Abstract
Mycobacterium avium subsp. paratuberculosis and Mycobacterium avium subsp. avium are antigenically and genetically similar organisms; however, they differ in their virulence for cattle. M. avium subsp. paratuberculosis causes a chronic intestinal infection leading to a chronic wasting disease termed paratuberculosis or Johne's disease, whereas M. avium subsp. avium causes only a transient infection. We compared the response of bovine monocyte-derived macrophages to ingestion of M. avium subsp. paratuberculosis and M. avium subsp. avium organisms by determining organism survival, superoxide and nitric oxide production, and expression of the cytokines tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), interleukin-8 (IL-8), IL-10, IL-12, and granulocyte-monocyte colony-stimulating factor (GM-CSF). Unlike M. avium subsp. paratuberculosis, macrophages were able to kill approximately half of the M. avium subsp. avium organisms after 96 h of incubation. This difference in killing efficiency was not related to differences in nitric oxide or superoxide production. Compared to macrophages activated with IFN-γ and lipopolysaccharide, macrophages incubated with M. avium subsp. paratuberculosis showed greater expression of IL-10 and GM-CSF (all time points) and IL-8 (72 h) and less expression of IL-12 (72 h), IFN-γ (6 h), and TNF-α (6 h). When cytokine expression by macrophages incubated with M. avium subsp. paratuberculosis was compared to those of macrophages incubated with M. avium subsp. avium, M. avium subsp. paratuberculosis-infected cells showed greater expression of IL-10 (6 and 24 h) and less expression of TNF-α (6 h). Therefore, the combination of inherent resistance to intracellular degradation and suppression of macrophage activation through oversecretion of IL-10 may contribute to the virulence of M. avium subsp. paratuberculosis in cattle.
Paratuberculosis (Johne's disease) is a chronic progressive enteritis of wild and domestic ruminants caused by infection with Mycobacterium avium subsp. paratuberculosis (9, 17). It is likely that calves become infected in utero or during the first few months of life as a result of ingestion of fecal material or milk containing M. avium subsp. paratuberculosis organisms (8). The organisms are taken up through M-cells in the small intestine and are phagocytized by macrophages within the lamina propria (24). The majority of infected animals appear to eliminate the infection, but some become chronically infected and enter a phase of subclinical infection. After several years, some chronically infected cattle develop clinical disease (8, 9). Intestinal lesions in paratuberculosis are characterized by loose aggregates of epithelioid macrophages and giant cells (9). Lymphocytes are conspicuously absent from lesions, and tubercle formation, which is characteristic of other mycobacterial infections, does not occur.
Several in vitro studies have evaluated the interaction of M. avium subsp. paratuberculosis organisms with monocyte-derived macrophages (2, 7, 30, 31, 34, 35). The results of these studies indicate that, although they are readily phagocytized, M. avium subsp. paratuberculosis organisms are not killed by bovine macrophages (34, 35). M. avium subsp. paratuberculosis organisms are sensitive to killing by nitric oxide, but the amount of nitric oxide produced by bovine macrophages is much less than that needed for effective killing (34). Pretreatment with gamma interferon (IFN-γ) or granulocyte-monocyte colony-stimulating factor (GM-CSF) restricted growth of M. avium subsp. paratuberculosis in monocytes but not in monocyte-derived macrophages (35). These data indicate that bovine macrophages have a limited capacity to kill M. avium subsp. paratuberculosis organisms and that resistance to infection may be related to the capacity of macrophages to induce an effective immune response.
Little is known about the capacity of infected bovine macrophages to generate an immune response. Infected cells have been shown to secrete the proinflammatory cytokines tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), and IL-6 (2). Our previous studies have shown that bovine macrophages infected with M. avium subsp. paratuberculosis downregulate surface expression of major histocompatibility complex class I and class II molecules within 12 to 24 h after infection and that expression is not increased by subsequent incubation with IFN-γ (31). Therefore, M. avium subsp. paratuberculosis-infected macrophages may not effectively present antigen to T lymphocytes.
In the present study, we evaluated the capacity of M. avium subsp. paratuberculosis-infected bovine macrophages to express pro- and anti-inflammatory cytokines, to produce antimicrobial molecules, and to kill the organisms. We compared the response of M. avium subsp. paratuberculosis-infected macrophages to that of macrophages infected with M. avium subsp. avium. M. avium subsp. avium is an antigenically and genetically similar organisms but is relatively nonpathogenic for cattle (11, 21). M. avium subsp. avium organisms appear to infect cattle, but unlike cattle infected with M. avium subsp. paratuberculosis, cattle infected with M. avium subsp. avium typically mount an effective systemic immune response, form caseous granulomas, and eliminate the infection (11). Therefore, comparing the responses of bovine macrophages to these two organisms may identify biochemical processes that are critical for inducing an effective immune response.
MATERIALS AND METHODS
Bacteria.
M. avium subsp. paratuberculosis strain 19698 and M. avium subsp. avium strain 35716 were obtained from the American Type Culture Collection. These strains were isolated from naturally infected cows. The organisms were grown to approximately 108/ml, washed, resuspended in 7H9 broth containing oleic acid-albumin-dextrose-catalase (OADC; Difco Laboratories, Detroit, Mich.), Tween 80, mycobactin J (Allied Laboratories, Ames, Iowa), and 5% fetal bovine serum. Both organisms were stored at 4°C for up to 3 months. Viability of organisms was assessed at least once a month by use of standard colony-counting assays.
Cell culture procedure.
Sodium citrate-anticoagulated blood for monocyte isolation was collected from three adult nonlactating Holstein cows that tested negative for Johne's disease as determined by fecal culture (n = 2) and serum enzyme-linked immunosorbent assay (ELISA) tests. Blood was centrifuged, and the buffy coat was removed. Peripheral blood mononuclear cells were isolated by use of Percoll (58%) density gradient centrifugation, washed, and resuspended at 107 cells/ml (32). For preparation of monocyte-derived macrophages, 107 mononuclear cells were allowed to adhere to a 25-mm plastic tissue culture plate for 90 min, and nonadherent cells were washed off. Adherent cells were cultured for 6 days at 37°C and 5% CO2 in RPMI 1640 tissue culture medium supplemented with 10% fetal bovine serum. After 6 days, most adherent cells were stellate in shape, consistent with macrophage morphology.
Monocyte-derived macrophages were infected with M. avium subsp. paratuberculosis or M. avium subsp. avium organisms (10 bacilli/macrophage) on day 7 of culture. Negative control macrophages were incubated with culture medium only. Positive control macrophages were incubated with recombinant bovine interferon (100 U/ml; gift from Novartis Animal Health Inc., Basel, Switzerland) and lipopolysaccharide (LPS; 10 μg/ml) derived from Escherichia coli serotype O26:B6 (Sigma Chemical Co., St. Louis, Mo.).
Ingestion and intracellular survival of M. avium subsp. paratuberculosis and M. avium subsp. avium organisms.
Macrophages from each experiment were stained with Ziehl-Neelsen carbolfuchsin stain, which specifically stains mycobacterial organisms (10). The percentage of macrophages containing organisms was determined by use of light microscopy.
Organism survival was assessed by use of a standard colony-counting technique after 48 and 96 h of incubation (35). Macrophages were lysed by addition of saponin (1 mg/ml, final concentration), washed, and cultured on 7H9 agar plates supplemented with OADC, Tween 80, and mycobactin J. Colony counts were performed after approximately 4 weeks for M. avium subsp. avium and 8 weeks for M. avium subsp. paratuberculosis.
Macrophage nitric oxide and superoxide production.
After 6 or 24 h of incubation, superoxide and nitric oxide production by macrophages was quantified. The Griess assay method was used to detect nitric oxide (25). The cytochrome c reduction method was used to quantify superoxide anion (23).
Reverse transcriptase-PCR.
At 6, 24, or 72 h after addition of organisms or activating agent, supernatant was removed, culture plates were washed once with warm culture medium, and total RNA was isolated by the guanidinium isothiocyanate-phenol-chloroform procedure as previously described (1). A semiquantitative reverse transcriptase-PCR method with limited amplification of specific cDNA products was used for characterization of TNF-α, IL-8, IL-10, IL-12, GM-CSF, and IFN-γ expression (20). Cytokine expression by macrophages from three cows was evaluated. The reverse transcriptase reaction contained 0.74 μg of random hexamer primers, 3.0 μg of total RNA, 1× first-strand buffer, 10 mM dithiothreitol, and 0.5 mM dNTPs. Samples were heated to 42°C for 2 min, and 200 U of Superscript II reverse transcriptase (Gibco-BRL, Gaithersburg, Md.) was added to bring the final volume to 20 μl. The samples were further incubated at 42°C for 50 min, followed by incubation at 70°C for 15 min.
The PCR mixture contained 1× PCR buffer, 0.2 mM dNTPs, 1 μM each of the cytokine-specific forward and reverse primers (Table 1), 0.4 μCi of [32P]dCTP (3,000 Ci/mmol), 1.25 U of Amplitaq polymerase (Perkin Elmer, Foster City, Calif.), and 2 μl of first-strand cDNA template. Thermocycler conditions included 94°C for 2 min, 54°C for 30 s, and 72°C for 1 min. Multiple dilutions of input cDNA template demonstrated that under these conditions, the amount of cDNA product correlated directly with the amount of cDNA template (data not shown).
TABLE 1.
Primers used for semiquantitative RT-PCR
| Gene | Primers (5′ → 3′) (forward above reverse) | Product size (bp) |
|---|---|---|
| IL-8 | TGCCTCATGTACTGTGTGGG | 286 |
| GGGATAAAGAAACCAAGGCG | ||
| IL-10 | GTTGCCTGGTCTTCCTGGCTG | 482 |
| TATGTAGTTGATGAAGATGTC | ||
| IL-12 | GAGGATTTTCCTCCCAAAGC | 125 |
| CTTCTACAATGGGGCGTGTT | ||
| IFN-γ | TGTGATGTGCTCCATCCTGT | 164 |
| ATTCTGCTGTCTAGGCCCAA | ||
| TNF-α | TGGAGGGAGAAGGGATTCTT | 282 |
| AGGGGCTGGAGAAAGGGAAG | ||
| GM-CSF | CGTCTCTGAAAAGTTTGACTCCC | 300 |
| ACTTCTGGGCTGGTTCCC | ||
| β-Actin | ATCAAGCCCACAGGTACTGG | 141 |
| TCACATAGTGAGGCCGAGTG |
Furthermore, all cytokines were initially analyzed at 25, 27, and 30 cycles to demonstrate that amplification increased in a linear manner (data not shown). TNF-α, IL-8, IL-10, GM-CSF, and IFN-γ were amplified separately for 28 cycles with cytokine-specific forward and reverse primers (Table 1). IL-12 was amplified for 32 cycles. The products were separated on 5% nondenaturing acrylamide gels, and specific products were detected with a phosphoimaging system (Molecular Dynamics, Foster City, Calif.). Primers for β-actin were used to normalize the amount of cDNA product in each sample. Because mRNA for actin was more abundant than mRNAs for the cytokines evaluated, β-actin was amplified for 21 cycles.
Bioassay for TNF-α.
TNF-α bioactivity was analyzed by the WEHI-164 cytotoxicity assay as previously described (2, 26). WEHI-164 cells were grown to confluence in Dulbecco's modified Eagle's medium supplemented with 5% fetal bovine serum and 100 U of penicillin G at 37°C and 5% CO2. Supernatants from macrophage cultures were diluted 1:2 with culture medium, and 100 μl was added to triplicate wells. Recombinant human TNF-α (R&D Systems, Minneapolis, Minn.) and Dulbecco's modified Eagle's medium were used as positive and negative controls, respectively. Samples were analyzed at 570 nm with a microplate ELISA reader (Molecular Devices Corp., Menlo Park, Calif.). The concentration of TNF-α was calculated from a standard curve developed with recombinant human TNF-α.
Statistical analysis.
Expression of cytokine mRNA was analyzed for three cows. Data were analyzed by use of a paired t test and considered statistically significant at P < 0.05. For bioassays, experiments were conducted in triplicate, and mean data from each of three cows were analyzed by use of factorial analysis of variance. Means of interest were compared by use of the Bonferroni-Dunn F test.
RESULTS
Uptake and survival of M. avium subsp. paratuberculosis and M. avium subsp. avium organisms.
When macrophages were incubated with M. avium subsp. paratuberculosis organisms for 6, 48, or 96 h, 94% ± 4%, 98% ± 3%, and 96% ± 3%, respectively, of macrophages contained 1 or more organisms. When macrophages were incubated with M. avium subsp. avium organisms for 6, 48, or 96 h, 91% ± 6%, 89% ± 6%, and 86% ± 4%, respectively, of macrophages contained at least 1 organism.
The number of viable M. avium subsp. paratuberculosis and M. avium subsp. avium organisms was evaluated after 48 and 96 h of incubation. The number of viable M. avium subsp. paratuberculosis organisms stayed the same or increased slightly over time (Table 2). Alternatively, a progressive decrease in the number of viable M. avium subsp. avium organisms was observed (P < 0.05). Addition of IFN-γ to cultures had no effect on the number of viable M. avium subsp. paratuberculosis organisms, whereas the mean number of M. avium subsp. avium organisms tended to decrease (data not statistically significant).
TABLE 2.
Colony-forming units of M. avium subsp. paratuberculosis and M. avium subsp. avium after incubation of 107 organisms in vitro with approximately 106 bovine monocyte-derived macrophages for 48 or 96 ha
| Strain | Treatment | Mean count (107 CFU) (% change)
|
|
|---|---|---|---|
| 48 h | 96 h | ||
| M. avium subsp. paratuberculosis | None | 0.98, 1.04, 1.09 (103) | 1.00, 1.04, 1.13 (106) |
| IFN-γ | 0.94, 1.19, 1.04 (106) | 0.87, 1.06, 1.13 (102) | |
| M. avium subsp. avium | None | 0.76, 0.88, 0.83 (82) | 0.36, 0.61, 0.53 (50)* |
| IFN-γ | 0.67, 0.84, 0.85 (78) | 0.20, 0.49, 0.54 (41)* | |
Values represent means of three replicate cultures for each of three cows. Values in parentheses represent percent change for all three cows compared to time zero samples. *, statistically different (P < 0.05) from time zero values.
Superoxide and nitric oxide production by macrophages ingesting M. avium subsp. paratuberculosis and M. avium subsp. avium organisms.
Compared to uninfected macrophages, macrophages incubated with M. avium subsp. paratuberculosis or M. avium subsp. avium showed no increase in superoxide production and only a slight increase in nitric oxide production at 12 and 24 h after addition of organisms (Table 3). When M. avium subsp. paratuberculosis-infected macrophages were compared to M. avium subsp. avium-infected macrophages, no differences in superoxide or nitric oxide production were identified. Addition of IFN-γ to macrophages incubated with M. avium subsp. paratuberculosis or M. avium subsp. avium resulted in an increase in nitric oxide production, but superoxide production decreased.
TABLE 3.
Production of superoxide and nitric oxide by bovine macrophages incubated for 24 h with M. avium subsp. paratuberculosis or M. avium subsp. avium organisms (10 organisms/macrophage) with or without IFN-γa
| Organism | Treatment | Mean superoxide production (nmol/mg of protein) ± SD | Mean nitric oxide production (μM) ± SD |
|---|---|---|---|
| Uninfected control | None | 45 ± 1 | 0 |
| M. avium subsp. paratuberculosis | None | 42 ± 8 | 2.0 ± 0.3 |
| IFN-γ | 19 ± 3* | 6.9 ± 0.5* | |
| M. avium subsp. avium | None | 41 ± 5 | 2.5 ± 0.3 |
| IFN-γ | 23 ± 3* | 7.8 ± 0.6* |
Values represent means for three cows. *, statistically significant (P < 0.05) compared to addition of organism alone.
Cytokine expression.
Cytokine mRNA expression was analyzed at 6, 24, and 72 h after addition of mycobacterial organisms (Table 4). PCR products were not obtained from samples that did not contain reverse transcriptase, indicating that the samples were not contaminated with genomic DNA. Negative controls consisted of macrophages incubated with RPMI medium, and positive controls consisted of macrophages incubated with IFN-γ and LPS. β-Actin was used as a control to adjust for the amount of input RNA.
TABLE 4.
Changes in cytokine mRNA expression by resting (control) and IFN-γ-and LPS-activated bovine macrophages (activated) and macrophages incubated with M. avium subsp. paratuberculosis (M. a. ptb) or M. avium subsp. avium (M. a. a.)a
| Cytokine | Treatment | Relative density
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 h
|
24 h
|
72 h
|
|||||||||||
| 1 | 2 | 3 | Change | 1 | 2 | 3 | Change | 1 | 2 | 3 | Change | ||
| IL-10 | Control | 612 | 960 | 1,104 | 632 | 1,117 | 894 | 448 | 493 | 524 | |||
| Activated | 2,206 | 2,507 | 3,879 | 3.2 | 2,074 | 2,547 | 2,173 | 2.5 | 482 | 575 | 669 | 1.2 | |
| M. a. ptb. | 6,900 | 9,905 | 11,638 | 12.2*† | 5,140 | 7,417 | 6,788 | 7.2*† | 3,710 | 3,803 | 4,552 | 8.3* | |
| M. a. a. | 2,287 | 4,227 | 4,968 | 4.2 | 2,004 | 4,219 | 3,158 | 3.5 | 3,110 | 3,551 | 3,183 | 6.7* | |
| IL-12 | Control | 0 | 0 | 0 | |||||||||
| Activated | 728 | 823 | 730 | 719 | 509 | 622 | 658 | 788 | 783 | ||||
| M. a. ptb. | 801 | 746 | 802 | 765 | 673 | 710 | 0* | ||||||
| M. a. a. | 608 | 302 | 364 | ND | 734 | 792 | 0* | ||||||
| INF-γ | Control | 144 | 296 | 241 | 168 | 183 | 206 | 87 | 0 | 72 | |||
| Activated | 1,232 | 1,607 | 1,379 | 6.2 | 446 | 398 | 375 | 2.2 | 102 | 100 | 54 | 1.5 | |
| M. a. ptb. | 378 | 482 | 403 | 1.9* | 342 | 361 | 451 | 2.0 | 174 | 199 | 207 | 3.5* | |
| M. a. a. | 447 | 452 | 386 | 1.9* | 189 | 178 | 321 | 1.2 | 163 | 203 | 154 | 3.1* | |
| TNF-α | Control | 56 | 154 | 59 | 57 | 42 | 78 | 40 | 62 | 87 | |||
| Activated | 358 | 266 | 272 | 3.4 | 181 | 238 | 326 | 4.2 | 109 | 79 | ND | 1.5 | |
| M. a. ptb. | 112 | 181 | 137 | 1.6*† | 184 | 132 | 218 | 3.0 | 45 | 66 | 74 | 0 | |
| M. a. a. | 338 | 313 | 348 | 3.8 | 192 | 229 | 239 | 3.8 | 0 | 152 | 45 | 0 | |
| IL-8 | Control | 178 | 40 | 35 | 136 | 41 | 59 | 298 | 184 | 196 | |||
| Activated | 2,013 | 358 | 395 | 11.0 | 1,769 | 845 | 928 | 14.9 | 296 | 251 | 225 | 1.1 | |
| M. a. ptb. | 2,243 | 679 | 787 | 14.7 | 2,245 | 1,712 | 1,678 | 24.0 | 1,317 | 662 | 681 | 4.0* | |
| M. a. a. | 1,841 | 752 | 722 | 13.1 | 1,723 | 1,421 | 1,503 | 19.8 | 982 | 878 | 851 | 4.0* | |
| GM-CSF | Control | 284 | 168 | 225 | 114 | 176 | 152 | 96 | 0 | 139 | |||
| Activated | 433 | 304 | 255 | 1.4 | 178 | 173 | 104 | 1.0 | 115 | 73 | 90 | 1.2 | |
| M. a. ptb. | 1,732 | 1,524 | 1,261 | 6.6* | 742 | 548 | 563 | 4.2* | 413 | 382 | 369 | 4.9*† | |
| M. a. a. | 1,802 | 1,330 | 2,342 | 8.1* | 659 | 621 | 679 | 4.5* | 158 | 177 | 172 | 2.1 | |
Values represent relative density for each of three cows (cows 1, 2, and 3). Change values represent mean fold change compared to the control. *, statistically significant (P < 0.05) compared to activated macrophages; †, statistically different (P < 0.05) compared to M. avium subsp. avium-infected macrophages. ND, not determined.
Relative changes in IL-10 expression for each of the treatments were consistent between experimental replicates (n = 3; Fig. 1, Table 4). Negative control samples had detectable IL-10 expression, and positive control samples had a two- to fourfold increase compared to the negative control. M. avium subsp. paratuberculosis-infected macrophages showed much greater expression of IL-10 at all three time points compared to the positive control. M. avium subsp. paratuberculosis-infected macrophages also showed greater mean IL-10 expression compared to M. avium subsp. avium-infected macrophages at all time points. The difference was statistically significant at 6 and 24 h.
FIG. 1.
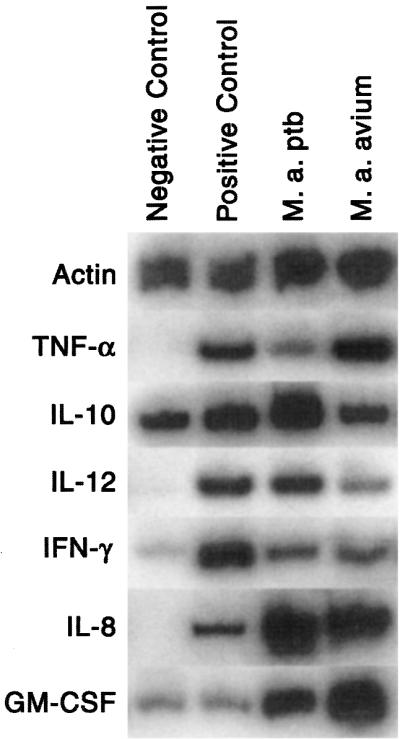
Cytokine mRNA expression by bovine macrophages 6 h after addition of medium (negative control), IFN-γ and LPS (positive control), M. avium subsp. paratuberculosis (M. a. ptb) organisms, or M. avium subsp. avium (M. a. a)organisms. Data are representative of results for three cows.
IL-12 expression by negative control macrophages was undetectable at all time points (Table 4, Fig. 1). Positive control cells and cells incubated with M. avium subsp. paratuberculosis or M. avium subsp. avium had similar increases in IL-12 expression at 6 and 24 h. However, at 72 h, positive control macrophages showed sustained expression of IL-12, whereas expression by M. avium subsp. paratuberculosis- and M. avium subsp. avium-infected macrophages was undetectable.
In positive control samples, IFN-γ expression was high at 6 h but decreased progressively thereafter, approaching negative-control values at 72 h (Fig. 1, Table 4). The increase in IFN-γ expression by M. avium subsp. paratuberculosis- and M. avium subsp. avium-infected macrophages was much less than that by the positive control at 6 h but was greater than that by the positive control at 72 h.
TNF-α expression was high at 6 h in positive control macrophages and macrophages incubated with M. avium subsp. avium organisms (Table 4, Fig. 1). Alternatively, TNF-α expression by macrophages incubated with M. avium subsp. paratuberculosis organisms was lower at 6 h compared to macrophages incubated with M. avium subsp. avium. At 72 h, TNF-α expression was low for all samples.
IL-8 was highly expressed by positive control macrophages and macrophages incubated with M. avium subsp. paratuberculosis and M. avium subsp. avium organisms (Fig. 1, Table 4). Mean values for M. avium subsp. paratuberculosis- and M. avium subsp. avium-infected macrophages were slightly greater at 6 and 24 h compared to the positive-control samples, and the difference was statistically significant at 72 h.
Positive control macrophages showed minimal increase in expression of GM-CSF compared to the negative control (Fig. 1, Table 4). However, M. avium subsp. paratuberculosis- and M. avium subsp. avium-infected macrophages showed a marked increase in GM-CSF expression, with expression by M. avium subsp. paratuberculosis-infected macrophages being greater than that by M. avium subsp. avium-infected macrophages at 72 h.
TNF-α bioactivity.
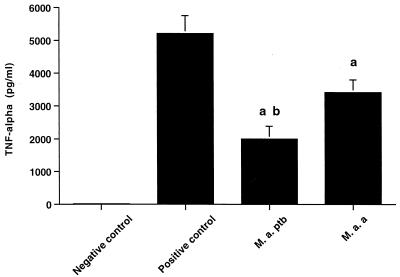
TNF-α bioactivity in cell culture supernatants was measured by the WEHI cell cytotoxicity assay at the 6-h time point (Fig. 2). TNF-α bioactivity was low in negative control cells but increased in samples incubated with IFN-γ and LPS. Compared to the positive control macrophages, macrophages incubated with M. avium subsp. paratuberculosis or M. avium subsp. avium had lower TNF-α bioactivity. Macrophages incubated with M. avium subsp. paratuberculosis also had lower TNF-α bioactivity than macrophages incubated with M. avium subsp. avium.
FIG. 2.
TNF-α bioactivity produced by bovine macrophages 6 h after addition of medium only (negative control), IFN-γ and LPS (positive control), M. avium subsp. paratuberculosis (M. a. ptb) organisms, or M. avium subsp. avium (M. a. a) organisms. Data a represent the mean ± standard deviation of two separate experiments on each of three cows. All tests were conducted in triplicate, and results were averaged. a, statistically different from positive control (P < 0.05); b, statistically different from M. avium subsp. avium-infected macrophages (P < 0.05).
DISCUSSION
Currently, susceptibility and resistance to mycobacterial infection is viewed as a struggle between strategies of the organism to evade intracellular killing mechanisms and the capacity of macrophages to become activated, generate a microbicidal response, and initiate a Th1-type immune response (27, 33). However, the interplay of host and organism factors that determine whether the organism is eliminated or establishes a persistent infection is poorly understood. To investigate these mechanisms with regard to paratuberculosis, we studied the response of bovine macrophages to the causative organism, M. avium subsp. paratuberculosis, and to the genetically similar but less pathogenic organism M. avium subsp. avium. Because M. avium subsp. avium appears to cause only a transient infection in cattle whereas M. avium subsp. paratuberculosis causes a persistent infection in some cattle, we hypothesized that this comparison would be informative.
Differences identified that may contribute to virulence included the capacity of bovine macrophages to kill M. avium subsp. avium but not M. avium subsp. paratuberculosis organisms and differential cytokine expression. The difference in killing efficiency was not related to differences in organism uptake or superoxide or nitric oxide production. Treatment of macrophages with IFN-γ increased nitric oxide production but had no effect on killing of M. avium subsp. paratuberculosis organisms and little effect on killing of M. avium subsp. avium organisms. These data indicate that bovine macrophages have an inherently greater capacity to kill M. avium subsp. avium organisms which is independent of induction of an acquired immune response.
Cytokine production by infected macrophages may also contribute to the virulence of M. avium subsp. paratuberculosis. Compared to macrophages activated with IFN-γ and LPS, macrophages incubated with M. avium subsp. paratuberculosis had greater expression of IL-10 and GM-CSF (all time periods) and IL-8 (72 h) and less expression of IL-12 (72 h), IFN-γ (6 h), and TNF-α (6 h). Previous studies have documented that the TNF-α/IL-10 balance is a major determinant of macrophage activation status (4, 5, 22, 15, 29). TNF-α is a major activator of both inflammatory and immune responses and is important in granuloma formation and initiation of a protective immune response in mycobacterial infections (14, 19, 15, 16, 28). Alternatively, IL-10 is a major anti-inflammatory and anti-immune cytokine. IL-10 has been shown to inhibit macrophage activation, cytokine secretion, microbicidal activity, and differentiation to dendritic cells (3, 6, 12, 13, 15). The effect of IL-10 is mediated in part through inhibition of TNF-α and IL-12 production by macrophages and inhibition of IFN-γ expression by T-lymphocytes. Therefore, overexpression of IL-10 could be responsible for the M. avium subsp. paratuberculosis-associated reduction in TNF-α and IFN-γ expression observed in this study (18). Unfortunately, the neutralizing anti-bovine IL-10 antibodies needed to directly test this hypothesis are not commercially available.
IL-12 expression was not different at 6 or 24 h but was lower in M. avium subsp. paratuberculosis-infected macrophages at 72 h than in positive-control macrophages. These data suggest that release of IL-12 is not sustained by M. avium subsp. paratuberculosis-infected macrophages. A recent study of M. avium subsp. avium-infected human macrophages also reported that infection resulted in progressive suppression of IL-12 production in vitro (31). Furthermore, IL-12 expression decreased progressively in spleens of mice infected with M. avium subsp. avium (31). Because IL-12 plays a central role in initiation of a Th1-type immune response, failure to sustain IL-12 production could blunt induction of an effective acquired immune response.
In conclusion, major differences in the response of bovine macrophages to infection with M. avium subsp. paratuberculosis and M. avium subsp. avium consist of the selective capacity to kill M. avium subsp. avium organisms and an altered pattern of cytokine expression. Greater expression of IL-10 and lesser expression of TNF-α by M. avium subsp. paratuberculosis-infected macrophages would be expected to inhibit macrophage activation and microbicidal activity.
Acknowledgments
This work was supported by a grant from the Minnesota Agricultural Experiment Station Rapid Response Fund.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Abrahamsen, M. S., T. G. Clark, and M. W. White. 1993. An improved method for isolating RNA from coccidian parasites. J. Parasitol. 81:107-109. [PubMed] [Google Scholar]
- 2.Adams, J. L., C. J. Czyprynski, and M. T. Collins. 1994. Mycobacterial cell wall components induce the production of TNF-α, IL-1, and IL-6 by bovine monocytes and murine macrophage cell line RAW 264.7. Microb. Pathog. 16:401-411. [DOI] [PubMed] [Google Scholar]
- 3.Allavena, P. L., L. Piemonti, D. Longoni, S. Bernasconi, A. Stoppacciaro, L. Ruco, and A. Mantovani. 1998. IL-10 prevents the differentiation of monocytes to dendritic cells but promotes their maturation to macrophages. Eur. J. Immunol. 28:359-368. [DOI] [PubMed] [Google Scholar]
- 4.Balcewicz-Sablinska, M. K., H. Gan, and H. G. Remold. 1999. Interleukin-10 produced by macrophages inoculated with Mycobacterium avium attenuates mycobacteria-induces apoptosis by reduction of TNF-α activity. J. Infect. Dis. 180:1230-1237. [DOI] [PubMed] [Google Scholar]
- 5.Beltan, E., L. Horgen, and N. Rastogi. 2000. Secretion of cytokines by human macrophages upon infection by pathogenic and non-pathogenic mycobacteria. Microb. Pathog. 28:313-318. [DOI] [PubMed] [Google Scholar]
- 6.Bogdan, C., Y. Vodovotz, and C. Nathan. 1991. Macrophage deactivation by IL-10. J. Exp. Med. 174:1549-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheville, N. F., J. Hostetter, B. V. Thomsen, F. Simutis, Y. Vanloubbeeck, and E. Steadham. 2001. Intracellular trafficking of Mycobacterium avium s. paratuberculosis in macrophages. Dtsch. Tieraerztl. Wochenschr. 108:236-242. [PubMed] [Google Scholar]
- 8.Chiodini, R. J., H. J. van Kruiningen, and R. Merkal. 1984. Ruminant paratuberculosis (Johne's disease): the current status and future prospects. Cornell Vet. 74:218-262. [PubMed] [Google Scholar]
- 9.Clarke, C. J. 1997. The pathology and pathogenesis of paratuberculosis in ruminants and other species. J. Comp. Pathol. 116:217-261. [DOI] [PubMed] [Google Scholar]
- 10.Coles, E. H. 1974. Veterinary clinical pathology, 2nd ed., p. 589-590. W. B. Saunders Co., Philadelphia, Pa.
- 11.de Lisle, G. W., G. F. Yates, and M. A. Joyce. 1998. Case report and DNA characterization of Mycobacterium avium isolated from multiple animals with lesions in a beef cattle herd. J. Vet. Diagn. Med. 10:283-284. [DOI] [PubMed] [Google Scholar]
- 12.De Smedt, T., M. van Mechelen, G. de Becker, J. Urbain, O. Leo, and M. Moser. 1997. Effects of interleukin-10 on dendritic cell maturation and function. Eur. J. Immunol. 27:1229-1238. [DOI] [PubMed] [Google Scholar]
- 13.Donnelly. R. P., H. Dickensheets, and D. S. Finbloom. 1999. The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J. Interferon Cytokine Res. 19:563-573. [DOI] [PubMed] [Google Scholar]
- 14.Eriks, I. S., and C. L. Emerson. 1997. Temporal effect of tumor necrosis factor alpha on murine macrophages infected with Mycobacterium avium. Infect. Immun. 65:2100-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giacomini, E., I. Elisabetta, L. Ferroni, M. Miettinen, L. Fattorini, G. Orefici, I. Julkunen, and E. M. Coccia. 2001. Infection of human macrophages and dendritic cells with Mycobacterium tuberculosis induces a differential cytokine gene expression that modualtes T cell responses. J. Immunol. 166:7033-7041. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Flores, R., R. Tamez-Guerra, S. D. Tucker, and R. T. Mehta. 1999. Bidirectional effects of IFN-gamma on growth of Mycobacterium avium complex in murine peritoneal macrophages. J. Interferon Cytokine Res. 17:331-336. [DOI] [PubMed] [Google Scholar]
- 17.Harris, N. B., and R. G. Barletta. 2001. Mycobacterium avium subsp. paratuberculosis in veterinary medicine. Clin. Microbiol. Rev. 14:489-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isler, P., B.G. de Rochemonteix, F. Songeon, N. Boehringer, and L. P. Nicod. 1999. Interleukin-12-production by human alveolar macrophages is controlled by the autocrine production of IL-10. Am. J. Respir. Cell Mol. Biol. 20:270-281. [DOI] [PubMed] [Google Scholar]
- 19.Kindler, V., A. P. Sappino, G. E. Grau, P. F. Piguet, and P. Vassalli. 1989. The inducing role of tumor necrosis factor in the development of bacterial granulomas during BCG infection. Cell 56:731-739. [DOI] [PubMed] [Google Scholar]
- 20.Lafluer, R., M. S. Abrahamsen, and S. K. Maheswaran. 1998. The biphasic mRNA expression pattern of bovine interleukin-8 in Pasteurella haemolytica lipopolysaccharide-stimulated alveolar macrophages is primarily due to tumor necrosis factor alpha. Infect. Immun. 66:4087-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marsh, I., R. Whittington, and D. Cousins. 1999. PCR-restriction endonuclease analysis for identification and strain typing of Mycobacterium avium subsp. paratuberculosis and Mycobacterium avium subsp. avium based on polymorphisms in IS1311. Mol. Cell. Probes 13:115-126. [DOI] [PubMed] [Google Scholar]
- 22.McHugh, S. L., Y. Tamamoto, T. W. Klein, and H. Friedman. 2000. Murine macrophages differentially produce proinflammatory cytokines after infection with virulent vs. avirulent Legionella pneumophila. J. Leukoc. Biol. 67:863-868. [DOI] [PubMed] [Google Scholar]
- 23.Metcalf, J. A., J. I. Gallin, W. M. Nauseef, and R. K. Root. 1986. Laboratory manual of neutrophil function, 1st ed. Raven Press, New York, N.Y.
- 24.Momotani, E., D. L. Whipple, A. B. Theirmann, and N. F. Cheville. 1988. Role of M cells and macrophages in the entrance of Mycobacterium paratuberculosis into dome of ileal Peyer's patches in calves. Vet. Pathol. 25:131-137. [DOI] [PubMed] [Google Scholar]
- 25.Murthunga, M., P. M. Preston, and K. J. Sumption. 1998. Nitric oxide produced by Cowdria ruminantium-infected bovine pulmonary endothelial cells in vitro is stimulated by gamma interferon. Infect. Immun. 66:2115-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rashid, J., D. J. Weiss, S,K, Maheswaran, and M. P. Murtaugh. 1996. In vitro expression and inhibition of procoagulant activity produced by bovine alveolar macrophages and peripheral blood cells. Vet. Res. Commun. 20:519-531. [DOI] [PubMed] [Google Scholar]
- 27.Rhoades, E. R., and H. J. Ullrich. 2000. How to establish a lasting relationship with your host: lessons learned from Mycobacterium spp. Immunol. Cell Biol. 78:301-310. [DOI] [PubMed] [Google Scholar]
- 28.Sano, C., K. Sato, T. Shimizu, H. Kajitani, H. Kawauchi, and H. Tomioka. 1999. The modulating effects of proinflammatory cytokines interferon-gamma (IFN-gamma) and tumor necrosis factor-alpha (TNF-alpha), and immunoregulating cytokines IL-10 and transforming growth factor-beta (TGF-beta), on antimicrobial activity of murine peritoneal macrophages against Mycobacterium avium-intracellulare complex. Clin. Exp Immunol 115:435-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarmento, A., and R. Appelberg. 1995. Relationship between virulence of Mycobacterium avium strains and induction of tumor necrosis factor alpha production in infected mice and in in vitro-cultured mouse macrophages. Infect. Immun 63:3759-3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stabel, J. R. 1996. Production of γ-interferon by peripheral blood mononuclear cells: an important diagnostic tool for detection of subclinical paratuberculosis. J. Vet Diagn Invest 8:345-350. [DOI] [PubMed] [Google Scholar]
- 31.Wagner, D., F. J. Sangari, S. Kim, M. Petrofsky, L. E. Bermidez. 2002. Mycobacteriun avium infection results in progressive suppression of the interleukin-12 production in vitro and in vivo. J. Leuko Biol 71:80-88. [PubMed] [Google Scholar]
- 32.Weiss, D. J., O. A. Evanson, D,.J. McClenahan, M. S. Abrahansen, and B. K. Walcheck. 2001. Regulation of expression of major histocompatibility antigens by bovine macrophages infected with Mycobacterium avium subsp. paratuberculosis or Mycobacterium avium subsp. avium. Infect. Immun 69:1002-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wigginton, J. E., and D. Kirschner. 2001. A model to predict cell-mediated immune regulatory mechanisms during human infection with Mycobacterium tuberculosis. J. Immunol 166:1951-1967. [DOI] [PubMed] [Google Scholar]
- 34.Zao, B., M. T. Collins, and C. J. Czuprynski. 1997. Effects of gamma interferon and nitric oxide on the interaction of Mycobacterium avium subsp paratuberculosis with bovine monocytes. Infect. Immun. 65:176-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zurbrick, B. G., D. M. Follett, and C. J. Czuprynski. 1988. Cytokine regulation of the intracellular growth of Mycobacterium paratuberculosis in bovine monocytes. Infect. Immun. 56:1692-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]