Abstract
The kinetics and homing characteristics of T-cell responses in humans after mucosal immunizations have not been well characterized. Therefore, we have investigated the magnitude and duration of such responses as well as the homing receptor expression of antigen-specific peripheral blood T cells by using an oral model vaccine, i.e., the live, attenuated Salmonella enterica serovar Typhi vaccine (Ty21a). Eight volunteers were each given three doses of the vaccine 2 days apart, and blood samples, from which CD4+ and CD8+ T cells were selected by the use of magnetic beads, were collected before vaccination and at regular intervals thereafter. To purify the potentially antigen-specific gut-homing T cells, CD45RA− integrin β7+ cells were further sorted by flow cytometry. The sorted cells were then stimulated in vitro with the serovar Typhi vaccine strain, and the proliferation of cells and the cytokine production were measured. Following vaccination, there was a large increase in both the proliferation of and the gamma interferon (IFN-γ) production by blood T cells stimulated with the vaccine strain. The responses were seen among both CD4+ and CD8+ T cells, although the CD8+ cells produced the largest amounts of IFN-γ. Peak responses were seen 7 to 14 days after the onset of vaccination. Furthermore, most of the IFN-γ produced by both CD4+ and CD8+ cells emanated from cells with the potential to home to mucosal tissues, as the integrin β7-expressing memory T cells produced around 10-fold more IFN-γ than the remaining populations. In conclusion, we demonstrate that oral vaccination with a live oral bacterial vaccine induces antigen-specific CD4+ and CD8+ memory T cells, almost all of which express the gut-homing integrin β7.
Although the kinetics and homing characteristics of B-cell responses in humans after mucosal immunizations have been extensively studied, mucosal T-cell responses after oral vaccination have been investigated in only a few studies (9, 19, 22, 23, 24). Oral vaccination with the attenuated Salmonella enterica serovar Typhi strains Ty21a (Vivotif) and CVD908 has been shown to induce proliferative responses, production of gamma interferon (IFN-γ), and antibody-dependent antibacterial activity by peripheral blood lymphocytes in the majority of vaccinated subjects at 3 weeks postvaccination (19, 21). Furthermore, a more recent report demonstrated that volunteers vaccinated with two doses of the CVD908 strain developed CD8+ cytotoxic-T-lymphocyte precursors, which after a short in vitro expansion were capable of killing serovar Typhi-infected cells (18). Except in that study, the relative contributions of CD4+ and CD8+ T cells in the response to oral vaccinations have not been investigated and the kinetics and homing patterns of the T-cell responses have not been evaluated in any detail.
A central event in the homing of lymphocytes to different tissues is the adhesion of lymphocytes by means of surface-homing receptors to binding structures, so-called addressins, on endothelial cells. Because some of the addressins are distributed in a tissue-restricted manner, tissue-specific homing of cells to corresponding homing receptors can occur. Some of the homing receptors that have been identified are α4β7, which guides cells to the mucosal lamina propria (17); l-selectin, which guides cells to peripheral lymph nodes (6); and cutaneous lymphocyte antigen, which guides cells to the skin (1).
The aim of this study was to determine the kinetics of circulating, mucosa-derived CD4+- and CD8+-T-cell responses after oral vaccination as well as the expression of intestinal homing receptors on T cells activated by the vaccine. As a model vaccine, we chose the live, attenuated serovar Typhi vaccine Ty21a, which previously has been shown to induce reliable antibody responses in intestinal secretions as well as circulating, vaccine-specific B cells and T cells (11, 12, 20, 21, 23).
MATERIALS AND METHODS
Subjects and vaccinations.
Eight healthy adult individuals, six women and two men (26 to 53 years of age; median age, 37), without any history of serovar Typhi infection or vaccination against typhoid fever, volunteered to participate in the study. The volunteers were given an oral capsule containing 2 × 109 to 5 × 109 live serovar Typhi Ty21a bacteria (Vivotif) on days 0, 2, and 4. Fifty milliliters of heparinized blood was collected from each volunteer before the first vaccination on day 0 and then on days 7, 14, and 21. Blood samples were also collected from the first two volunteers on days 3, 29, and 35.
The study was approved by the Ethical Committee for Human Research at Göteborg University, and informed consent was obtained from each volunteer before participation.
Cell preparation.
Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation on Ficoll-Paque (Pharmacia, Uppsala, Sweden). The cells were washed three times with phosphate-buffered saline (PBS) and resuspended in culture medium (Iscove's medium supplemented with 5% heat-inactivated human AB+ serum, 1% l-glutamine, and 1% gentamicin). From six of the volunteers, CD4+ and CD8+ cells were isolated from the PBMC by positive selection by immunomagnetic techniques. Magnetic beads coated with mouse anti-CD4 antibodies (Dynabeads M-450 CD4; Dynal, Oslo, Norway) were mixed with PBMC in equivalent numbers in 8 ml of culture medium. The suspension was incubated with gentle mixing at 4°C for 30 min. The CD4+ population was separated with a magnet, washed in PBS, and suspended in 2 ml of culture medium. The remaining cells were then incubated with magnetic beads coated with anti-CD8 antibodies (Dynabeads M-450 CD8; Dynal) at a bead-to-PBMC ratio of 0.5:1, incubated, and separated as described above. The attached beads were removed from the positively selected CD4+ and CD8+ cells by the addition of polyclonal goat anti-mouse immunoglobulin G (IgG) (Detachabead; Dynal) and incubated with shaking at room temperature for 45 min, after which they were washed in culture medium.
The purities of the cell populations were monitored by incubation with anti-CD4-fluorescein isothiocyanate, anti-CD8-phycoerythrin, and anti-CD3-peridinin chlorophyll a protein (all from Becton Dickinson and Co. [BD], San Jose, Calif.) followed by analysis with a flow cytometer (FACSCalibur; BD).
Staining and cell sorting.
In an attempt to purify the gut-homing memory cells, we sorted the T cells based on the expression of CD45RA and β7 integrin. Antibodies to β7 integrin were used because they were commercially available and because peripheral blood T cells expressing the β7 chain also express the α4β7 heterodimer (17). Suspensions of purified CD4+ or CD8+ cells were incubated for 30 min on ice with phycoerythrin-conjugated anti-β7 antibodies (clone FIB504; BD Pharmingen, San Jose, Calif.) and fluorescein isthiocyanate-conjugated anti-CD45RA antibodies (BD) in culture medium, washed once, and resuspended in fluorescence-activated cell sorter buffer (PBS supplemented with 0.1% bovine serum albumin [BSA], 1 mM EDTA, and 0.05% sodium azide). The cell sorting was performed using a FACSVantage SE (BD) equipped with a 488-nm-wavelength argon laser, at a sheath pressure of 22 lb/in2; the sorted cell populations were collected in fluorescence-activated cell sorter buffer supplemented with 5% human AB+ serum, and the cells were kept at 4°C during and after the sorting. The sorted cells were thereafter washed twice with PBS and resuspended in culture medium. The viability of the sorted cells, as determined by trypan blue exclusion, was always above 98%.
Bacterial culture.
Viable serovar Typhi Ty21a bacteria were obtained by dissolving a vaccine capsule (Vivotif) in 3 ml of PBS for 10 min. The suspension was then plated onto horse blood agar plates and incubated overnight at 37°C. The bacteria were then subcultured once on new horse blood agar plates, harvested, and stored at −70°C. When live bacteria were to be used for T-cell stimulation, a vial of the bacteria was thawed and cultured overnight and suspended in PBS. Heat-killed bacteria were obtained by incubating bacteria suspended in PBS in an 80°C water bath for 1 h. UV-killed bacteria were obtained by exposing a bacterial suspension in a petri dish to UV light for 25 min. The suspensions of heat-killed and UV-killed bacteria were then stored at 4°C and used throughout the study.
Bacterial antigens.
Crude membrane preparations (MP) of serovar Typhi Ty21a were made by sonication followed by differential centrifugation as previously described (3). Crude lipopolysaccharide was prepared by hot phenol-water extraction (25).
Cell culture.
To obtain antigen-presenting cells (APC), the CD4+- and CD8+-T-cell-depleted PBMC remaining after the magnetic separation were plated in round-bottomed, 96-well plates (NUNC, Aarhus, Denmark) at 105 cells/well and incubated for 2 h at 37°C. Thereafter, the nonadherent cells were removed, the wells were washed twice with PBS, and 105 cells of the different T-cell subpopulations per well were added. Replicate wells received 106, 107, or 108 live serovar Typhi Ty21a cells/ml, 107 heat-killed or UV-killed Ty21a cells/ml, 20 μg of MP/ml, 5 μg of bacterial lipopolysaccharide/ml, 5 μg of phytohemagglutinin (PHA) (Murex Diagnostics Ltd., Temple Hill, United Kingdom)/ml as a positive control, or culture medium alone as a negative control. The plates were then incubated in 5% CO2 at 37°C for 5 days. Forty-eight hours after the initiation of culture, 100 μl of the culture medium was removed and replaced by 100 μl of fresh culture medium. Three days later, 100 μl of the culture medium was removed and the wells were pulsed with 0.5 μCi of [3H]thymidine (Amersham, Arlington Heights, Ill.)/well for 8 h, after which the contents of the plates were frozen at −20°C. This material was later thawed, harvested onto nylon filters, and analyzed with a scintillation counter. The replicate supernatants collected (three or six wells) were pooled and stored at −70°C until they were analyzed for cytokine content.
Detection of cytokines.
The amounts of released IFN-γ, interleukin-10 (IL-10), and transforming growth factor β (TGF-β) in culture supernatants were measured with different enzyme-linked immunosorbent assays (ELISAs).
To determine the concentration of IFN-γ, 96-well Maxisorp plates (NUNC, Roskilde, Denmark) were coated with anti-human IFN-γ (1.5 μg/ml, clone 1D1k; Mabtech AB, Stockholm, Sweden) in 0.05 M carbonate buffer, pH 9.6, and kept at 4°C overnight. After the wells were blocked with 1% BSA in PBS at room temperature for 1 h, the cell culture supernatants were diluted in PBS-Tween 20 (0.05%) containing 0.1% BSA and were incubated in the plates at 4°C overnight; recombinant human IFN-γ (BD Pharmingen, San Diego, Calif.) was used as a reference to construct a standard curve. The plates were then incubated with biotinylated goat anti-human IFN-γ (0.3 μg/ml, clone 7B6-1; Mabtech) for 3 h at room temperature, followed by incubation with peroxidase-labeled Extravidin (Sigma, St. Louis, Mo.) for 1 h. The ELISA was developed with tetramethylbenzidine dihydrochloride (Sigma), the reaction was stopped with 1 M H2SO4, and fluorescence was measured with a spectrophotometer at a wavelength of 450 nm. Similarly, the concentrations of IL-10 and TGF-β were analyzed using reagents from BD Pharmingen (Opt-EIA) and R&D Systems (DuoSet; Abingdon, United Kingdom), respectively, and the assays were performed according to the recommendations of the manufacturers. To measure both active and latent forms of TGF-β, the samples were activated by the addition of 16 μl of 1 M HCl to each 80-μl sample, followed by incubation at room temperature for 10 min. The supernatants were then neutralized by adding 16 μl of a solution containing 1.4 M NaOH and 0.5 M HEPES and, after being mixed, were incubated for another 2 h at room temperature before the ELISA analysis. The standard curves and the cytokine concentrations were calculated using the computer software GraphPad Prism 3.0 (GraphPad Software, Inc., San Diego, Calif.).
Serum antibodies.
Levels of anti-serovar Typhi IgA and IgG were measured in sera by ELISA as follows: flat-bottomed polystyrene microtiter plates (NUNC) were coated with 0.01 mg of poly-l-lysine (Sigma)/ml and incubated for 30 min at room temperature. Thereafter, 108 serovar Typhi cells (strain SL 2404 grown on blood agar plates overnight) diluted in 1 ml of PBS were added. The plates were then centrifuged at 750 × g for 5 min, followed by gentle addition of 50 μl of 0.5% glutaraldehyde (Sigma) in PBS per well for 15 min at room temperature to fix the bacteria; the plates were then blocked by incubation with 10% fetal calf serum in PBS at 37°C for 30 min. Thereafter, serum samples were added and serially diluted in PBS-Tween 20 containing 0.1% BSA and incubated for 2 h at room temperature followed by incubation with a horseradish peroxidase-labeled rabbit anti-human IgA or anti-human IgG (Jackson). The ELISA was developed by the use of o-phenylenediamine dihydrochloride (Sigma) and H2O2 in a 0.1 M sodium citrate buffer, pH 4.5, for 30 min, followed by the addition of H2SO4 to stop the reaction. The plates were read in a spectrophotometer at 492 nm, and the end point titers were determined as the reciprocal interpolated dilution giving an absorbance of 0.4 above the background. A more-than-twofold increase in titer between pre- and postimmunization samples was chosen to signify seroconversion (10).
RESULTS
The kinetics of the antibody as well as T-cell immune responses in peripheral blood after oral administration of a live, attenuated serovar Typhi vaccine was studied in eight healthy adult Swedish volunteers.
Analysis of the serum antibody levels showed that seven out of the eight volunteers responded significantly, with a >2-fold increase in anti-serovar Typhi Ty21a IgA titers, and six out of eight showed an anti-Ty21a IgG response after vaccination (data not shown). The antibody responses in the different vaccinees peaked at either 7 or 14 days after the onset of vaccination; in each subject, the IgG and IgA titers peaked at the same time point. On average, the responders showed a sixfold increase in IgA and a threefold increase in anti-serovar Typhi IgG titers compared to prevaccination levels.
The kinetics and the nature of the T-cell response were studied after the positive selection of CD4+ and CD8+ subsets with magnetic beads. The purified populations were found to contain 99% CD4+ cells and 98% CD8+ cells, respectively (median values; n = 18). Stimulation of the purified T cells with heat-killed serovar Typhi showed that both the CD4+ and CD8+ cell populations responded to the vaccine strain with vigorous proliferation and IFN-γ production in most of the vaccines (seven of eight) (Table 1 and Fig. 1 and 2). The kinetics of the T-cell response was comparable to that observed for the serum antibody responses. Thus, the peak of the response was seen in either the day 7 or the day 14 samples, with a tendency for an earlier peak in the CD8+ cells than in the CD4+ cells. Stimulation of CD8+ cells with live serovar Typhi Ty21a bacteria resulted in somewhat higher levels of IFN-γ production than stimulation with heat-killed bacteria (a two- to fourfold difference), but the kinetics of CD4+ as well as CD8+ responses were similar regardless of whether the cells were stimulated with MP or live, UV-killed, or heat-killed serovar Typhi Ty21a bacteria. Only the data from stimulations with heat-killed bacteria are shown, as this antigen preparation resulted in the most reproducible and consistent results (Fig. 1 and 2). Stimulation of the APC, in the absence of T cells, with any of the antigen preparations or with live or killed bacteria did not result in detectable IFN-γ production (<20 pg/ml) or cell proliferation (<100 cpm).
TABLE 1.
Kinetics of the cellular immune response to serovar Typhi Ty21aa
| No. of days after vaccination | Frequency of respondersb
|
|
|---|---|---|
| T-cell proliferation | IFN-γ production | |
| 3 | 0/2 | 0/2 |
| 7 | 6/8 | 6/8 |
| 14 | 4/8 | 6/8 |
| 21 | 3/8 | 4/8 |
| 36 | 1/2 | 2/2 |
Peripheral blood T cells were stimulated with 107 heat-killed serovar Typhi Ty21a bacteria/ml.
A significant response is defined as a >2-fold increase in the rate of proliferation or IFN-γ production compared to the level before vaccination in either the CD4+- or the CD8+-T-cell population. Values are numbers of vaccinees who responded/total numbers of vaccinees.
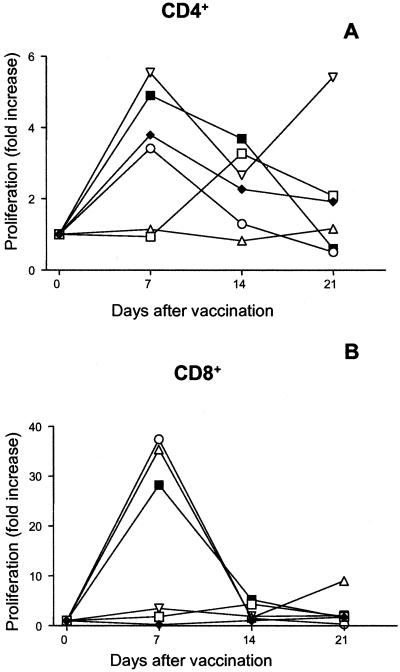
FIG. 1.
Purified peripheral blood CD4+ (A) or CD8+ (B) cells from volunteers vaccinated with serovar Typhi Ty21a were stimulated with 107 heat-killed Ty21a bacteria/ml in the presence of APC at different time points before and after vaccination. Five days after the antigen stimulation, the proliferative responses were measured and the results were normalized by division with the proliferation levels of PHA-stimulated cells. The data shown are fold increases of normalized rates of proliferation compared to the prevaccination levels. Prevaccination levels were as follows: for CD4+ cells, the median rate of proliferation was 13,900 cpm (range, 4,200 to 17,900 cpm); for CD8+ cells, the median rate of proliferation was 250 cpm (range, 80 to 640 cpm). Values from each of the six individual vaccinees are shown. A paired t test was performed to compare levels at the peak time point with the prevaccination levels. P = 0.0076 (A) and 0.055 (B).
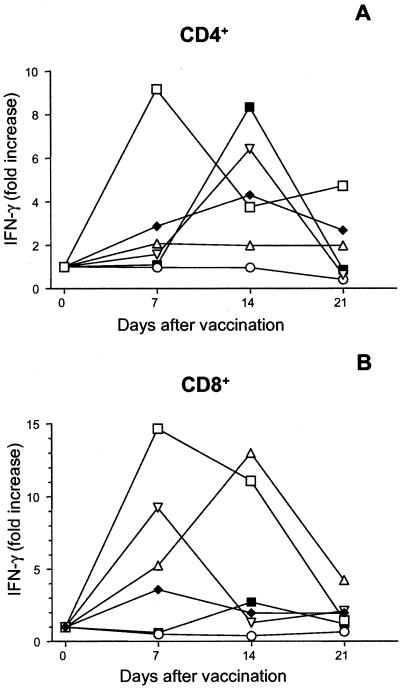
FIG. 2.
Purified peripheral blood CD4+ (A) or CD8+ (B) cells from volunteers vaccinated with serovar Typhi Ty21a were stimulated with 107 heat-killed Ty21a bacteria/ml in the presence of APC at different time points before and after vaccination. Two days after the antigen stimulation, culture supernatants were removed, values for the six replicate wells were pooled, and the IFN-γ levels were measured and normalized by division with the IFN-γ levels of PHA-stimulated cells. The data shown are fold increases of normalized IFN-γ production compared to prevaccination levels. Prevaccination levels were as follows: for CD4+ cells, the median rate of production was 390 pg/ml (range, <100 to 2,900 pg/ml); for CD8+ cells, the median rate of production was 1,600 pg/ml (range, <100 to 12,100 pg/ml). Values from each of the six individual vaccinees are shown. A paired t test was performed to compare levels at the peak time point with the prevaccination levels. P = 0.027 (A) and 0.045 (B).
The CD4+ cells exhibited a three- to sixfold rise in the rate of proliferation and a two- to ninefold increase in IFN-γ production at the peak time point compared to rates of proliferation and production before the vaccination. With regard to the CD8+-cell responses, the vaccinees clustered into two different groups (Table 2). Thus, cells from half of the volunteers produced low levels of IFN-γ before vaccination (<100 pg/ml) and their IFN-γ production increased up to 15-fold at day 7 or 14; in the other group, large amounts of IFN-γ (3,000 to 12,000 pg/ml) were produced after antigen stimulation even before vaccination and increases after vaccination varied. Only the latter subjects responded with increased proliferation of the CD8+ cells (proliferation was 30-fold higher after vaccination than before vaccination), whereas there was no relation between prevaccination IFN-γ levels and either anti-Ty21a IgA or IgG titers.
TABLE 2.
Response of CD8+ T cells to stimulation with serovar Typhi Ty21aa
| Volunteer | T-cell proliferation (cpm)
|
IFN-γ production (pg/ml)
|
||
|---|---|---|---|---|
| Preb | Fold increasec | Pre | Fold-increase | |
| 3 | 80 | 1.7 | <100 | 3.6 |
| 4 | 270 | 4.3 | <100 | 15 |
| 6 | 130 | 1.5 | <100 | 9.2 |
| 5 | 550 | 35 | 3,020 | 13 |
| 7 | 230 | 37 | 4,960 | 0.65 |
| 8 | 640 | 28 | 12,100 | 1.5 |
Peripheral blood CD8+ T cells and APC were stimulated with 107 heat-killed serovar Typhi Ty21a bacteria/ml.
Pre, results from samples taken before vaccination.
Results from the peak time points (7 to 21 days after the onset of vaccination) were divided by the prevaccination results.
The cell culture supernatants were also analyzed for the presence of IL-10 and TGF-β, and although both cytokines could be detected in the supernatants, no significant increases in their levels could be detected after vaccination (data not shown).
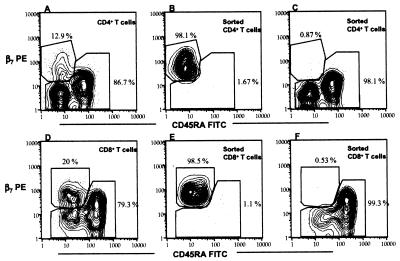
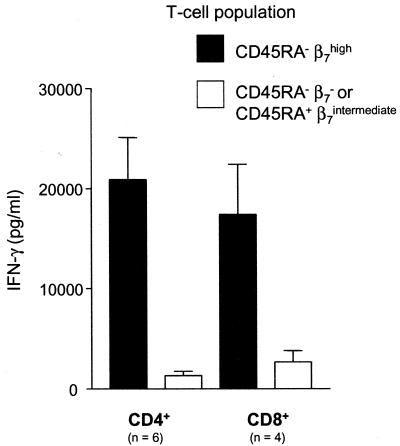
To further study the homing characteristics of the T cells responding to the vaccination, we sorted the CD4+ and CD8+ cells, based on the expression of the memory differentiation marker CD45RA and the mucosal homing receptor β7 integrin, into memory cells expressing β7 (CD45RA− β7high cells) and the remaining populations (CD45RA− β7−-CD45RA+ β7intermediate). After the sorting, the median purity was 97.7% (28 samples) for the CD45RA− β7high population and 99.1% (28 samples) for the remaining populations (Fig. 3). These T-cell subpopulations were then stimulated with serovar Typhi Ty21a bacteria in the presence of T-cell-depleted adherent cells and were analyzed for proliferative responses and cytokine production. Dramatic differences in the proliferative responses and levels of IFN-γ production of the different T-cell subsets sorted on the basis of integrin β7 and CD45RA expression were observed. The CD45RA− β7high cells were consistently more reactive to the serovar Typhi bacteria than were the CD45RA− β7-CD45RA+β7intermediate cells, a phenomenon demonstrated by significantly higher rates of proliferation and levels of IFN-γ production. Both CD4+ and CD8+ cells showed patterns similar to this (25-fold and 9-fold mean increases in levels of IFN-γ, respectively) (Fig. 4). However, there was no significant difference in responses to PHA stimulation between the sorted populations (data not shown).
FIG. 3.
Countour plots illustrating the staining of integrin β7 versus that of CD45RA. Peripheral blood mononuclear cells were purified by magnetic beads into CD4+ (A)- and CD8+ (D)-T-cell fractions. The cells were stained with antibodies to β7 and CD45RA and were sorted by flow cytometry into CD45RA− β7high (B and E) and CD45RA− β7−/CD45RA+ β7intermediate (C and F) populations. The percentages of the populations are shown at the gates; each figure contains data from at least 1,000 cells and depicts results of one representative experiment out of 15 (A to C) or 13 (D to F).
FIG. 4.
Purified peripheral blood CD4+ and CD8+ cells from volunteers vaccinated with serovar Typhi Ty21a were sorted by flow cytometry into CD45RA− β7high and CD45RA− β7−-/CD45RA+β7intermediate subsets. The sorted cells were then stimulated with 106 live Ty21a bacteria/ml in the presence of APC at different time points before and after vaccination. Two days after antigen stimulation, culture supernatants were removed, triplicate wells were pooled, and the IFN-γ levels were measured. The means ± standard errors of the means of values for IFN-γ production at the peak time points of the vaccinees tested (six CD4+ vaccinees and four CD8+ vaccinees) are shown. A paired t test was performed to compare levels between the sorted subsets. P = 0.0043 (A) and 0.046 (B).
DISCUSSION
In the present study we demonstrated that oral vaccination with live, attenuated serovar Typhi strain Ty21a induces a vigorous T-cell response in peripheral blood that peaks between 7 and 14 days after vaccination. This response has characteristics of a Th1 response, such as the production of large amounts of IFN-γ but no IL-10 or TGF-β. The finding that both CD4+ and CD8+ T cells contribute to the response extends results of previous studies of T-cell activation following vaccination with the serovar Typhi Ty21a strain. In those studies, an increase in proliferation of, and IFN-γ production by, peripheral blood lymphocytes was seen 3 weeks after vaccination (19, 23). In accordance with the low capacity of CD8+ T cells to proliferate without help from CD4+ T cells, we observed that the CD8+ T cells proliferated more slowly than the CD4+ population after antigen stimulation (median counts per minute were 1,600 and 22,000, respectively). In contrast, IFN-γ was produced in similar amounts by CD4+ and CD8+ cells when the cells were stimulated with killed serovar Typhi bacteria or MP antigens. Live bacteria induced higher IFN-γ production levels in the CD8+ than in the CD4+ T cells (a fourfold difference), but since this was observed both before and after vaccination, the magnitudes of responses were comparable for the two T-cell subsets. The observation might indicate that although the vaccine strain retains only about 3% of the invasiveness of the wild-type strain (8), the live bacteria could still partially invade the APC and therefore get better access to the major histocompatibility complex class I (MHC-I)-presenting pathway than could the killed bacterial cells in our system.
Another interesting observation is that the CD8+ cells from three out of six of the vaccinees, none of whom had been vaccinated before, produced substantial amounts of IFN-γ in response to the inactivated vaccine strain before vaccination. Furthermore, these vaccinees were the only subjects whose CD8+ cells responded with increased proliferation. The prevaccination response was probably not attributable to earlier encounters with serovar Typhi bacteria, as the CD8+-cell response was not related to the preimmune antibody titers. Instead, it is feasible that the reactivity of the T cells in preimmune samples may be due to cross-reactivity of the CD8+ T cells from these subjects with other intestinal bacteria. This possibility is supported by a recent study with mice, where MHC-Ib-restricted CD8+ T cells specific for an N-formyl peptide of another intracellular pathogen, Listeria monocytogenes, were shown to display an unexpectedly high degree of cross-reactivity with formylated peptides from other bacteria (16). If such a high cross-reactivity may also be exhibited by human MHC-Ib-restricted CD8+ T cells, this could explain why in some of the vaccinees the CD8+ but not the CD4+ T cells produced large amounts of IFN-γ already before the vaccination. Another possibility is that the high baseline IFN-γ production of CD8+ T cells in some of the vaccinees is due to an antigen-specific bystander activation of the CD8+ T cells. It was recently shown with mice that infection or in vitro stimulation with a bacterial pathogen could induce IL-12 production by cells of the innate immune system, which triggered CD8+ T cells to secrete large amounts of IFN-γ in the absence of antigen stimulation (14). The existence of a similar phenomenon in our study is suggested by the fact that the cells cultured from the subjects whose CD8+ T cells responded to serovar Typhi stimulation with the production of large amounts (>3,000 pg/ml) of IFN-γ before vaccination also exhibited more than fivefold-higher levels of IL-12 than did the cells cultured from other subjects (data not shown). This elevated innate IL-12 response of some subjects might have induced a higher baseline IFN-γ secretion by the CD8+ T cells (14), which would mask the antigen-specific IFN-γ response to the vaccine. The higher innate response of the APC could also, by increased costimulation of CD8+ T cells, allow for the substantially higher proliferation of the CD8+ T cells seen in these subjects after vaccination (Fig. 1B). However, further experiments are needed to elucidate whether the secreted IL-12 is actually the cause of the higher IFN-γ production.
Homing of lymphocytes to the intestinal lamina propria has previously been shown to be mediated by α4β7 integrin, which binds to its endothelial ligand MadCAM-1 (2). On T cells, α4β7 is expressed at high density on a gut-homing subset of memory T cells and at intermediate levels on naïve T cells (13). Because of different glycosylation patterns between MadCAM-1 on high endothelial venules of Peyer's patches and on endothelial cells of the lamina propria, the differential expression of α4β7 leads to different migration patterns of naïve and memory T cells (5). Thus, although MadCAM-1 is expressed on endothelial cells throughout the intestine, the naïve T cells can transmigrate out only through the high endothelial venules of Peyer's patches, where the induction of intestinal immune responses takes place, while the α4β7-expressing memory T cells can pass all MadCAM-1-expressing endothelial cells and thus end up at the effector sites of the gut, throughout the entire lamina propria (4). Therefore, to elucidate vaccine-specific responses and to purify the vaccine-induced, mucosally derived T cells, we further sorted the peripheral blood CD8+ or CD4+ T cells based on the expression of β7 integrin and CD45RA; the expression of the latter is lacking on the majority of memory and effector T cells (15). The sorted T-cell subsets were stimulated in vitro with serovar Typhi Ty21a, and the proliferation and cytokine production were measured. We could then show that the CD45RA− β7high T cells, which are the mucosally homing memory cells, were indeed responsible for almost all the reactivity against the vaccine strain, as they produced considerably higher levels of IFN-γ than did the remaining population. This was the case for both CD4+ and CD8+ cells. The homing receptor expression of the responding T cells explains the relatively short duration of the T-cell response observed: the antigen-specific T cells quickly leave the circulation and home back to the intestinal mucosa, where some of them persist as memory cells. Similarly, it has been observed that vaccine-induced circulating B cells are found only in peripheral blood for a very short time, 5 to 9 days, after oral vaccination (7). Our results are consistent with those of two other studies showing that the majority of the circulating antigen-specific CD4+ T cells in rotavirus-infected or Helicobacter pylori-infected subjects belongs to the β7high subset (16, 18). Furthermore, it has also been shown that the antigen-specific CD4+ T cells induced by feeding the cells a soluble protein antigen express β7 (13). However, the present study is the first to show that both CD4+ and CD8+ T cells activated by an oral vaccine preferentially express β7 integrin. Furthermore, it has not previously been shown that this phenomenon also involves effector-type T cells that secrete large amounts of cytokines. These findings may be important in selecting future vaccines for the induction of mucosal T-cell responses.
Acknowledgments
This study was supported by grants from the Swedish Research Council, an EC council (MUCIMM), AstraZeneca, Adlerbert's science foundation, Tore Nilson's foundation for medical research, and Magnus Bergvall's foundation.
Strain SL2404 was kindly provided by Bruce Stocker, Stanford University School of Medicine. We thank Jan Kilhamn for setting up the ELISA and Kerstin Andersson for skillful technical assistance.
Editor: R. N. Moore
REFERENCES
- 1.Berg, E. L., T. Yoshino, L. S. Rott, M. K. Robinson, R. A. Warnock, T. K. Kishimoto, L. J. Picker, and E. C. Butcher. 1991. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J. Exp. Med. 174:1461-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berlin, C., E. L. Berg, M. J. Briskin, D. P. Andrew, P. J. Kilshaw, B. Holzmann, I. L. Weissman, A. Hamann, and E. C. Butcher. 1993. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 74:185. [DOI] [PubMed] [Google Scholar]
- 3.Bölin, I., H. Lönroth, and A.-M. Svennerholm. 1995. Identification of Helicobacter pylori by immunological dot blot method based on reaction of a species-specific monoclonal antibody with a surface-exposed protein. J. Clin. Microbiol. 33:381-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandtzaeg, P., I. N. Farstad, and L. Helgeland. 1998. Phenotypes of T cells in the gut. Chem. Immunol. 71:1-26. [DOI] [PubMed] [Google Scholar]
- 5.Butcher, E. C., and L. J. Picker. 1996. Lymphocyte homing and homeostasis. Science 272:60-66. [DOI] [PubMed] [Google Scholar]
- 6.Camerini, D., S. P. James, I. Stamenkovic, and B. Seed. 1989. Leu-8/TQ1 is the human equivalent of the Mel-14 lymph node homing receptor. Nature 342:78-82. [DOI] [PubMed] [Google Scholar]
- 7.Czerkinsky, C., A.-M. Svennerholm, M. Quiding, R. Jonsson, and J. Holmgren. 1991. Antibody-producing cells in peripheral blood and salivary glands after oral cholera vaccination of humans. Infect. Immun. 59:996-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dragunsky, E. M., E. Rivera, H. D. Hochstein, and I. S. Levenbook. 1990. In vitro characterization of Salmonella typhi mutant strains for live oral vaccines. Vaccine 8:263-268. [DOI] [PubMed] [Google Scholar]
- 9.Husby, S., J. Mestecky, Z. Moldoveanu, S. Holland, and C. O. Elson. 1994. Oral tolerance in humans. T cell but not B cell tolerance after antigen feeding. J. Immunol. 152:4663-4670. [PubMed] [Google Scholar]
- 10.Jertborn, M., A.-M. Svennerholm, and J. Holmgren. 1986. Saliva, breast milk, and serum antibody responses as indirect measures of intestinal immunity after oral cholera vaccination or natural disease. J. Clin. Microbiol. 24:203-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kantele, A. 1990. Antibody-secreting cells in the evaluation of the immunogenicity of an oral vaccine. Vaccine 8:321-326. [DOI] [PubMed] [Google Scholar]
- 12.Kantele, A., M. Häkkinen, Z. Moldoveanu, A. Lu, E. Savilahti, R. D. Alvarez, S. Michalek, and J. Mestecky. 1998. Differences in immune responses induced by oral and rectal immunizations with Salmonella typhi Ty21a: evidence for compartmentalization within the common mucosal immune system in humans. Infect. Immun. 66:5630-5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kantele, A., J. Zivny, M. Hakkinen, C. O. Elson, and J. Mestecky. 1999. Differential homing commitments of antigen-specific T cells after oral or parenteral immunization in humans. J. Immunol. 162:5173-5177. [PubMed] [Google Scholar]
- 14.Lertmemongkolchai, G., G. Cai, C. A. Hunter, and G. J. Bancroft. 2001. Bystander activation of CD8+ T cells contributes to the rapid production of IFN-gamma in response to bacterial pathogens. J. Immunol. 166:1097-1105. [DOI] [PubMed] [Google Scholar]
- 15.Mitra, D. K., S. C. De Rosa, A. Luke, A. Balamurugan, B. K. Khaitan, J. Tung, N. K. Mehra, A. I. Terr, A. O'Garra, L. A. Herzenberg, and M. Roederer. 1999. Differential representations of memory T cell subsets are characteristic of polarized immunity in leprosy and atopic diseases. Int. Immunol. 11:1801-1810. [DOI] [PubMed] [Google Scholar]
- 16.Nataraj, C., G. R. Huffman, and R. J. Kurlander. 1998. H2M3wt-restricted,Listeria monocytogenes-immune CD8 T cells respond to multiple formylated peptides and to a variety of gram-positive and gram-negative bacteria. Int. Immunol. 10:7-15. [DOI] [PubMed] [Google Scholar]
- 17.Rott, L. S., M. J. Briskin, D. P. Andrew, E. L. Berg, and E. C. Butcher. 1996. A fundamental subdivision of circulating lymphocytes defined by adhesion to mucosal addressin cell adhesion molecule-1. Comparison with vascular cell adhesion molecule-1 and correlation with beta 7 integrins and memory differentiation. J. Immunol. 156:3727-3736. [PubMed] [Google Scholar]
- 18.Sztein, M. B., M. K. Tanner, Y. Polotsky, J. M. Orenstein, and M. M. Levine. 1995. Cytotoxic T lymphocytes after oral immunization with attenuated vaccine strains of Salmonella typhi in humans. J. Immunol. 155:3987-3993. [PubMed] [Google Scholar]
- 19.Sztein, M. B., S. S. Wasserman, C. O. Tacket, R. Edelman, D. Hone, A. A. Lindberg, and M. M. Levine. 1994. Cytokine production patterns and lymphoproliferative responses in volunteers orally immunized with attenuated vaccine strains of Salmonella typhi. J. Infect. Dis. 170:1508-1517. [DOI] [PubMed] [Google Scholar]
- 20.Tacket, C. O., G. Losonsky, D. N. Taylor, L. S. Baron, D. Kopecko, S. Cryz, and M. M. Levine. 1991. Lack of immune response to the Vi component of a Vi-positive variant of the Salmonella typhi live oral vaccine strain Ty21a in human studies. J. Infect. Dis. 163:901-904. [DOI] [PubMed] [Google Scholar]
- 21.Tagliabue, A., L. Nencioni, A. Caffarena, L. Villa, D. Boraschi, G. Cazzola, and S. Cavalieri. 1985. Cellular immunity against Salmonella typhi after live oral vaccine. Clin. Exp. Immunol. 62:242-247. [PMC free article] [PubMed] [Google Scholar]
- 22.Tagliabue, A., L. Villa, M. T. De Magistris, M. Romano, S. Silvestri, D. Boraschi, and L. Nencioni. 1986. IgA-driven T cell-mediated anti-bacterial immunity in man after live oral Ty 21a vaccine. J. Immunol. 137:1504-1510. [PubMed] [Google Scholar]
- 23.Viret, J.-F., D. Favre, B. Wegmüller, C. Herzog, J. U. Que, S. J. Cryz, Jr., and A. B. Lang. 1999. Mucosal and systemic immune responses in humans after primary and booster immunizations with orally administered invasive and noninvasive live attenuated bacteria. Infect. Immun. 67:3680-3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wennerås, C., A.-M. Svennerholm, and C. Czerkinsky. 1994. Vaccine-specific T cells in human peripheral blood after oral immunization with an inactivated enterotoxigenic Escherichia coli vaccine. Infect. Immun. 62:874-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westphal, O., and K. Jann. 1965. Bacterial lipopolysaccharide: extraction with phenol-water and further applications of the procedure, p. 83-92. In R. Whitler (ed.), Methods in carbohydrate chemistry. Academic Press, New York, N.Y.