the collections, we examined the femoral artery macroscopically and microscopically; no atherosclerotic lesions, including early fatty streaks, were observed after the 7-wk HCHF challenge. ECs were harvested from these biopsy samples and were cultured for 2–4 passages before functional measurements were conducted. The homogeneity of the ECs was confirmed by the cells’ typical cobble stone morphology (Figure 2A), by positive fluorescence staining of the ECs with anti–von Willenbrand factor (Figure 2B), and by Dil-LDL-cholesterol uptake assays (Figure 2C). No vascular smooth muscle cell contamination was observed, as shown by negative staining with an antibody to smooth muscle cell α-22 actin (Figure 2D). The same antibody positively stained baboon vascular smooth muscle cells (Figure 2E), the morphology of which differed from that of the ECs (Figure 2F).
FIGURE 2.
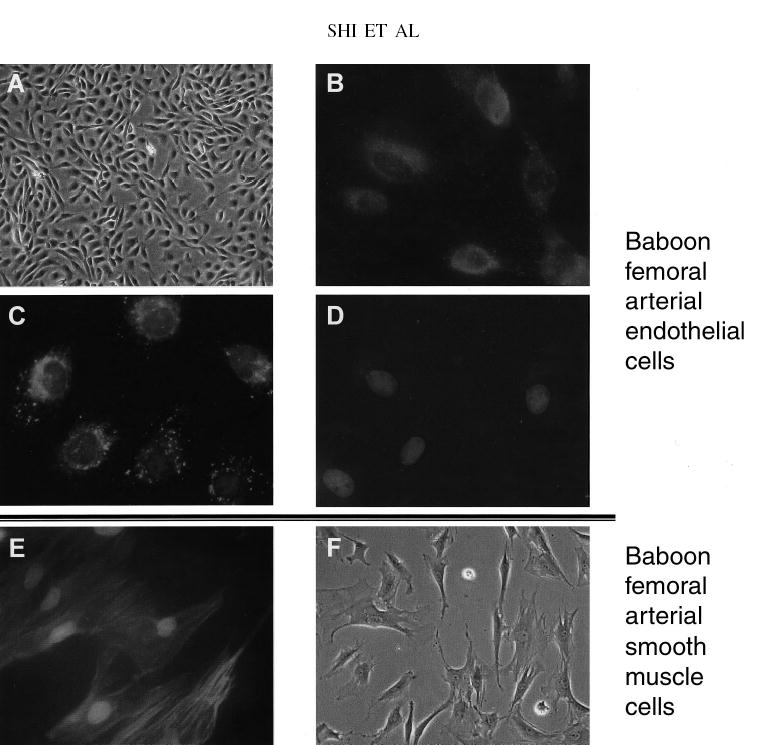
The homogeneity of collected baboon femoral artery endothelial cells (ECs) was confirmed with the use of both morphologic analyses and immunofluorescence analyses. Cells from all 10 baboons were examined for their endothelial origin, and representative images are shown. (A) Isolated ECs showed a typical cobblestone shape under a microscope (magnification ×100). (B) Immunofluorescence staining of baboon ECs with antibody to von Willenbrand factor (Sigma, St Louis, MO). A 200-μL cell suspension from the harvest was seeded on a Lab-Tek culture chamber (Nalgene Nunc International, Rochester, NY) coated with 1% gelatin. After the cells reached 80–90% confluence, they were fixed with 2% formalin in phosphate-buffered saline at room temperature for 20 min. After the samples were air-dried, the cells were blocked with 10% normal serum and incubated with antibody to von Willenbrand factor antibody at a 1:400 dilution. The primary antibodies were detected by FITC-labeled secondary antibody (Santa Cruz, CA), and images were taken with an Eclipse E800 microscope (Nikon Inc, Melville, NY). (C) Uptake of labeled LDL cholesterol. ECs cells were seeded on Lab-Tek culture chambers (Nalgene Nunc International) and grown to 80–90% confluence. The cells were incubated at 37 °C for 4 h with medium containing 10 mg fluorescence-labeled LDL cholesterol/mL (Biomedical Technologies Inc, Stoughton, MA) and 5% fetal calf albumin but no serum supplementation. The slides were fixed with 2% formalin for 20 min at room temperature, mounted with SlowFade (Molecular Probes, Eugene, OR), and observed under a Eclipse E800 fluorescence microscope (Nikon Inc). (D) Direct immunofluorescence staining in ECs incubated with antibody to smooth muscle cell α-22 actin conjugated with FITC at a 1:200 dilution (Sigma) for 1 h at room temperature. Other experimental conditions were the same as described for panel B. None of the cells stained positive. (E) Direct immunofluorescence staining in baboon femoral arterial vascular smooth muscle cells with antibody to smooth muscle cell α-22 actin conjugated with FITC. All cells under the visual field stained positive. (F) Baboon femoral artery smooth muscle cells were observed under a light microscope and showed clear morphologic differences from ECs.
We measured markers of endothelial function in both cell culture medium and EC extracts. As shown in Table 4, we found that the diet challenge increased MCP-1 release 2-fold compared with basal release. However, the concentrations of cytokines in the culture medium (including IL-1β, IL-8, RANTES, GM-CSF, and IFN-γ) were not significantly changed and the concentrations of RANTES, GM-CSF, and IFN-γ were below the detection limits. Soluble VCAM-1 and soluble ICAM-1 concentrations in the culture medium were also not significantly changed by the dietary challenge. However, mVCAM-1 and mE-selectin concentrations were significantly increased (2.5 and 2.6-fold, respectively, P < 0.01) after the dietary challenge compared with basal concentrations (Table 5), whereas the concentrations of released soluble cell adhesion molecules were not significantly changed (data not shown). Furthermore, eNOS concentrations after the 7-wk HCHF diet (36.4 ± 21.9 pg/μg protein) were significantly lower than basal concentrations (196.5 ± 29.7 pg/μg protein; P < 0.01). These findings illustrate that the early changes in endothelial dysfunction, which already exist at the cellular level, may not be detectable by measuring soluble markers in circulating blood. These dysfunctional changes were maintained in vitro even after 2–4 passages.
TABLE 4.
Concentrations of secreted soluble inflammatory markers in femoral artery endothelial cells harvested from 10 baboons after the basal diet or the high-cholesterol high-fat (HCHF) diet1
| Soluble marker | Basal diet | HCHF diet |
|---|---|---|
| IL-6 (pg/μg protein) | 8.1 ± 1.5 | 5.8 ± 1.1 |
| IL-8 (pg/μg protein) | 0.11 ± 0.05 | 0.09 ± 0.004 |
| GM-CSF (pg/μg protein) | <0.01 | <0.01 |
| INF-γ (pg/μg protein) | <0.01 | <0.01 |
| MCP-1 (pg/μg protein) | 0.21 ± 0.11 | 0.44 ± 0.132 |
| RANTES (pg/μg protein) | <0.01 | <0.01 |
| IL-1β (pg/μg protein) | 0.01 ± 0.01 | 0.01 ± 0.01 |
| sVCAM-1 (ng/mL) | 86.0 ± 5.2 | 94.2 ± 7.0 |
| sICAM-1 (ng/mL) | 64.2 ± 2.4 | 64.3 ± 1.9 |
All values are . IL, interleukin; GM-CSF, granulocyte-macrophage colony-stimulating factor; INF-γ, interferon γ; MCP-1, monocyte chemoattractant protein 1; RANTES, regulated on activation normal T cell expressed and secreted factor; IL-1β, interleukin 1β; sVCAM-1, soluble vascular cell adhesion molecule 1; sICAM-1, soluble intercellular cell adhesion molecule 1.
Significantly different from the basal diet, P < 0.01 (paired Student’s t test).
TABLE 5.
Changes in membrane-bound vascular cell adhesion molecule 1 (mVCAM-1) membrane-bound E-selectin (mE-selectin), and endothelial nitric oxide synthase (eNOS) concentrations in baboon femoral artery endothelial cell (BFAEC) extracts after exposure to various stimuli in vitro1
| mVCAM-1
|
mE-selectin
|
eNOS
|
||||
|---|---|---|---|---|---|---|
| Basal diet | HCHF diet | Basal diet | HCHF diet | Basal diet | HCHF diet | |
| No treatment | 0.77 ± 0.22 | 1.85 ± 0.532 | 0.08 ± 0.12 | 0.21 ± 0.182 | 196.5 ± 29.7 | 36.4 ± 21.92 |
| TNF-α, 10 ng/mL | 41.69 ± 13.223 | 16.13 ± 3.574 | 19.57 ± 22.62 | 7.68 ± 4.80 | 29.7 ± 21.83 | 4.3 ± 2.9 |
| LPS, 1 μg/mL | 17.18 ± 7.423 | 4.71 ± 1.113 | 5.01 ± 9.58 | 0.85 ± 0.81 | 10.0 ± 5.53 | 6.3 ± 2.8 |
| Native LDL cholesterol, 100 μg/mL | 3.22 ± 0.453 | 2.01 ± 0.82 | 0.09 ± 0.07 | 0.09 ± 0.01 | 28.5 ± 18.03 | 2.3 ± 1.3 |
| oxLDL cholesterol, 100 μg/mL | 2.17 ± 0.513 | 1.65 ± 0.43 | 0.01 ± 0.01 | 0.04 ± 0.02 | 3.5 ± 2.54 | 3.7 ± 1.7 |
All values are ; n = 10 baboons. HCHF, high-cholesterol, high-fat; TNF-α, tumor necrosis factor α; LPS, lipopolysaccharide; oxLDL, oxidized LDL.
Significantly different from the basal diet, P < 0.05.
Significantly different from no treatment of endothelial cells collected either after the basal diet or after the HCHF diet (paired Student’s t test with Bonferroni correction for multiple comparisons):
P < 0.05,
P < 0.01.
Effects of dietary challenges on endothelial responses to proinflammatory stimuli
We also tested the hypothesis that the 7-wk HCHF dietary challenge programmed the ECs to respond differently after a rechallenge in vitro. To test this hypothesis, we stimulated the ECs in vitro with both inflammatory risk factors (TNF-α and lipopolysaccharide) and cholesterol factors (native LDL cholesterol and oxLDL cholesterol) to measure cellular responses, which included changes in the concentrations of cytokines and cell adhesion molecules. As shown in Table 5, TNF-α caused a 54-fold increase in mVCAM-1 expression compared with no treatment in ECs that were collected from baboons before the diet challenge. However, in ECs collected after the dietary challenge, the degree of endothelial response to TNF-α was dramatically reduced (8.7-fold increase in VCAM-1 expression compared with no treatment). Compared with no treatment, TNF-α caused a 225-fold increase in the expression of mE-selectin in EC cells collected before the dietary challenge and a 37-fold increase in EC cells collected after the dietary challenge (Table 5). A similar pattern was also observed in ECs that were challenged with lipopolysaccharide. Lipopolysaccharide caused a 22-fold increase in mVCAM-1 concentrations compared with basal VCAM-1 concentrations in ECs collected before the dietary challenge and a 2.5-fold increase in mVCAM-1 concentrations compared with basal concentrations in ECs collected after the dietary challenge. The changes in VCAM-1 and mE-selectin concentrations induced by native LDL-cholesterol and oxLDL-cholesterol treatments were not as striking. Compared with no treatment, treatment with native LDL cholesterol and oxLDL-cholesterol caused a 4.1-fold and 2.8-fold increase, respectively, in mVCAM-1 concentrations in the ECs obtained before the dietary challenge; no increase was observed in the cells prepared after the 7-wk dietary challenge. Compared with changes in mVCAM-1 and mE-selectin concentrations, eNOS concentrations were dramatically reduced by the treatment of ECs with TNF-α, lipopolysaccharide, and native LDL and oxLDL cholesterol. In ECs collected before the HCHF dietary challenge, eNOS concentrations decreased from 196.5 ± 29.7 pg/μg protein in untreated cells to 29.7 ± 21.8 pg/μg protein (P = 0.019) after TNF-α treatment, 10.0 ± 5.5 pg/μg protein after lipopolysaccharide treatment (P = 0.010), 28.5 ± 18.0 pg/μg protein after native LDL-cholesterol treatment (P = 0.014), and 3.5 ± 2.5 pg/μg protein after oxLDL-cholesterol treatment (P = 0.005). Similar decreases also tended to be observed in ECs collected after the 7-wk HCHF dietary challenge. However, because the untreated eNOS concentrations were low (36.4) and variation was high, none of the reductions by TNF-α, lipopolysaccharide, or LDL cholesterol were statistically significant.
We also observed that in vitro stimulation with TNF-α, lipopolysaccharide, native LDL cholesterol, or oxLDL cholesterol activated ECs to release inflammatory factors, as shown in Table 6. Similar to the response patterns described above, the responses in inflammatory markers to TNF-α, lipopolysaccharide, and LDL-cholesterol stimulations were reduced in ECs obtained after the HCHF dietary challenge (Table 6). For example, treatment with TNF-α caused a 34-fold increase of MCP-1 production in the ECs obtained before the dietary challenge but only a 10-fold increase in the HCHF diet-challenged cells (P = 0.02, 2-factor ANOVA). These findings are consistent with a preconditioning effect in which prior activation may cause the cells to better tolerate additional challenges. Alternatively, if inflammatory responses are part of the normal endothelial defense against adverse stimuli, such as lipopolysaccharide, then the prior in vivo exposure to the HCHF diet appears to impair the function of ECs.
TABLE 6.
Changes in secreted soluble inflammatory markers in baboon femoral artery endothelial cells challenged in vitro1
| In vitro stimulus
|
|||||
|---|---|---|---|---|---|
| Cytokine | No treatment | TNF-α | LPS | Native LDL | oxLDL |
| IL-6 | |||||
| 0 wk | 6.9 ± 1.7 | 10.5 ± 2.42 | 10.3 ± 2.42 | 5.1 ± 1.6 | 6.2 ± 1.5 |
| 7 wk | 5.8 ± 1.1 | 7.9 ± 0.9 | 8.6 ± 1.1 | 5.3 ± 2.0 | 4.3 ± 0.6 |
| IL-8 | |||||
| 0 wk | 0.12 ± 0.32 | 12.8 ± 2.13 | 9.1 ± 3.32 | 2.4 ± 2.2 | 2.1 ± 1.9 |
| 7 wk | 0.1 ± 0.04 | 6.5 ± 0.64 | 4.8 ± 0.94 | 0.1 ± 0.02 | 0.17 ± 0.08 |
| GM-CSF | |||||
| 0 wk | <0.01 | 0.25 ± 0.12 | 0.11 ± 0.06 | <0.01 | <0.01 |
| 7 wk | <0.01 | 0.1 ± 0.013 | 0.03 ± 0.012 | <0.01 | <0.01 |
| INF-γ | |||||
| 0 wk | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| 7 wk | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| MCP-15 | |||||
| 0 wk | 0.35 ± 0.08 | 12.02 ± 2.44 | 7.0 ± 2.12 | 1.4 ± 0.53 | 0.85 ± 0.30 |
| 7 wk | 0.70 ± 0.14 | 7.35 ± 0.774 | 6.9 ± 1.13 | 1.6 ± 1.2 | 2.18 ± 1.26 |
| RANTES | |||||
| 0 wk | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| 7 wk | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
All values are , n = 10 baboons. TNF-α, tumor necrosis factor α; LPS, lipopolysaccharide; oxLDL, oxidized LDL; IL, interleukin; GM-CSF, granulocyte-macrophage colony-stimulating factor; INF-γ, interferon γ; MCP-1, monocyte chemoattractant protein 1; RANTES, regulated on activation normal T cell expressed and secreted factor.
Significantly different from no treatment at the same week (paired Student’s t test) with Bonferroni correction for multiple comparisons:
P < 0.05,
P < 0.01,
P < 0.001.
There was a significant time-by-treatment interaction only for MCP-1 concentrations in endothelial cell treated with TNF-α based on 2-factor ANOVA.
DISCUSSION
Consistent with our previous observations (11), the HCHF diet can effectively increase total blood cholesterol and non-HDL cholesterol concentrations in baboons. HDL-cholesterol concentrations were also increased in these baboons. The increase in HDL-cholesterol concentrations could partially attenuate the risk of atherosclerosis that is associated with elevated non-HDL-cholesterol concentrations. Elevated serum cholesterol concentrations may trigger inflammatory responses in the blood circulation and locally in vascular walls (13, 14). Our results show that the HCHF diet also results in gross systemic inflammatory responses, as indicated by markedly elevated cytokine concentrations. Because of the in vivo nature of our experimental model, the elevated cytokine concentrations in serum could be derived either from circulating white blood cells or from any tissue source, such as endothelium (15). On the basis of the amount of cytokine release in serum compared with that in cell culture supernatant fluid, it appears that ECs may significantly contribute to the increases in serum MCP-1 concentrations but contribute less to increases in the concentrations of circulating RANTES, TNF-α, IL-6, and IL-8.
We also noted that different inflammatory markers may have different metabolic responses in baboons challenged with an HCHF diet. For example, at the designated time points in the course of the HCHF challenge, we observed no significant changes in the concentrations of CRP, a widely regarded risk factor for cardiovascular disease. Our results were consistent with our previous study of a different group of baboons that found that CRP concentrations were not increased by a 7-wk HCHF dietary challenge (11). Together, these findings suggest that the response in CRP concentrations may be an early event. CRP concentrations may have increased initially but may have already returned to baseline concentrations after 3 wk of the HCHF diet because concentrations of CRP were also not increased after 7 wk of dietary challenge. Alternatively, the HCHF diet may not be sufficient or may not have been fed for a sufficient duration to induce changes in CRP concentrations. The increases in IL-6 and TNF-α concentrations persisted only for a short period; the highly elevated concentrations at 3 wk had returned to baseline concentrations by the end of the 7-wk dietary challenge. Technical errors are unlikely to be responsible for this finding because these 2 cytokines were measured with all the other cytokines in the same multiplex Luminex assays, and samples from all 3 time points were measured in the same assay. IL-8 and MCP-1 concentrations, however, were high at 3 wk and remained high at the end of the 7-wk dietary challenge.
The mechanisms underlying the different responses over time of different cytokines are not clear. It is possible that the high IL-6 and TNF-α responses, which are mainly produced by macrophages and monocytes (16), were the result of acute responses to the HCHF challenge. These cytokines normally return to baseline concentrations once the acute phase responses are attenuated (17). Prolonged increases in the concentrations of these cytokines are most likely associated with either severe damage of tissues after trauma or invasion of the body by pathogenic organisms (18). The HCHF diet may trigger acute stress responses when baboons are suddenly exposed to high quantities of fat and cholesterol. However, the acute reactions may be replaced by more chronic and vascular-specific inflammatory reactions if the high-fat, high-cholesterol serum environment persists (19). Furthermore, the pattern of increased chemokine concentrations (IL-8 and MCP-1) but decreased cytokine concentrations (TNF-α and IL-6) over the 7-wk dietary challenge could indicate a unique inflammatory signaling cascade in nonhuman primates, which certainly deserves additional investigation. This pattern of changes may be responsible for the lack of increase in CRP concentrations during the HCHF diet period.
Our study additionally showed that soluble VCAM-1 and soluble ICAM-1 concentrations in sera were not changed significantly over the entire course of the HCHF-diet period. However, mVCAM-1 and mE-selectin concentrations, which were measured from the BFAECs, were significantly increased after 7 wk of the HCHF diet (Table 4). This observation is consistent with earlier findings in mice (14) and suggests that some early endothelial dysfunctional changes triggered by the HCHF diet may not be severe enough to be observed in the corresponding circulating markers. The endothelial dysfunction was also illustrated by the dramatic inhibition of eNOS protein concentrations during the HCHF diet period. It is possible that prolonged duration of the HCHF diet may cause sustained endothelial damage and increase circulating concentrations of adhesion molecules. Interestingly, the damaging effects of the HCHF diet on in vivo cells are still detectable in vitro even after 2–4 passages. This persistence is additionally reflected in the decreased in vitro responses to inflammatory or cholesterol factor challenges. Although the mechanism for this apparent attenuation is not known, the findings are consistent with a preconditioning phenomenon (20). When ECs are exposed to an adverse environment in vivo, protective mechanisms may be activated to maintain the functional integrity of the cells. When these cells are exposed to the same risk factors again in vitro, the endogenous defense mechanisms may be more effectively and promptly activated than before so that dysfunctional changes are slowed. The preconditioning phenomenon may be a protective mechanism in which low-dose exposure to certain adverse environmental factors (eg, infection) could prepare the vascular tissue to withstand subsequent high-dose exposures. However, similar to all immune responses, preconditioning could also trigger an overreaction, which can be destructive as well.
One of the limitations of our study was the lack of control baboons that went through the same experimental period as the HCHF-challenged baboons. The advantage of the cross-sectional control design was that environmental and time-dependent factors could be controlled. One of the drawbacks in the cross-sectional design, however, was that between-individual variations may have confounded the findings. However, our study was based on a longitudinal observation within the same baboons over a defined period of time, which had the advantage of minimizing between-subject variations in the measured biomarkers that were not related to the dietary challenges.
In summary, our results clearly show that the HCHF diet resulted not only in increased serum cholesterol concentrations but also in inflammation and endothelial dysfunction. Although the changes in endothelial dysfunction were not detectable directly in the circulating blood, they were present in ECs, as marked by decreased eNOS expression, elevated MCP-1 secretion, and elevated mVCAM-1 and mE-selectin concentrations. Prior in vivo exposure to the HCHF diet attenuated the in vitro responses of the ECs to the same risk factors for atherosclerosis. The preconditioning phenomenon induced by the prior in vivo exposure to atherosclerotic risk factors (eg, the HCHF diet) may have significant clinical relevance for the susceptibility to atherogenesis and merits additional investigation.
QS participated in the experimental design, the collection of endothelial cells, the functional analyses, the data analyses, and the writing of the manuscript. JFVB was responsible for the cholesterol measurements. CJ was responsible for the cytokine measurements. KR, MML, and LT were responsible for the animal dietary challenges, the blood collections, and the femoral artery biopsies. RSK was involved in the preparation of LDL cholesterol and oxLDL cholesterol and in the experimental design. DLR contributed to eNOS-related analyses and to endothelial confirmation analyses and participated in the experimental design, the interpretation of results, and the revision of the manuscript. JLVB contributed to the experimental design, the interpretation of the results, and the writing of the manuscript. XLW was responsible for the original concept of the study, the study design, the data analyses, the interpretation of the results, and the writing of the manuscript. None of the authors had any conflicts of interest.
Footnotes
From the Departments of Genetics (QS, JFVB, CJ, DLR, and JLVB), Comparative Medicine (KR, MML, and LT), and Physiology and Medicine (RSK) and the Southwest National Primate Research Center (KR, MML, and JLVB), the Southwest Foundation for Biomedical Research, San Antonio, Texas, and the Division of Cardiothoracic Surgery, Michael E DeBakey Department of Surgery, Baylor College of Medicine, Houston, Texas (XLW).
Supported by grants P01 HL28972, P51 RR13986, and R01 HL66053 from the NIH; XLW is an AHA Established Investigator (AHA0400031).
Reprints not available. Address correspondence to XL Wang, MS NAB 2010, Baylor College of Medicine, One Baylor Plaza, Houston, TX 77030. E-mail:xlwang@bcm.tmc.edu.
References
- 1.Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–5. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 2.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 3.Libby P, Aikawa M. Mechanisms of plaque stabilization with statins. Am J Cardiol. 2003;91:4B–8. doi: 10.1016/s0002-9149(02)03267-8. [DOI] [PubMed] [Google Scholar]
- 4.Hubbard AK, Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic Biol Med. 2000;28:1379–86. doi: 10.1016/s0891-5849(00)00223-9. [DOI] [PubMed] [Google Scholar]
- 5.van de Stolpe A, van der Saag PT. Intercellular adhesion molecule-1. J Mol Med. 1996;74:13–33. doi: 10.1007/BF00202069. [DOI] [PubMed] [Google Scholar]
- 6.Cines DB, Pollak ES, Buck CA, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–61. [PubMed] [Google Scholar]
- 7.Rainwater DL, Kammerer CM, Mahaney MC, et al. Localization of genes that control LDL size fractions in baboons. Atherosclerosis. 2003;168:15–22. doi: 10.1016/s0021-9150(03)00051-0. [DOI] [PubMed] [Google Scholar]
- 8.Kushwaha RS, McGill HC., Jr Diet, plasma lipoproteins and experimental atherosclerosis in baboons (Papio sp. ) Hum Reprod Update. 1998;4:420–9. doi: 10.1093/humupd/4.4.420. [DOI] [PubMed] [Google Scholar]
- 9.Ziouzenkova O, Sevanian A, Abuja PM, Ramos P, Esterbauer H. Copper can promote oxidation of LDL by markedly different mechanisms. Free Radic Biol Med. 1998;24:607–23. doi: 10.1016/s0891-5849(97)00324-9. [DOI] [PubMed] [Google Scholar]
- 10.Esterbauer H, Ramos P. Chemistry and pathophysiology of oxidation of LDL. Rev Physiol Biochem Pharmacol. 1996;127:31–64. doi: 10.1007/BFb0048264. [DOI] [PubMed] [Google Scholar]
- 11.Wang XL, Rainwater DL, Mahaney MC, Stocker R. Cosupplementation of vitamin E and coenzyme Q10 reduces circulating markers of inflammation in baboons. Am J Clin Nutr. 2004;80:649–55. doi: 10.1093/ajcn/80.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Felux D, VandeBerg J, Wang XL. Discordance of endothelial nitric oxide synthase in the arterial wall and its circulating products in baboons: interactions with redox metabolism. Eur J Clin Invest. 2003;33:288–95. doi: 10.1046/j.1365-2362.2003.01143.x. [DOI] [PubMed] [Google Scholar]
- 13.Liao F, Andalibi A, deBeer FC, Fogelman AM, Lusis AJ. Genetic control of inflammatory gene induction and NF-kappa B-like transcription factor activation in response to an atherogenic diet in mice. J Clin Invest. 1993;91:2572–9. doi: 10.1172/JCI116495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grainger DJ, Mosedale DE, Metcalfe JC, Bottinger EP. Dietary fat and reduced levels of TGFbeta1 act synergistically to promote activation of the vascular endothelium and formation of lipid lesions. J Cell Sci. 2000;113:2355–61. doi: 10.1242/jcs.113.13.2355. [DOI] [PubMed] [Google Scholar]
- 15.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 16.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 17.Pinto FJ. The value of intravascular ultrasound in interventional cardiology. Rev Port Cardiol. 1999;18:I97–104. [PubMed] [Google Scholar]
- 18.Grimble RF. Inflammatory response in the elderly. Curr Opin Clin Nutr Metab Care. 2003;6:21–9. doi: 10.1097/00075197-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Naderali EK, Fatani S, Williams G. Chronic withdrawal of a high-palatable obesity-inducing diet completely reverses metabolic and vascular abnormalities associated with dietary-obesity in the rat. Atherosclerosis. 2004;172:63–9. doi: 10.1016/j.atherosclerosis.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 20.Cheng JZ, Sharma R, Yang Y, et al. Accelerated metabolism and exclusion of 4-hydroxynonenal through induction of RLIP76 and hGST5. 8 is an early adaptive response of cells to heat and oxidative stress. J Biol Chem. 2001;276:41213–23. doi: 10.1074/jbc.M106838200. [DOI] [PubMed] [Google Scholar]


