Abstract
The long-term burial of organic carbon in sediments results in the net accumulation of oxygen in the atmosphere, thereby mediating the redox state of the Earth's biosphere and atmosphere. Sediment microbial activity plays a major role in determining whether particulate organic carbon is recycled or buried. A diverse consortium of microorganisms that hydrolyze, ferment, and terminally oxidize organic compounds mediates anaerobic organic matter mineralization in anoxic sediments. Variable temperature regulation of the sequential processes, leading from the breakdown of complex particulate organic carbon to the production and subsequent consumption of labile, low-molecular weight, dissolved intermediates, could play a key role in controlling rates of overall organic carbon mineralization. We examined sediment organic carbon cycling in a sediment slurry and in flow through bioreactor experiments. The data show a variable temperature response of the microbial functional groups mediating organic matter mineralization in anoxic marine sediments, resulting in the temperature-driven decoupling of the production and consumption of organic intermediates. This temperature-driven decoupling leads to the accumulation of labile, low-molecular weight, dissolved organic carbon at low temperatures and low-molecular weight dissolved organic carbon limitation of terminal metabolism at higher temperatures.
Keywords: carbon cycle, fermentation, terminal metabolism, sulfate reduction
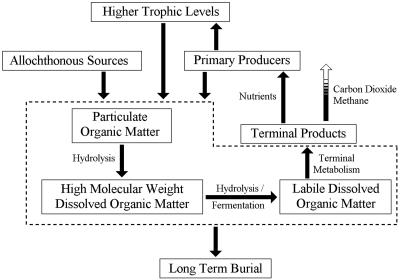
Microbes performing the terminal mineralization of organic carbon to metabolic end products (Fig. 1) are limited largely to labile, low-molecular weight (LMW), dissolved organic matter [LMW-dissolved organic carbon (DOC)] (molecular mass, <600 Da) that can be transported across cellular membranes (1). Particulate organic matter is initially broken down into high-molecular weight dissolved organic matter through extracellular enzymatic hydrolysis. High-molecular weight dissolved organic matter is further hydrolyzed and fermented to LMW-DOC, such as volatile fatty acids (VFAs) (2-4), that are available for terminal metabolism (Fig. 1) (2, 5, 6). Despite the importance of hydrolysis/fermentation in organic matter mineralization, relatively little is known about the environmental controls on this process.
Fig. 1.
Schematic showing organic matter dynamics in aquatic ecosystems. Inputs of organic matter are derived from allochthonous and autochthonous sources. The dashed line indicates the suite of microbial processes that mineralize organic matter. Particulate organic carbon is hydrolyzed to high-molecular weight DOC, which is then further hydrolyzed and fermented to labile LMW-DOC. Terminal metabolizers then mineralize labile LMW-DOC to terminal end products. Organic matter recycling provides inorganic nutrient sources to support production in aquatic ecosystems and influences the amount of organic carbon subject to long-term burial.
We examined the regulation of organic carbon mineralization in anoxic coastal marine sediments. A large fraction (55%) of global sediment organic matter oxidation occurs in coastal marine sediments, even though they account for only 7.5% of the total area of marine sediments (7, 8). High metabolic rates in these sediments deplete oxygen within millimeters of the sediment-water interface, and the majority of organic matter mineralization proceeds via anaerobic pathways (9), mainly sulfate reduction (9-12). The organic carbon consumed by sulfate-reducing bacteria is often VFAs, such as acetic acid (5, 6).
Temperature and substrate availability influence the rates and seasonal patterns of microbial activity (13) and, hence, organic matter mineralization (14). For instance, rates of sulfate reduction in coastal sediments generally follow a seasonal pattern similar to that of temperature (10, 15). Although sulfate reduction and other anaerobic terminal metabolic processes depend on the labile organic carbon substrates produced by fermentation, the temperature dependence of LMW-DOM production by hydrolysis/fermentation and the consumption by terminal metabolism may be quite different. In this study, we explored the temperature responses of hydrolysis/fermentation and terminal metabolism within a microbial community by using two separate approaches, slurry incubations and bioreactor experiments. Here, we document different temperature-activity relationships between these two microbial functional groups and discuss the effect of this temperature-driven decoupling of organic carbon mineralization on sediment carbon cycling.
Materials and Methods
The Slurry Experiment. In December 2004, sediment from a 2-4-cm depth, pooled from several intact sediment cores obtained from Umbrella Creek (Satilla River, Georgia), was homogenized under anoxic conditions. Slurries were generated by mixing sediment with anoxic artificial porewater (2:1 water/sediment). The artificial porewater contained 292 mM NaCl, 12.6 mM MgCl2, 0.9 mM CaCl2, 4 mM NH4Cl, 1.2 mM KH2PO4, 5.7 mM KCl, and 9.5 mM NaHCO3 (pH 7.6). Two slurries were prepared; one was amended with sulfate (5 mM Na2SO4) and the other was sulfate-free. Slurries were mixed gently and centrifuged, and the supernatant was removed and discarded. This rinsing procedure was repeated four times. The final slurry was distributed into headspace-free 6-ml vials. Vials were sealed in a container filled with N2 and placed at 5°C, 12°C, 15°C, 22°C, and 34°C for a 3-day equilibration. Next, acetate (2 mM C in the form of sodium acetate) was added to the vials containing sulfate, and high-molecular weight DOC (2 mM C in the form of dextran, a dextrose polysaccharide, with a molecular mass of 500,000 Da) was added to the sulfate-free vials. Fifteen vials were prepared for each temperature and slurry treatment (sulfate or sulfate-free). Sampling occurred over a time course, and at each time-point, three vials were centrifuged, and the supernatant was sampled to determine concentrations of dissolved inorganic carbon (DIC), DOC, sulfate, VFAs, and methane (Table 1).
Table 1. Sampling, preservation, and analytical methods for CH4, DIC, DOC, 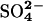 , VFAs, and Br-.
, VFAs, and Br-.
| Analyte | Sample | Preservation | Analytical method |
|---|---|---|---|
| Aqueous measurements | |||
| CH4 | Unfiltered in He purged headspace vial | 1.47 M phosphoric acid | Shimadzu GC 14A flame ionization detector gas chromatograph (16) |
| DIC | Unfiltered | Run immediately | Shimadzu TOC 5000 IR gas analyzer |
| DOC | 0.2-μm filtered | 0.1 M nitric acid, refrigerated | Shimadzu TOC 5000 high-temperature combustion IR gas analyzer after sparging with CO2-free air (16) |
| SO42– | 0.2-μm filtered | 0.1 M nitric acid, refrigerated | Dionex ion chromatograph (16) |
| VFA* | 0.2-μm filtered | Frozen | Modified 2-nitrophenylhydrazide derivatization and HPLC (17) |
| Br– | 0.2-μm filtered | 0.1 M zinc acetate | Colorimetric (Lachat Quikchem 8000 autoanalyzer method 30-135-21-1B) |
| Sediment solid-phase measurements | |||
| PLFA | Sediment | Frozen (–80°C) | Phospholipid extraction followed by gas chromatographic analysis (18) |
PLFA, phospholipid fatty acid.
VFA measured include acetic, propionic, lactic, formic, glycolic, butyric, isobutyric succinic, valeric, and isovaleric
Potential rates of hydrolysis/fermentation were determined in sulfate-free slurries amended with the high-molecular weight DOC polysaccharide. Potential rates of terminal metabolism were determined in slurries amended with sulfate and acetate. Rates were calculated from the change in substrate (sulfate and DOC) and product (DIC, methane, and VFA) concentration over time (see Rates and Reaction Pathways).
Bioreactor Experiments. The results from the slurry experiment reflected the response of a single sediment microbial community to changes in temperature. To investigate whether a similar response was observed over a seasonal temperature cycle, flow-through bioreactor experiments were conducted by using sediments from the same site, collected four times over a year. Rates of hydrolysis/fermentation and terminal metabolism were determined in carbon-amended bioreactors (2 mM C in the form of dextran with a molecular mass of 500,000 Da) and in control bioreactors (no carbon addition).
Intact sediment cores (8.8-cm i.d.) were obtained in April 2004 (20°C), May 2004 (27°C), August 2004 (29°C), and January 2005 (12°C). Sediment from the 2- to 4-cm depth of four intact cores was extruded and placed into bioreactors (2-cm height × 8.8-cm i.d.) (19). Duplicate bioreactors were used for each treatment. A peristaltic pump circulated anoxic artificial porewater, which had the same chemical composition noted above with the addition of 1 mM bromide (Br-), with or without dextran through the bioreactors at a rate of 10 ml·h-1. The entire assembly was placed in an anoxic container that was housed in an incubator at in situ temperature. Water exiting the bioreactors was sampled at least once daily for 10 days. The sample was obtained by placing the bioreactor outflow tubing in a glass vial for 2 h, allowing overflow of at least 1 vial volume. This sample was used for analysis of pH, DIC, sulfate, methane, VFA, DOC, and Br- (Table 1). Flow rate was monitored throughout the experiment. The Br- breakthrough was used to validate diffuse flow; the concentrations and flow rates were used to calculate reaction rates. At termination, bioreactors were disassembled, and sediment was immediately frozen (-80°C) for bacterial phospholipid fatty acid (PLFA) analysis (19).
Rates and Reaction Pathways. Rates of metabolic processes (μmol·cm-3 day-1) in slurries were calculated from the linear change in concentration over time, based on the original volume (cm3) of sediment. Net rates of DIC (RDIC), CH4 (RCH4), DOC (RDOC), and VFA (RVFA) production and 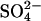 consumption (RSO4) in bioreactors (μmol·cm-3 day-1) were estimated by the change in concentration between the reactor outflow (Cout) and inflow (Cin), correcting for flow rate (Q) (Eq. 1). Rates in dextran-amended bioreactors (RA) were corrected for rates in control bioreactors (RC) to estimate the production from dextran-carbon (RD), thus reflecting net rates and allowing us to differentiate between hydrolysis/fermentation of dextran vs. naturally occurring sediment organic matter (Eq. 2). RVFA-D represents VFA production from dextran, and RVFA-C represents VFA production from endogenous organic matter.
consumption (RSO4) in bioreactors (μmol·cm-3 day-1) were estimated by the change in concentration between the reactor outflow (Cout) and inflow (Cin), correcting for flow rate (Q) (Eq. 1). Rates in dextran-amended bioreactors (RA) were corrected for rates in control bioreactors (RC) to estimate the production from dextran-carbon (RD), thus reflecting net rates and allowing us to differentiate between hydrolysis/fermentation of dextran vs. naturally occurring sediment organic matter (Eq. 2). RVFA-D represents VFA production from dextran, and RVFA-C represents VFA production from endogenous organic matter.
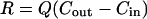 |
[1] |
and
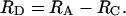 |
[2] |
Rates of terminal metabolism were calculated from changes in substrates 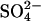 and products (DIC and CH4) in two ways:
and products (DIC and CH4) in two ways:
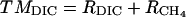 |
[3] |
and
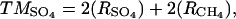 |
[4] |
where terminal metabolism, TM, on a per carbon basis is calculated from either DIC production (TMDIC; see Eq. 3), which is the sum of DIC and CH4 production (RDIC and RCH4, respectively), or from sulfate (TMSO4; see Eq. 4), which is double the rate of sulfate uptake (RSO4) and RCH4, reflecting the stoichiometry of these microbial processes. RCH4 was always negligible (<1%) in these experiments. TMDIC matched TMSO4 within 5% in all cases, indicating that sulfate reduction was the dominant mode of terminal metabolism and that fermentative production of DIC was insignificant (i.e., terminal respiration processes largely or entirely produced the DIC).
Rates of hydrolysis/fermentation (HFD; see Eq. 5) in the slurry and dextran-addition bioreactor experiments were calculated from the rate of terminal metabolism and the rate of VFA production (TMD and RVFA-D, respectively; corrected for controls) on a per carbon basis:
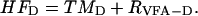 |
[5] |
This calculated value of HFD is a minimum estimate because intermediates produced by fermentation and available to terminal metabolizers other than the measured VFAs, such as alcohols or monosaccharides, were not considered. Rates of hydrolysis/fermentation of natural particulate organic matter in control bioreactors (HFC) were determined from rates of terminal metabolism, as well as the net DOC (RDOC) and VFA (RVFA-C) production rates in the control bioreactors:
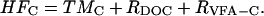 |
[6] |
Note that there was no net DOC production in the dextran-amended slurry and bioreactor experiments when corrected for DOC production in the controls and the added dextran; therefore, DOC was not included in the calculation of the rate of HFD (Eq. 5). HFD (Eq. 5) is the rate of hydrolysis/fermentation of the added dextran, whereas HFC (Eq. 6) is the rate of hydrolysis/fermentation of natural particulate sediment organic matter. RVFA-C was <1% of RDOC in control bioreactors.
Apparent Activation Energies. The apparent activation energies (Ea) of TM and hydrolysis/fermentation, HF, were calculated by using the Arrhenius equation (15):
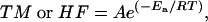 |
[7] |
where A is a constant, R is the gas constant, and T is temperature (in K). The slope of the natural log of the rate against T-1 equals -Ea/R. Data from August (29°C) were not included in calculations of Ea for the control bioreactors given the sharp decline in rates and observed carbon limitation of the microbial population (see Results). When rates in control bioreactors were calculated on a per microgram of PLFA basis for all time points (including August), plots of ln(TM) and ln(HF) vs. T-1 were linear (r2 ≈ 0.9), and the Ea of TM was still ≈50% that of HF.
Results
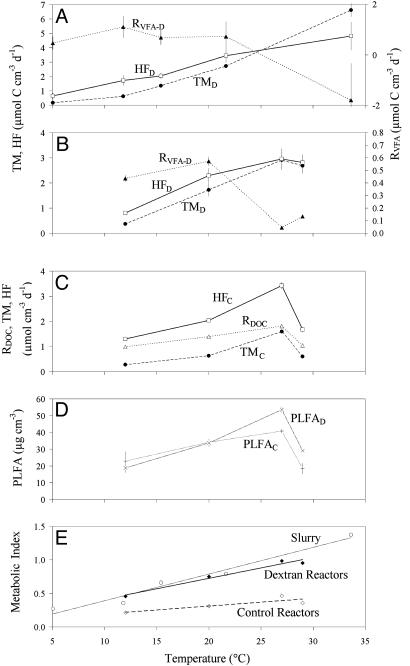
In the slurry experiment, rates of both potential hydrolysis/fermentation and potential terminal metabolism, mainly (97%) sulfate reduction, increased strongly above ≈20°C, whereas the net rate of VFA production decreased with increasing temperature (Fig. 2A). From 10% (at 5°C) to >90% (at 34°C) of the added dextran-carbon was converted to VFAs, mainly acetic and propionic acids, which together accounted for >80% of the net VFA production. Hydrolysis/fermentation and terminal metabolism exhibited significantly different temperature responses. Rates of hydrolysis/fermentation exceeded rates of terminal metabolism at temperatures <25°C (p < 0.05; differences between means were evaluated with a Student t test). At 25°C, rates of terminal metabolism and hydrolysis/fermentation were equal, and at temperatures exceeding 25°C, rates of terminal metabolism exceeded rates of hydrolysis/fermentation (p < 0.05).
Fig. 2.
Rates, microbial abundance, and the metabolic index (MI). Net rates (±SD) of hydrolysis/fermentation (HF; □), terminal metabolism (TM; •), and VFA (RVFA; ▴) or DOC (RDOC; ▵) production in slurries (A) and bioreactors [dextran amended (B) and the control (C)]. Rates in dextran bioreactors reflect rates of dextran breakdown. Rates in control bioreactors reflect particulate organic matter breakdown. (D) Sediment PLFA content (±SD) in bioreactors [dextran (PLFAD; ×) and the control (PLFAC; +)]. (E) MI reflects the ratio of rates of TM to HF in slurries (○) and bioreactors [dextran (♦) and the control (⋄)].
Results from the bioreactor experiments validated the slurry results (Fig. 2 B and C). Potential rates of hydrolysis/fermentation of the added high-molecular weight dextran were significantly greater than rates of terminal metabolism at 12°C and 20°C (p < 0.05), resulting in the net VFA production. Acetic and propionic acids accounted for >80% of the VFAs produced in dextran-amended bioreactors, echoing the results obtained in the slurry experiment. In control bioreactors, net rates of hydrolytic/fermentative DOC production from particulate organic matter and terminal metabolism increased between 12°C and 27°C (Fig. 2C). Rates of terminal metabolism, hydrolysis/fermentation, and net DOC production declined sharply from 27°C to 29°C (May to August).
Bacterial PLFAs were used to evaluate changes in microbial abundance. PLFA concentrations increased in control bioreactors from winter to early summer (Fig. 2D). In late summer, a marked drop in microbial biomass coincided with decreased rates of terminal metabolism, hydrolysis/fermentation, and net DOC production (Fig. 2C). A significant increase (p < 0.05) in PLFA abundance was observed in dextran-amended bioreactors in May (30% increase) and August (>50% increase) (Fig. 2D).
The efficiency of coupling between hydrolysis/fermentation and terminal metabolism is described here by the metabolic index (MI), which reflects the ratio of the respective process rates [MI = (TM/HF)]. As MI approaches zero, VFA production outpaces consumption, leading to the net accumulation of VFAs. When production and consumption of VFAs are tightly coupled, there is little or no net VFA production, and MI approaches 1. At temperatures <14°C, MI was ≈0.5 in both slurries and dextran-amended bioreactors, indicating that rates of dextran hydrolysis/fermentation were twice the rates of terminal metabolism (Fig. 2E). At temperatures >25°C, MI either approached (bioreactor) or exceeded (slurry) 1 (Fig. 2E). MI in control bioreactors was always <0.5 (Fig. 2E). The apparent activation energy, Ea, for terminal metabolism by sulfate reduction was similar between experiments [89.2 (slurry), 82.7 (control), and 85.5 (dextran) kJ·mol-1; average = 85.8 kJ·mol-1] and was significantly higher (p < 0.05) than Ea for hydrolysis/fermentation [47.8 (slurry), 45.6 (control), and 53.4 (dextran) kJ·mol-1; average = 48.9 kJ·mol-1)].
Discussion
Marine sediments are a globally significant reservoir of organic carbon. Organic matter mineralization, which influences the long-term burial of organic carbon and, thereby, regulates atmospheric oxygen concentrations (20), requires coupling between several functional groups of microorganisms (21). In this article, we report the unexpected discovery of temperature-driven decoupling of key microbial groups involved in organic carbon mineralization. We observed a greater temperature sensitivity of sulfate-reducing bacteria, whose activity dominates the anaerobic terminal metabolic pathway in marine sediments, than the temperature sensitivity of the microorganisms involved in the hydrolysis/fermentation of complex organic matter. This variable temperature control on rates of hydrolysis/fermentation vs. rates of terminal metabolism resulted in net VFA production at temperatures <25°C and net VFA consumption at temperatures >25°C, as evidenced by the LMW-DOC limitation of sulfate-reducing terminal metabolizers during summer.
Organic carbon limitation of sulfate-reducing bacterial activity during early and late summer was apparent from the MI and PFLA data obtained in dextran-amended bioreactors. During colder winter months, MI in dextran-amended and control bioreactors was ≈0.5, showing that rates of hydrolysis/fermentation exceeded those of terminal metabolism by about a factor of 2. In contrast, during summer, MI in dextran-amended bioreactors was ≈1, whereas MI in control bioreactors remained at ≈0.5. In the dextran-amended bioreactors, MI could not be >1 because rates were control-corrected and the VFA substrates for terminal metabolism were derived exclusively from dextran hydrolysis/fermentation. In slurries amended with acetate, however, terminal metabolism rates reflected potential activity, and MI exceeded 1, suggesting that potential terminal metabolic rates exceeded those of hydrolysis/fermentation.
The MI in control bioreactors was always <0.5, meaning that terminal metabolizers consumed <50% of the DOC generated by hydrolysis/fermentation of natural particulate organic matter. The close coupling of hydrolysis/fermentation and terminal metabolism in the dextran-amended bioreactors when T was >25°C showed that the sediment microbial community was capable of the rapid oxidation of labile LMW-DOC. Therefore, we hypothesize that much of the DOC produced in control bioreactors was refractory and unavailable to terminal metabolizers on the time scale of these experiments.
The significant (p < 0.05) increase of microbial abundance in response to dextran addition, evidenced by the increase in sediment PLFA content (Fig. 2D), corresponded to times when MI in dextran-amended bioreactors was ≈1, indicating the rapid and complete consumption of fermentation products by terminal metabolizers (Fig. 2E). We interpret this carbon-stimulated increase in PFLA to be indicative of carbon limitation of sediment microbes in early and late summer. In winter and early spring, no such increase in PFLA content in the dextran-amended sediments was observed, suggesting that the microbial population was not limited by organic carbon availability during the colder months.
The Ea values reported for sulfate reduction (82.7-89.2 kJ·mol-1) in this article are similar to those reported in the literature (36-132 kJ·mol-1) (12, 15). Similarly, the Ea values we report for hydrolysis/fermentation of organic matter (45.6-53.4 kJ·mol-1) are similar to those reported for organic matter degradation in marine sediments (54-125 kJ·mol-1) (22). However, our results differentiate the Ea value for terminal metabolic microbial populations vs. hydrolytic/fermentative microbial populations. Most geochemical models assume that the Ea value of anoxic terminal metabolism reflects the response of the entire microbial community (15). The data presented here indicate that this approach is not necessarily valid because the functional groups mediating distinct phases of organic matter mineralization may exhibit different temperature responses.
Differential temperature control of the hydrolytic/fermentative and terminal metabolic microbial communities provides a clear mechanism for decoupling the production and consumption of labile LMW-DOC intermediates. This phenomenon is consistent with the observed (23, 24) accumulation of labile VFAs in anoxic marine sediments for which no mechanism had been identified (25). Although shifts in the microbial community composition could explain varying patterns of substrate abundance (26), VFA accumulation at low temperatures in the absence of apparent microbial population transitions or substrate limitations has been documented (6, 23-25, 27), and differential temperature responses of functional microbial groups can explain such results. The temperature-limited capacity of the hydrolytic/fermentative production of labile LMW-DOC during the summer months may generate a “bottleneck” in organic matter mineralization, leading to carbon limitation of the terminal metabolic microbial community at certain times of year.
Anoxic habitats occur throughout the aquatic and terrestrial biospheres, and processing of organic matter in these habitats contributes substantially to the global cycling of carbon, nutrients, and other elements (Fig. 1) (2, 7, 9, 20, 21). Variable temperature responses of key functional microbial components may be a fundamental feature of the anoxic microbial community involved in organic matter mineralization and has significant implications for organic matter turnover within and across ecosystems and geographic zones and with respect to global climate change. Rates of methanogenesis, the dominant terminal metabolic pathway in freshwater environments, increase with increasing temperature (25, 28, 29). Acetate accumulation in freshwater methanogenic environments at a low temperature (25) may reflect temperature-driven decoupling of hydrolysis/fermentation and methanogenic terminal metabolism. Although the response of methanogenesis to temperature changes may be complicated by changes in the relative importance of different methanogenic pathways or methanogen population shifts (25, 28), the available data show clearly that methanogenesis rates are sensitive to changes in temperature; therefore, temperature-driven decoupling of production and consumption of LMW-DOC may occur in freshwater environments as well as in coastal marine sediments.
Whether the temperature-driven decoupling for temperate marine sediments applies to other geographic zones (e.g., tropical environments) remains to be determined. The data presented here show clearly that small changes in temperature affect the efficiency of organic matter turnover in anoxic marine sediments. Differential temperature regulation of key phases of organic matter mineralization may also influence system-level coupling between other processes [e.g., when water column primary production depends on regenerated inorganic nutrients derived from sediment mineralization of organic matter (Fig. 1)].
Acknowledgments
We thank D. Albert, J. Dai, H. Ding, M. Erickson, J. Menken, W. Porubsky, T. Roberts, V. Samarkin, and M. Sun for analytical assistance and C. Meile, H. Schulz, J. Edmonds, T. Hollibaugh, P. Megonigal, M. Moran, B. Orcutt, and two anonymous reviewers for providing comments that substantially improved the paper. The data presented here are part of a Ph.D. dissertation by N.B.W. This work was supported by the Georgia Sea Grant Program and by the National Science Foundation's Long-Term Ecological Research Program.
Author contributions: N.B.W. and S.B.J. designed research; N.B.W. performed research; and N.B.W. and S.B.J. wrote the paper.
Conflict of interest statement: No conflicts declared.
Abbreviations: LMW, low molecular weight; DOC, dissolved organic carbon; VFA, volatile fatty acid; DIC, dissolved inorganic carbon; PLFA, phospholipid fatty acid; MI, metabolic index.
References
- 1.Weiss, M. S., Abele, U., Weckesser, J., Welte, W., Schiltz, E. & Schultz, G. E. (1991) Science 254, 1627-1630. [DOI] [PubMed] [Google Scholar]
- 2.Fenchel, T. M. & Findlay, B. J. (1995) Ecology and Evolution of Anoxic Worlds (Oxford Univ. Press, London).
- 3.Arnosti, C., Repeta, D. J. & Blough, N. V. (1994) Geochim. Cosmochim. Acta 58, 2639-2652. [Google Scholar]
- 4.Burdige, D. J., Skoog, A. & Gardner, K. (2000) Geochim. Cosmochim. Acta 64, 1029-1041. [Google Scholar]
- 5.Sørensen, J. Christensen, D. & Jørgensen, B. B. (1981) Appl. Environ. Microbiol. 42, 5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wellsbury, P. & Parkes, R. J. (1995) FEMS Microbiol. Ecol. 17, 85-94. [DOI] [PubMed] [Google Scholar]
- 7.Middelburg, J. J., Soetaert, K. & Herman, P. M. J. (1997) Deep-Sea Res. 44, 327-344. [Google Scholar]
- 8.Wollast, R. (1991) in Ocean Margin Processes in Global Change, eds. Mantoura, R. F. C., Martin, J. M. & Wollast, R. (Wiley, Chichester, U.K.), pp. 365-381.
- 9.Canfield, D. E. (1993) in Interactions of C, N, P and S Biogeochemical Cycles and Global Change, eds. Wollast, R., Mackenzie, F. T. & Chou, L. (Springer, Berlin), pp. 333-363.
- 10.King, G. M. (1988) Limnol. Oceanogr. 33, 376-390. [Google Scholar]
- 11.Roden, E. E. & Tuttle, J. H. (1993) Mar. Ecol. Prog. Ser. 93, 101-118. [Google Scholar]
- 12.Roden, E. E., Tuttle, J. H., Boynton, J. H. & Kemp, W. M. (1995) J. Mar. Res. 53, 799-819. [Google Scholar]
- 13.Pomeroy, L. R. & Wiebe, W. J. (2001) Aquat. Microb. Ecol. 23, 187-204. [Google Scholar]
- 14.Westermann, P. (1996) World J. Microbiol. Biotech. 12, 497-503. [DOI] [PubMed] [Google Scholar]
- 15.Westrich, J. T. & Berner R. A. (1988) Geomicrobiol. J. 6, 99-117. [Google Scholar]
- 16.Weston, N. B., Porubsky, W. P., Samarkin, V. A., Erickson, M., MacAvoy, S. E. & Joye, S. B. (2005) Biogeochemistry, in press.
- 17.Albert, D. B. & Martens, C. S. (1997) Mar. Chem. 56, 27-37. [Google Scholar]
- 18.Boschker, H. T. S., de Brouwer, J. F. C. & Cappenberg, T. E. (1999) Limnol. Oceanogr. 44, 309-319. [Google Scholar]
- 19.Roychoudhury, A. N., Viollier, E. & Van Cappellen, P. (1998) Appl. Geochem. 13, 269-280. [Google Scholar]
- 20.Berner, R. A. (2003) Nature 426, 323-326. [DOI] [PubMed] [Google Scholar]
- 21.Megonigal, J. P., Hines, M. E. & Visscher, P. T. (2005) in Biogeochemistry, Treatise on Geochemistry, ed. Schlesinger, W. H. (Elsevier, Amsterdam), Vol. 8, pp. 317-424. [Google Scholar]
- 22.Middelburg, J. J., Klaver, G., Nieuwenhuize, J., Wielemaker, A., de Haas, W., Vlug, T. & van der Nat, J. F. W. A. (1996) Mar. Ecol. Prog. Ser. 132, 157-168. [Google Scholar]
- 23.Wu, H., Green, M. & Scranton, M. I. (1997) Limnol. Oceanogr. 42, 705-713. [Google Scholar]
- 24.Albert, D. B., Taylor, C. & Martens, C. S. (1995) Deep-Sea Res. 42, 1239-1260. [Google Scholar]
- 25.Fey, A. & Conrad, R. (2000) Appl. Environ. Microbiol. 66, 4790-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoehler, T. M., Albert, D. B., Alperin, M. J. & Martens, C. S. (1999) Limnol. Oceanogr. 44, 662-667. [Google Scholar]
- 27.Arnosti, C. & Jørgensen, B. B. (2003) Mar. Ecol. Prog. Ser. 249, 15-24. [Google Scholar]
- 28.Conrad, R. (2002) Nutr. Cycl. Agroecosys. 64, 59-69. [Google Scholar]
- 29.Nozhevnikova, A. N., Holliger, C., Ammann, A. & Zehnder, A. J. B. (1997) Water Sci. Tech. 36, 57-64. [Google Scholar]