Abstract
The virulence of different isolates of Mycobacterium has been associated with two morphologically distinguishable colonial variants: opaque (SmOp) and transparent (SmTr). In this report we used an in vitro assay to compare macrophage (Mφ) responses to SmOp and SmTr Mycobacterium fortuitum variants, taking advantage of the fact that these variants were derived from the same isolate. Cells preactivated or not with gamma interferon (IFN-γ) were infected with SmOp or SmTr M. fortuitum. We showed that SmOp and SmTr induced different levels of nitric oxide (NO) production by IFN-γ-stimulated Mφ. Indeed, the amount of IFN-γ-induced NO production by J774 cells was 4.8 to 9.0 times higher by SmOp (23.1 to 37.7 μM) compared to SmTr infection (3.9 to 4.8 μM) (P = 0.0332), indicating that virulent SmTr bacilli restricted NO production. In addition, IFN-γ-induced NO production by Mφ was higher when correlated with reduction of only avirulent SmOp bacillus viability. SNAP (S-nitroso-N-acetyl-dl-penicillamine)-induced NO production did not modify SmTr viability, indicating its resistance to nitrogen radicals. Electron microscopy studies were performed to evaluate the capacity of phagosomes to fuse with lysosomes labeled with bovine serum albumin-colloidal gold particles. By 24 h postinfection, 69% more phagosome-containing SmOp variant had fused with lysosomes compared to the SmTr-induced phagosomes. In conclusion, these data indicate that virulent SmTr bacilli may escape host defense by restricting IFN-γ-induced NO production, resisting nitrogen toxic radicals, and limiting phagosome fusion with lysosomes.
Fast-growing mycobacteria have increasingly been recognized as a human pathogen rather than mere colonizers. Among the rapidly growing mycobacteria found in abundance in the environment, strains associated with human diseases are restricted mainly to the Mycobacterium fortuitum-Mycobacterium chelonae complex (42). Human diseases are most commonly caused by accidental inoculation of contaminated materials (16). Disseminated cutaneous lesions have been frequently associated with immunosuppression (17, 18, 41). It has been noted that treatment of infections due to nontuberculous mycobacteria remains difficult, in part because mycobacteria are resistant to many of the first-line tuberculosis agents and in part because so few other agents are available for therapy (40).
Mycobacterium virulence is variable, depending not only on species but also on strains of a given species (3). Several studies have shown that mycobacteria, which grow on a solid medium, yield distinguishable colonial morphotypes. The smooth, flat, transparent variant (SmTr) has been associated with greater virulence (23, 31) and lower susceptibility to many antimicrobial agents, whereas the smooth, domed, opaque (SmOp) colony is avirulent (31, 34). Few studies have compared the virulence levels of different colonial variants of a single Mycobacterium isolate (21, 22), and until now no study has been performed to define M. fortuitum virulence determinants.
Pathogenic mycobacteria, if not destroyed by the host innate immune defense, survive and multiply inside macrophages (Mφ) within membrane-bound compartments that do not acidify and have a restricted ability to fuse with lysosomes (35). Host cells can also participate in the effective phase of the infection, acting in the modulation of innate and acquired immune responses (13, 24). Mφ increase their antimycobacterial activity when activated by IFN-γ (29), a cytokine produced by both natural killer cells and T helper-1 lymphocytes (39). This activity implies the induction of microbicidal molecules such as NO by Mφ (1, 6, 10). Furthermore, it has been demonstrated that IFN-γ associated with lipopolysaccharide (LPS) can induce the maturation of Mycobacterium phagosomes to phagolysosomes in mouse macrophages (32, 38).
In the present study, we postulated that the ability of mycobacteria to live inside phagosomes of infected Mφ is dependent on bacillus virulence. In order to investigate this possibility, we used an in vitro assay to compare very close microorganisms, the SmTr and SmOp of a single M. fortuitum isolate, with regard to their ability to infect Mφ, to inhibit Mφ-induced microbicidal molecules such as NO, and to restrict the fusion of their vacuoles with lysosomes. In contrast to SmOp, the more virulent SmTr M. fortuitum strain induced lower NO production in vitro by gamma interferon (IFN-γ)-activated Mφ as well as phagosomes with lower fusigenicity.
MATERIALS AND METHODS
Cells and culture medium.
The mouse Mφ cell line J774E clone (kindly provided by P. D. Stahl, Washington University) was maintained at 37°C in 5% CO2 in RPMI 1640 (Sigma, St. Louis, Mo.) supplemented with 10% fetal bovine serum (HyClone, Logan, Utah, or Gibco, Rockville, Md.), 2.0 g of sodium bicarbonate/liter, 25 mM HEPES, 1.8 μM 6-thioguanine, 100 IU of penicillin/ml, and 100 μg of streptomycin/ml (Sigma).
Bacteria.
The SmOp M. fortuitum variant was isolated from naturally infected C57BL/6 mice. To obtain SmTr, nude nu/nu mice were inoculated intravenously with 108 opaque colonial M. fortuitum variant bacteria. After 30 days of infection, bacteria were harvested from the livers and spleens of infected nude nu/nu mice, and only SmTr variant was grown on Middlebrook 7H10 agar plates (Difco Laboratories, Detroit, Mich.) supplemented with 10% oleic acid-albumin-dextrose-catalase (Difco Laboratories). Aliquots of SmOp or SmTr mycobacteria were frozen at −70°C. When required, the frozen samples were quickly thawed, vortex mixed, and adjusted to the desired titer in cell culture medium. Both M. fortuitum SmOp and SmTr variants were characterized by PCR. Briefly, a loopful of mycobacteria was suspended in 0.4 ml of TET (10 mM Tris-HCl [pH 7.5], 1 mM EDTA) with 1% Triton X-100 and subjected to three cycles of boiling and freezing at −20°C. Ten microliters of the lysates was used for the PCRs. This procedure was based on the enzymatic amplification of the hsp65 gene, followed by digestion with BstEII and HaeIII (36). Amplifications were performed in 50-μl volume reactions containing 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2, 10% glycerol, 200 μM concentrations of deoxynucleoside triphosphates, a 0.5 μM concentration of each primer (Tb11 [5′-ACCAACGATGGTGTCCAT] and Tb12 [5′-CTTGTCGAACCGCATACCCT]), and 1.25 U of Taq polymerase (Gibco, Grand Island, N.Y.). The amplification mixture was submitted to an initial denaturation at 95°C for 10 min; followed by 45 cycles of denaturation at 95°C for 1 min, annealing at 60°C for 1 min, and extension at 72°C for 1 min; followed in turn by a final extension step at 72°C for 7 min.
Cell activation and infection.
Cells (5 × 105 per ml) in complete medium without antibiotics (infection medium) were plated in 24-well culture plates (Costar, Cambridge, Mich.), containing or not containing 13-mm-diameter glass coverslips (Glasstécnica Imp., São Paulo, Brazil), in duplicates or triplicates. Before 24 h of M. fortuitum infection, J774 cells were pretreated or not pretreated with 50 to 400 IU of recombinant IFN-γ (rIFN-γ; PharMingen, San Diego, Calif., or R&D Systems, Minneapolis, Minn.)/ml in the absence or presence of 1 mM aminoguanidine (AMG; Sigma), a competitive inhibitor of nitric oxide synthase. For electron microscopy (EM) experiments, cells were plated in plastic culture petri dishes (35 mm; Costar). Thereafter, Mφ were infected for an additional 24 h with SmOp or SmTr M. fortuitum strains at a 10:1 mycobacterium/Mφ ratio. At 24 h postinfection, Mφ cultures were fixed with 2.5% glutaraldehyde in phosphate-buffered saline for 20 min, and then glass coverslips containing infected cells were allowed to air dry and were Ziehl-Neelsen stained as previously described (8). To determine the percentage of infected cells, triplicate coverslips for each treatment group were examined by light microscopy (magnification, ×1,000), and at least 200 Mφ were counted per coverslip.
Evaluation of bacillus viability.
At 24 h postinfection, culture supernatants were gently collected and infected J774 cells were washed twice with warm RPMI 1640 medium supplemented with 25 mM HEPES to remove noninternalized mycobacteria. In order to liberate intracellular bacilli, infected J774 cells were detached, counted, and then lysed by addition of saline containing 0.05% sodium dodecyl sulfate (SDS) and protease inhibitors (p-nitrophenyl p′-guanidinobenzoate, phenylmethylsulfonyl fluorete, Nα-p-tosyl-l-lysine chloromethyl ketone, and N-tosyl-l-phenylanine chloromethyl ketone) (Sigma) for 20 min at room temperature (RT). The number of viable mycobacteria per dish was determined by plating serial 10-fold dilutions of cell lysates on Middlebrook 7H10 agar (Difco Laboratories). Colonies were counted after incubation at 37°C for 10 to 15 days, and the results were expressed as the number of CFU per 105 cells.
Measurement of NO2−.
To detect NO production by Mφ, its stable end product, the NO2− content was determined by Griess reaction (15). Briefly, conditioned medium was collected and centrifuged (12,000 × g) for 3 min. Aliquots (50 μl) of conditioned medium were then distributed in a 96-well microtiter plate (Costar), and equal volumes of Griess reaction solution (1% sulfanilamide and 0.1% naphthyl-ethylenediamine in 2.5% phosphoric acid) were added. The reaction was allowed to proceed for 10 min at RT, and the optical density at 540 nm was measured. The amounts of NO in the samples were calculated by extrapolation from a sodium nitrite standard curve prepared for each experiment.
Quantification of phagosome-lysosome fusion by EM.
In order to label cell lysosomes, Mφ were incubated with 15 nM bovine serum albumin conjugated to colloidal gold particles (BSA-gold) for 3 h, and then the cells were washed, activated or not activated with IFN-γ as described above, and chased for an additional 18 h in infection medium at 37°C and 5% CO2. At 24 h postinfection, the cells were fixed and processed for EM as previously described (14). Briefly, infected cells were fixed at 4°C with 2.5% glutaraldehyde (Polyscience, Warrington, Pa.) in 0.1 mM cacodylate buffer (pH 7.2) plus 0.1 M sucrose, 5 mM CaCl2, and 5 mM MgCl2 (Sigma). After a 1-h treatment with wash buffer, cells were postfixed for 1 h at RT with 1% osmium tetroxide in 0.1 mM cacodylate buffer devoid of sucrose. They were then scraped off, concentrated in 2% agar in cacodylate buffer, and treated for 1 h at RT with 1% uranyl acetate in Veronal buffer. Samples were dehydrated in a graded series of acetone solutions and embedded in Epon. Thin sections were sequentially stained with 2% uranyl acetate in distillate water and lead citrate. As previously described, EM counting of BSA-gold-labeled phagosomes that fused with lysosomes was performed to assess fusion (11). Briefly, we performed two cuts 10 μm apart from each other, and at least 150 phagosomes were counted. Phagosome fusion with lysosomes was considered when at least one BSA-gold particle was found inside the vacuoles.
NO-releasing agent treatment. An NO-releasing agent, SNAP (S-nitroso-N-acetyl-dl-penicillamine), was purchased from Calbiochem (La Jolla, Calif.). SNAP was dissolved in dimethyl sulfoxide (Sigma) at a concentration of 100 mM, stored at −20°C, and freshly dissolved and diluted with RPMI 1640 immediately before addition to the cultures. Prior to 24 h of infection, 100 μM SNAP was added to the cultures.
Statistical analysis.
All experiments were done in duplicate or triplicate and independently repeated at least three times. The results of the experiments were expressed as the mean ± the standard error of the mean (SEM) of three or more experiments. The Student t test or one-way analysis of variance (ANOVA) was used to analyze significance. Linear regression analysis was used to determine the correlation between intracellular bacillus viability and NO production in response to different IFN-γ concentrations. A P value of <0.05 was considered significant.
RESULTS
Intracellular uptake of M. fortuitum SmOp and SmTr variants was similar in J774 cells.
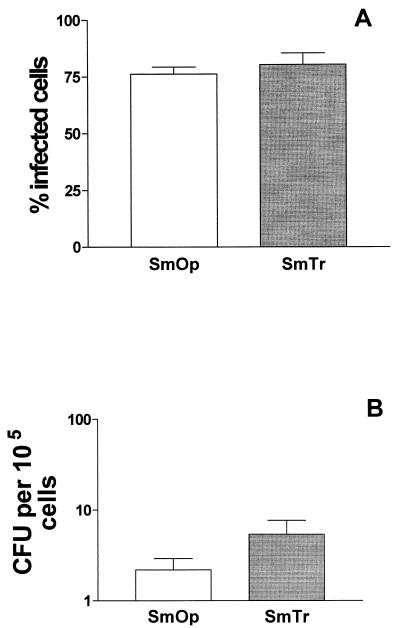
The SmOp and SmTr ability to infect resting J774 cells was evaluated. Cells were incubated with SmOp or SmTr variant, and 24 h later the percentage of infected cells was determined by optical microscopic counting. As shown in Fig. 1A, the percentages of SmOp- and SmTr-infected cells were similar: 76.2% ± 3.1% for SmOp-infected cells and 80.3% ± 5.1% for cells infected with SmTr bacilli.
FIG. 1.
Intracellular uptake of M. fortuitum SmOp and SmTr was similar in J774 cells. (A) Percentage of M. fortuitum SmOp- or SmTr-infected J774 cells. Cells were infected with SmOp or SmTr bacilli at a ratio of 10:1 and, 24 h later, fixed and Ziehl-Neelsen stained. Microscopic counting was performed to determine the percentage of SmOp- or SmTr-infected cells (P = 0.4892). Bars represent the mean ± the SEM of 12 and 5 independent experiments with SmOp and SmTr, respectively. (B) SmOp or SmTr viability in nonstimulated Mφ. Cells were infected as described above and lysed 24 h later. Cell supernatants were then serially diluted and plated in 7H10 Middlebrook medium. The M. fortuitum viability was determined by CFU counts after 10 days of plate incubation (P = 0.1565). Bars represent the mean ± the SEM of eight independent experiments with SmOp and six independent experiments with SmTr. P values were determined by the Student t test.
Although the percentages of Mφ infected with either SmOp or SmTr were similar, the infective capacity could only be assessed by determining the number of intracellular viable bacilli. At 24 h postinfection, cells were lysed to release intracellular mycobacteria, and an aliquot of the Mycobacterium-containing medium was serially diluted and plated on Middlebrook agar. Although there were 2.5 more SmTr (5.4 ± 2.3 CFU/105 Mφ) than SmOp (2.2 ± 0.7 CFU/105 Mφ) intracellular viable bacilli, this difference was not statiscally significant (Fig. 1B).
SmOp variant potentialized NO production by IFN-γ-treated J774 cells.
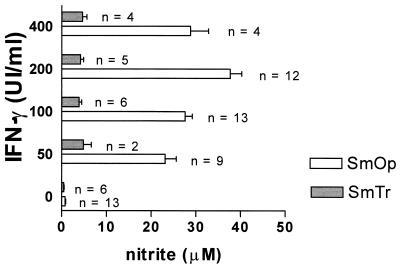
The ability of reactive nitrogen intermediates, such as NO, to inhibit Mycobacterium growth is controversial (2, 5) and has not been investigated in the control of fast-growing mycobacteria. The capacity of SmOp and SmTr variants to induce NO production by activated J774 Mφ cell line was examined. To induce NO, cells were treated with different concentrations of rIFN-γ, which ranged from 50 to 400 IU/ml, for 18 h and then infected with SmOp or SmTr colonies. At 24 h postinfection, the NO2− content was determined by using the Griess reaction. Control nonstimulated cells infected with SmOp (0.8 ± 0.5 μM) or SmTr (0.5 ± 0.3 μM) variants produced insignificant nitrite levels (Fig. 2). IFN-γ treatment greatly increased NO production only by cells infected with SmOp Mycobacterium variant (Fig. 2). Indeed, the amount of IFN-γ-induced NO production by J774 cells was 4.8 to 9.0 times higher with SmOp (23.1 to 37.7 μM) compared to SmTr infection (3.9 to 4.8 μM; P = 0.0332). Interestingly, NO levels produced by SmTr-infected cells pretreated with IFN-γ were similar to the levels produced by control noninfected IFN-γ-treated J774 cells (3.8 ± 1.2 μM, n = 8), indicating that only the SmOp variant potentialized IFN-γ-induced nitrogen radical production.
FIG. 2.
M. fortuitum SmOp and SmTr induced different levels of NO production by infected J774 cells. Cells were treated with different concentrations of rIFN-γ (50 to 400 IU/ml) for 18 h and then infected with SmOp or SmTr colonies. At 24 h postinfection, cell supernatants were collected, and the NO2− concentrations were determined by using the Griess reaction (P = 0.0332, ANOVA). Control cells treated with 100 IU of rIFN-γ/ml produced 3.8 ± 1.2 μM (n = 8) NO2−. The bars represent the mean ± the SEM of (n) independent experiments.
NO production by rIFN-γ-treated J774 cells resulted from iNOS activity, since 1 mM AMG completely abrogated NO production by activated Mφ infected with either M. fortuitum SmOp or SmTr (0.9 ± 0.4 or 1.6 ± 0.4 μM, respectively).
IFN-γ-induced NO reduced SmOp uptake.
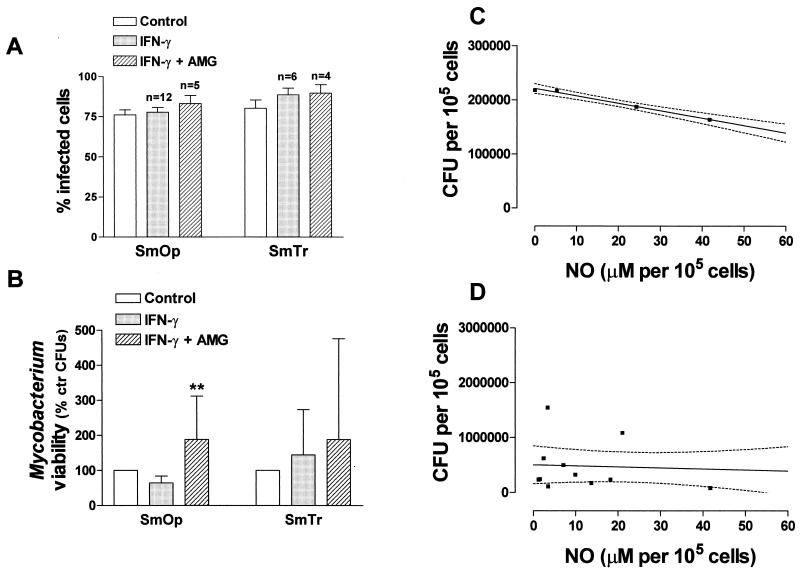
To determine whether IFN-γ-induced NO production by Mφ had any effect on the capacity to infect cells, we measured the percentage of infection and intracellular viability of SmOp or SmTr mycobacteria by adding rIFN-γ associated or not associated with AMG. Neither rIFN-γ addition nor NO inhibition affected the percentage of both SmOp- and SmTr-infected Mφ (Fig. 3A). Intracellular viability of the two variants was then assessed in control and IFN-γ-treated cells. IFN-γ treatment decreased SmOp viability in seven of eight experiments. However, the reduction of 35.8% ± 7.0% (n = 8) in viability was not statistically significant compared to nontreated controls. AMG was then added to rIFN-γ-treated cells, and CFU were determined 24 h postinfection. Interestingly, NO inhibition led to increases of 88.4% ± 43.8% in SmOp intracellular viability compared to bacillus viability in control nontreated Mφ and of 169.4% ± 66.3% compared to cells only pretreated with IFN-γ (P = 0.0075, ANOVA), indicating NO participation in SmOp IFN-γ-induced killing (Fig. 3B). A quantitative assessment of the strength of the association between SmOp intracellular viability and NO production in response to different IFN-γ concentrations was made via linear regression analysis. The reduction in SmOp intracellular viable bacilli in the first moment after infection was highly correlated with the level of produced NO by IFN-γ-stimulated J774 cells (r = 0.9961 and P = 0.0039; Fig. 3C). On the other hand, SmTr viability was not affected by treatment with either IFN-γ alone or IFN-γ plus AMG (Fig. 3B), and there was no correlation between the numbers of SmTr intracellular bacilli and NO production in response to IFN-γ treatment (r = 0.1293 and P = 0.6737), indicating SmTr prevention to induce reactive nitrogen radicals (Fig. 3D).
FIG. 3.
IFN-γ-induced NO reduced SmOp uptake. (A) Percentage of J774 infected cells, treated or not treated with either rIFN-γ (100 IU/ml) alone or with rIFN-γ plus AMG (1 mM). Cells were treated, infected, and stained as indicated in the legend for Fig. 1. The P values were 0.4669 for SmOp-infected cells and 0.2623 for SmTr-infected cells (ANOVA; n = number of experiments). (B) SmOp or SmTr viability in J774 cells pretreated with rIFN-γ alone or with rIFN-γ plus AMG. Cells were infected and lysed, and cell supernatants were plated in agar as indicated in the legend for Fig. 1. Control viability was considered 100% (2.2 ± 0.7 CFU/105 cells for SmOp-infected cells and 5.4 ±2.3 CFU/105 cells for SmTr-infected cells). The results represent the percentage of viability related to control cells (P = 0.0075 and P = 0.7066 [ANOVA] for SmOp and SmTr, respectively). The bars represent the mean ± the SEM of six independent experiments for SmOp-infected cells and eight independent experiments for SmTr-infected cells. (C) A strong linear correlation between NO produced by SmOp-infected J774 cells pretreated with 50 to 200 IU of IFN-γ/ml and the number of intracellular viable bacilli (CFU/105 cells) was demonstrated. Each point represents the mean of two to eight independent experiments (r = 0.9961 and P = 0.0039). (D) Absence of linear correlation between NO produced by SmTr-infected J774 cells pretreated with 100 IU of IFN-γ/ml and the number of intracellular viable bacilli (CFU/105 cells). Each point represents one experiment (n = 13, r = 0.1293, and P = 0.6737).
Since rIFN-γ-induced NO had a consistent but slight effect on SmOp and no effect on SmTr infection, we sought to determine whether higher NO concentrations could decrease intracellular Mycobacterium viability. To address this issue, cells were treated with SNAP, an exogenous NO donor. At 24 h postinfection, the NO concentration achieved higher levels in SNAP-treated cells than in rIFN-γ-treated cells (108.7 μM [n = 5] and 208.5 μM [n = 2] for SmTr and SmOp, respectively). SNAP treatment reduced SmOp intracellular viability by 70.7% ± 29.3% (n = 2) compared to control cells. On the other hand, SmTr viability was even slightly higher (21.5% ± 48.0, n = 5) in response to elevated NO concentrations in SNAP-treated cells.
SmOp and SmTr colonies induced phagosomes with different fusion capacities in the J774 cell line.
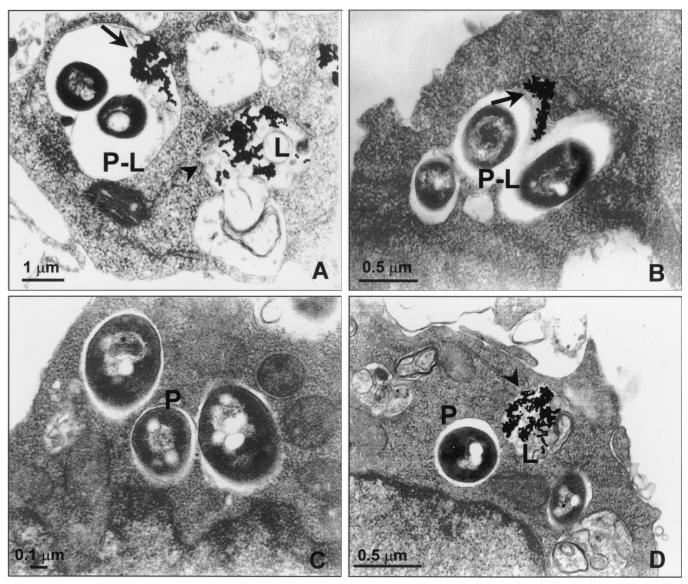
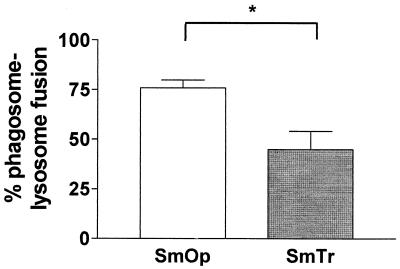
EM observations showed that there were morphological differences between SmOp- and SmTr-induced phagosomes. SmOp bacteria were found in large phagocytic vacuoles, whereas the SmTr bacilli were observed within tight phagosomes (Fig. 4). Since it has been demonstrated that Mycobacterium survival is related to restricted phagosome-lysosome fusion (35), we sought to determine whether there were differences between SmOp- and SmTr-induced phagosomes with regard to their ability to fuse with lysosomes. EM counting of BSA-gold-labeled phagosomes that fused with lysosomes was performed to assess fusion. Interestingly, the extent of fusion of SmOp variant-containing phagosomes with lysosomes was 69% higher than for those induced by SmTr colonies in resting Mφ (P = 0.0409) (Fig. 5). Surprisingly, although IFN-γ-induced NO production was able to reduce SmOp viability, IFN-γ addition to the cultures did not modify the fusion ability of either SmOp- or SmTr-containing phagosomes with lysosomes. In addition, AMG added to IFN-γ-treated cells did not modify phagosome-lysosome fusion despite the restoration of SmOp intracellular viability (Table 1).
FIG. 4.
Ultrastructural characterization of SmOp- and SmTr-induced phagosomes. To label lysosomes, J774 cells were incubated with 15-nm BSA-gold for 3 h and then washed and infected with SmOp or SmTr variants of M. fortuitum. At 24 h postinfection, cells were fixed and processed for EM as indicated in Material and Methods. (A and B) J774 cells infected with SmOp in phagosomes that fuse with BSA-gold labeled lysosomes. (C and D) J774 cells infected with SmTr inside phagosomes that restrict fusion with lysosomes. Arrows (panels A and B), BSA-gold inside phagolysosomes; arrowheads (panels A and D), BSA-gold inside lysosomes; P, phagosomes; P-L, phagolysosomes; L, lysosomes.
FIG. 5.
SmOp- and SmTr-induced phagosomes with different fusion capacities in the J774 cell line. Cells were cultured, infected, and processed for EM as indicated in Fig. 4. After EM, we performed two cuts 10 μm apart from each other for each sample, and at least 150 phagosomes were counted. Phagosome fusion with lysosomes was considered when at least one BSA-gold particle was found inside vacuoles. The percentage of phagosomes that fused with lysosomes was quantified. The graph shows that SmOp variant-containing phagosomes have a greater ability to fuse with lysosomes compared to those induced by SmTr colonies. Bars represent mean ± SEM of three independent experiments with SmOp and four independent experiments with SmTr (P = 0.0409). The P value was determined by using the Student t test.
TABLE 1.
Treatment with IFN-γ or with IFN-γ plus AMG did not modify phagosome-lysosome fusion
| Treatment | Mean % phagosome-lysosome fusion ± SEM (n)a
|
|
|---|---|---|
| SmOp | SmTr | |
| Control | 75.90 ± 3.87 (3) | 44.88 ± 9.18 (4) |
| IFN-γ | 72.57 ± 6.94 (3) | 46.83 ± 9.63 (4) |
| IFN-γ + AMG | 69.00 ± 6.50 (2) | 47.90 ± 11.52 (3) |
Values are means ± the SEM (n = number of experiments) of the percentage of fusion of SmOp- or SmTr-induced phagosomes with BSA-gold-labeled lysosomes in the J774 Mφ cell line treated with IFN-γ alone (100 IU/ml) or with IFN-γ plus AMG (1 mM). After EM, phagosome fusion with lysosomes was assessed as indicated in the legend for Fig. 5. We did not detect statistical differences between control nontreated cells and IFN-γ- or IFN-γ-AMG-treated cells. P values were 0.7457 for SmOp-infected cells and 0.9774 for SmTr-infected cells (ANOVA).
DISCUSSION
In the present study we used an in vitro assay to compare Mφ responses to two different M. fortuitum colony morphotypes, taking advantage of the fact that they were derived from the same isolate. The association of distinct colonial morphotypes and bacillus virulence has been already described, although most of the observations compared relative virulence from different isolates (9, 23, 30). In the present study, the more virulent SmTr morphotype was converted from an SmOp M. fortuitum that has been used to infect nu/nu BALB/c mice. As demonstrated for both M. avium and M. tuberculosis (25-27, 31, 33, 34), we showed that the M. fortuitum variants described here also displayed distinct virulence profiles. Virulence depended, at least in part, on the capacity of bacilli to prevent NO production by activated Mφ and on the nature of the compartment they induced in infected Mφ. The possible mechanisms to explain these differences have not been investigated but may be associated with distinct cell wall components, such as acidic polysaccharide present in the virulent bacillus that is absent in the avirulent bacillus and in the recently described virulent bacillus inhibition of signaling pathways that results in decreased production of host mycobacterial killing mechanisms (28, 37).
Both M. fortuitum SmOp and SmTr variants were able to infect the J774 Mφ cell line. Although we detected almost three times more SmTr intracellular viable mycobacteria than SmOp bacilli in infected Mφ, this difference was not statistically significant. Previous studies comparing macrophage ability to phagocytose mycobacteria displaying distinct virulence showed no differences at 24 h postinfection in Mφ ability to take up bacilli and in the number of intracellular viable bacilli. At 4 to 7 days after infection, however, the number of virulent mycobacteria was higher than the number of avirulent mycobacteria (9, 10, 21, 26). Since our aim was to study the early stages of infection, we did not evaluate later times and cannot exclude that similar differences exist in our in vitro model.
We showed for the first time that SmOp and SmTr variants induced different levels of NO production by IFN-γ-stimulated Mφ and that IFN-γ treatment only reduced SmOp intracellular viability. Indeed, IFN-γ-induced NO production by cells infected with avirulent bacilli was significantly greater than by cells infected with virulent mycobacteria and decreased by 36% the intracellular viability of SmOp variant, an effect completely reverted by AMG. Furthermore, the amount of NO produced by cells treated with IFN-γ strongly correlated with a decrease in SmOp intracellular viability (r = 0.9961 and P = 0.0039). In addition, SmOp and not SmTr intracellular viability was affected in the presence of higher NO concentrations. Together, these data suggest that SmTr virulence depends on SmTr prevention to induce reactive nitrogen intermediates by Mφ. It is possible that ligands present in the SmOp cell wall but absent in the SmTr cell wall interact with receptors in the Mφ surface and then transduce a second signal responsible for induction of higher NO production. In addition, this interaction can induce Mφ to produce cytokines known to potentialize NO production by Mφ (19, 20). In SmTr resistance to nitrogen intermediates, it has been demonstrated that M. avium-M. intracellulare complex strains and a pathogenic M. tuberculosis isolate express the noxRI gene that confers bacillus resistance to reactive nitrogen intermediates (12).
In experiments to determine the nature of SmOp- and SmTr-induced phagosomes, we demonstrated that, by 24 h postinfection, 69% more phagosome-containing SmOp bacilli had fused with lysosomes versus the SmTr-induced phagosomes. These data demonstrated that SmTr-containing phagosomes, compared to SmOp-induced phagosomes, more strongly restricted fusion with lysosomes. Our data are in agreement with studies showing that phagosome-containing dead bacilli have greater fusion capacity than those containing live mycobacteria (4, 7) and that pathogenic mycobacteria primarily reside in nonacidified, nonlysosomal compartments (38). In this report the avirulent M. fortuitum variant lived in phagosomes that progressed more rapidly through the endocytic pathway to fuse with lysosomes than the virulent variant. It is important to note that phagosomes containing the virulent M. fortuitum bacilli displayed greater fusion capacity with lysosomes compared to what has been described about fusion capacity of phagosomes containing M. tuberculosis or M. avium (35, 43). This can be explained by the fact that these mycobacteria are known to be more virulent than M. fortuitum. Together, these data suggest that fusion capacity with lysosomes is an important parameter to define not only Mycobacterium viability (38) but also the virulence of mycobacteria derived from the same isolate.
Neither the addition of IFN-γ nor the inhibition of NO production by AMG modified the fusion ability of virulent and avirulent phagosomes with lysosomes. Schaible et al. (32) demonstrated that the addition of IFN-γ and lipopolysaccharide to M. avium-infected cells induced phagosomes that reduced intraphagosomal pH in correlation with an increased accumulation of proton-ATPases without any change in Mycobacterium viability. These authors concluded that in their system the functional translocation toward more lysosomal compartments appeared to precede any marked drop in microbial viability, suggesting that IFN-γ plus lipopolysaccharide-induced phagosome-lysosome fusion is the product of an alteration in Mφ physiology rather than a consequence of microbial death (32). Our results indicate that in cells infected with avirulent SmOp, NO produced in response to IFN-γ-mediated activation was able to inhibit intracellular bacillus viability before any enhancement in phagosomal fusigenicity with lysosomal compartments. Furthermore, by 24 h postinfection with virulent SmTr M. fortuitum, IFN-γ treatment neither modified bacillus viability nor phagosome fusion with lysosomes. The differences between our findings and the previous study (32) may be due to the use of different Mycobacterium species with different virulence profiles.
In conclusion, we demonstrated for the first time that in an early phase of infection virulent bacilli, in contrast to avirulent bacilli, from a single isolate escape host defense by resisting NO, failing to induce nitrogen toxic radicals, and restricting phagosome maturation to phagolysosomes, host responses known to be important for controlling Mycobacterium infection.
Acknowledgments
This study was supported by grant 520630/96-3 from Conselho Nacional de Desenvolvimento Tecnológico, Brazil (CNPq).
We are grateful to Cláudia Ida Brodskyn and Manoel Barral-Netto for critically reading the manuscript. We thank Washington Luis Conrado dos Santos for invaluable discussions and Chantal de Chastellier for invaluable suggestions for the EM experiments.
Editor: J. D. Clements
REFERENCES
- 1.Adams, L. B., S. G. Franzblau, Z. Vavrin, J. B. J. Hibbs, and J. L. Krahenbuhl. 1991. l-Arginine-dependent macrophage effector functions inhibit metabolic activity of Mycobacterium leprae. J. Immunol. 147:1642-1646. [PubMed] [Google Scholar]
- 2.Appelberg, R., and I. M. Orme. 1993. Effector mechanisms involved in cytokine-mediated bacteriostasis of Mycobacterium avium infections in murine macrophages. Immunology 80:352-359. [PMC free article] [PubMed] [Google Scholar]
- 3.Appelberg, R., A. Sarmento, and A. G. Castro. 1995. Tumour necrosis factor-alpha (TNF-α) in the host resistance to mycobacteria of distinct virulence. Clin. Exp. Immunol. 101:308-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker, L. P., K. M. George, S. Falkow, and P. L. Small. 1997. Differential trafficking of live and dead Mycobacterium marinum organisms in macrophages. Infect. Immun. 65:1497-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bermudez, L. E. 1993. Differential mechanisms of intracellular killing of Mycobacterium avium and Listeria monocytogenes by activated human and murine macrophages: the role of nitric oxide. Clin. Exp. Immunol. 91:277-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan, J., Y. Xing, R. S. Magliozzo, and B. R. Bloom. 1992. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 175:1111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clemens, D. L., and M. A. Horwitz. 1995. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J. Exp. Med. 181:257-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crowle, A. J., and M. May. 1981. Preliminary demonstration of human tuberculoimmunity in vitro. Infect. Immun. 31:453-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowle, A. J., A. Y. Tsang, A. E. Vatter, and M. H. May. 1986. Comparison of 15 laboratory and patient-derived strains of Mycobacterium avium for ability to infect and multiply in cultured human macrophages. J. Clin. Microbiol. 24:812-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denis, M. 1991. Interferon-gamma-treated murine macrophages inhibit growth of tubercle bacilli via the generation of reactive nitrogen intermediates. Cell. Immunol. 132:150-157. [DOI] [PubMed] [Google Scholar]
- 11.Desjardins, M., N. N. Nzala, R. Corsini, and C. Rondeau. 1997. Maturation of phagosomes is accompanied by changes in their fusion properties and size-selective acquisition of solute materials from endosomes. J. Cell Sci. 110:2303-2314. [DOI] [PubMed] [Google Scholar]
- 12.Ehrt, S., M. U. Shiloh, J. Ruan, M. Choi, S. Gunzburg, C. Nathan, Q. Xie, and L. W. Riley. 1997. A novel antioxidant gene from Mycobacterium tuberculosis. J. Exp. Med. 186:1885-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flesch, I. E., J. H. Hess, I. P. Oswald, and S. H. Kaufmann. 1994. Growth inhibition of Mycobacterium bovis by IFN-γ stimulated macrophages: regulation by endogenous tumor necrosis factor-alpha and by IL-10. Int. Immunol. 6:693-700. [DOI] [PubMed] [Google Scholar]
- 14.Frehel, C., C. Offredo, and C. de Chastellier. 1997. The phagosomal environment protects virulent Mycobacterium avium from killing and destruction by clarithromycin. Infect. Immun. 65:2792-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green, L. C., D. A. Wagner, J. Glogowski, P. L. Skipper, J. S. Wishnok, and S. R. Tannenbaum. 1982. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 126:131-138. [DOI] [PubMed] [Google Scholar]
- 16.Griffith, D. E., W. M. Girard, and R. J. Wallace. 1993. Clinical features of pulmonary disease caused by rapidly growing mycobacteria: an analysis of 154 patients. Am. Rev. Respir. Dis. 147:1271-1278. [DOI] [PubMed] [Google Scholar]
- 17.Hadjiliadis, D., A. Adlakha, and U. B. Prakash. 1999. Rapidly growing mycobacterial lung infection in association with esophageal disorders. Mayo Clin. Proc. 74:45-51. [DOI] [PubMed] [Google Scholar]
- 18.Hoy, J. F., K. V. Rolston, R. L. Hopfer, and G. P. Bodey. 1987. Mycobacterium fortuitum bacteremia in patients with cancer and long-term venous catheters. Am. J. Med. 83:213-217. [DOI] [PubMed] [Google Scholar]
- 19.Kamijo, R., H. Harada, T. Matsuyama, M. Bosland, J. Gerecitano, D. Shapiro, J. Le, S. I. Koh, T. Kimura, and S. J. Green. 1994. Requirement for transcription factor IRF-1 in NO synthase induction in macrophages. Science 263:1612-1615. [DOI] [PubMed] [Google Scholar]
- 20.Martin, E., C. Nathan, and Q. W. Xie. 1994. Role of interferon regulatory factor 1 in induction of nitric oxide synthase. J. Exp. Med. 180:977-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meylan, P. R., D. D. Richman, and R. S. Kornbluth. 1990. Characterization and growth in human macrophages of Mycobacterium avium complex strains isolated from the blood of patients with acquired immunodeficiency syndrome. Infect. Immun. 58:2564-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michelini-Norris, M. B., D. K. Blanchard, C. A. Pearson, and J. Y. Djeu. 1992. Differential release of interleukin (IL)-1α, IL-1β, and IL-6 from normal human monocytes stimulated with a virulent and an avirulent isogenic variant of Mycobacterium avium-intracellulare complex. J. Infect. Dis. 165:702-709. [DOI] [PubMed] [Google Scholar]
- 23.Moehring, J. M., and M. R. Solotorowski. 1965. Relationship of colonial morphology to virulence for chickens of Mycobacterium avium and the nonphotochromogens. Am. Rev. Respir. Dis. 92:704-713. [DOI] [PubMed] [Google Scholar]
- 24.Molloy, A., and G. Kaplan. 1996. Cell-mediated immune response, p. 305-314. In W. N. Rom and S. M. Garay (ed.), Tuberculosis. Little, Brown & Co., Boston, Mass.
- 25.North, R. J., L. Ryan, R. LaCource, T. Mogues, and M. E. Goodrich. 1999. Growth rate of mycobacteria in mice as an unreliable indicator of mycobacterial virulence. Infect. Immun. 67:5483-5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paul, S., P. Laochumroonvorapong, and G. Kaplan. 1996. Comparable growth of virulent and avirulent Mycobacterium tuberculosis in human macrophages in vitro. J. Infect. Dis. 174:105-112. [DOI] [PubMed] [Google Scholar]
- 27.Pedrosa, J., M. Florido, Z. M. Kunze, A. G. Castro, F. Portaels, J. McFadden, M. T. Silva, and R. Appelberg. 1994. Characterization of the virulence of Mycobacterium avium complex (MAC) isolates in mice. Clin. Exp. Immunol. 98:210-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rastogi, N., C. Frehel, A. Ryter, H. Ohayon, M. Lesourd, and H. L. David. 1981. Multiple drug resistance in Mycobacterium avium: is the wall architecture responsible for exclusion of antimicrobial agents? Antimicrob. Agents Chemother. 20:666-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rook, G. A., J. Steele, M. Ainsworth, and B. R. Champion. 1986. Activation of macrophages to inhibit proliferation of Mycobacterium tuberculosis: comparison of the effects of recombinant gamma-interferon on human monocytes and murine peritoneal macrophages. Immunology 59:333-338. [PMC free article] [PubMed] [Google Scholar]
- 30.Saito, H., and H. Tomioka. 1988. Susceptibilities of transparent, opaque, and rough colonial variants of Mycobacterium avium complex to various fatty acids. Antimicrob. Agents Chemother. 32:400-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaefer, W. B., C. L. Davis, and M. L. Cohn. 1970. Pathogenicity of transparent, opaque, and rough variants of Mycobacterium avium in chickens and mice. Am. Rev. Respir. Dis. 102:499-506. [DOI] [PubMed] [Google Scholar]
- 32.Schaible, U. E., S. Sturgill-Koszycki, P. H. Schlesinger, and D. G. Russell. 1998. Cytokine activation leads to acidification and increases maturation of Mycobacterium avium-containing phagosomes in murine macrophages. J. Immunol. 160:1290-1296. [PubMed] [Google Scholar]
- 33.Schlesinger, L. S., T. M. Kaufman, S. Iyer, S. R. Hull, and L. K. Marchiando. 1996. Differences in mannose receptor-mediated uptake of lipoarabinomannan from virulent and attenuated strains of Mycobacterium tuberculosis by human macrophages. J. Immunol. 157:4568-4575. [PubMed] [Google Scholar]
- 34.Stormer, R. S., and J. O. D. Falkinham. 1989. Differences in antimicrobial susceptibility of pigmented and unpigmented colonial variants of Mycobacterium avium. J. Clin. Microbiol. 27:2459-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sturgill-Koszycki, S., P. H. Schlesinger, P. Chakraborty, P. L. Haddix, H. L. Collins, A. K. Fok, R. D. Allen, S. L. Gluck, J. Heuser, and D. G. Russell. 1994. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science 263:678-681. (Erratum, 263:1359.) [DOI] [PubMed] [Google Scholar]
- 36.Telenti, A., F. Marchesi, M. Balz, F. Bally, E. C. Bottger, and T. Bodmer. 1993. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J. Clin. Microbiol. 31:175-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tse, H. M., S. I. Josephy, E. D. Chan, D. Fouts, and A. M. Cooper. 2002. Activation of the mitogen-activated protein kinase signaling pathway is instrumental in determining the ability of Mycobacterium avium to grow in murine macrophages. J. Immunol. 168:825-833. [DOI] [PubMed] [Google Scholar]
- 38.Via, L. E., R. A. Fratti, M. McFalone, E. Pagan-Ramos, D. Deretic, and V. Deretic. 1998. Effects of cytokines on mycobacterial phagosome maturation. J. Cell Sci. 111(Pt. 7):897-905. [DOI] [PubMed] [Google Scholar]
- 39.Wakil, A. E., Z. E. Wang, J. C. Ryan, D. J. Fowell, and R. M. Locksley. 1998. Interferon gamma derived from CD4+ T cells is sufficient to mediate T helper cell type 1 development. J. Exp. Med. 188:1651-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wallace, R. J., J. L. J. Cook, J. Glassroth, D. E. Griffith, K. N. Olivier, and F. Gordon. 1997. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Official statement of the American Thoracic Society, March 1997. Medical Section of the American Lung Association. Am. J. Respir. Crit. Care Med. 156:S1-S25. [DOI] [PubMed] [Google Scholar]
- 41.Weinstein, R. A., H. M. Golomb, G. Grumet, E. Gelmann, and G. P. Schechter. 1981. Hairy cell leukemia: association with disseminated atypical mycobacterial infection. Cancer 48:380-383. [DOI] [PubMed] [Google Scholar]
- 42.Wolinsky, E. 1979. Nontuberculous mycobacteria and associated diseases. Am. Rev. Respir. Dis. 119:107-159. [DOI] [PubMed] [Google Scholar]
- 43.Xu, S., A. Cooper, S. Sturgill-Koszycki, T. van Heyningen, D. Chatterjee, I. Orme, P. Allen, and D. G. Russell. 1994. Intracellular trafficking in Mycobacterium tuberculosis and Mycobacterium avium-infected macrophages. J. Immunol. 153:2568-2578. [PubMed] [Google Scholar]