Abstract
Alveolar macrophages are likely the first cell type to encounter Mycobacterium tuberculosis in a pulmonary infection, resulting in the production of chemokines. In order to evaluate this response, alveolar macrophages harvested from nonvaccinated and Mycobacterium bovis BCG-vaccinated guinea pigs were infected in vitro with live M. tuberculosis H37Ra or H37Rv (multiplicity of infection, 1:1) or cultured with lipopolysaccharide (10 μg/ml) for 3, 12, and 24 h. Interleukin-8 (IL-8) and monocyte chemoattractant protein 1 (MCP-1) mRNA expression was determined by real-time PCR. Culture supernatants were assayed for guinea pig IL-8 protein by using a human IL-8 enzyme-linked immunosorbent assay kit. Alveolar macrophages harvested from BCG-vaccinated guinea pigs produced significantly more mRNA and protein for IL-8 than alveolar macrophages harvested from nonvaccinated guinea pigs at 12 and 24 h poststimulation or postinfection. Infection with attenuated M. tuberculosis (H37Ra) stimulated alveolar macrophages isolated from BCG-vaccinated guinea pigs to produce significantly more IL-8 mRNA than did alveolar macrophages infected with a virulent strain (H37Rv) at 12 and 24 h postinfection. Significant MCP-1 mRNA production was also detected in stimulated or infected alveolar macrophages; however, prior vaccination did not significantly affect levels of MCP-1 mRNA. Alveolar macrophages isolated from BCG-vaccinated guinea pigs produced significantly more IL-8 mRNA and protein when stimulated for 24 h with heat-killed H37Ra, heat-killed H37Rv, and H37Rv cell wall, but not mannose-capped lipoarabinomannan (ManLAM), than did cells stimulated with media alone. These observations indicate that prior vaccination may alter very early events in the M. tuberculosis-infected lung.
Despite the existence of the Mycobacterium bovis BCG vaccine, the death toll from tuberculosis (TB) exceeds 3 million individuals annually, making TB the leading infectious killer of adults worldwide (13). BCG is the only vaccine available at this time for the control of TB. Due to variations in the ability of BCG to protect uniformly against adult TB, new candidate vaccines are being tested in murine and guinea pig models (5, 47). The search for a better TB vaccine would be greatly facilitated by a clearer understanding of the mechanisms involved in vaccine-induced resistance in the guinea pig model of tuberculosis (27, 28, 29).
There are several indications that chemokines participate in the host defense of the lung against bacterial pathogens during which the effective response is characterized by recruitment and activation of inflammatory cells, such as granuloma formation during infection with Mycobacterium tuberculosis (8, 19, 21, 45, 55). Alveolar macrophages are the first cell type to encounter M. tuberculosis in a pulmonary infection, and these resident macrophages are known to produce cytokines and chemokines as a result of stimulation by M. tuberculosis (42, 49, 55). This production first causes an influx of proinflammatory neutrophils, which is then followed by an infiltration of monocytes and T lymphocytes.
The contribution of this cellular infiltrate to TB resistance, especially early events in infection like the accumulation of neutrophils, is poorly understood. While neutrophils can engulf mycobacteria, they are unable to efficiently control the infection (18, 41). Recent evidence suggests a crucial immunomodulatory role for neutrophils (46), corresponding with elevated levels of interleukin-12 (IL-12), tumor necrosis factor alpha (TNF-α), IL-1β, and gamma interferon (6, 34), as well as the production of chemokines that attract monocytes and T lymphocytes (40). This early neutrophil influx may be a critical first step in response to infection of the lung with M. tuberculosis, but little attention has been paid experimentally to this issue.
Chemokines can be categorized into subfamilies based upon the presence and position of four conserved cysteine residues near the N terminus. CXC chemokines have their first two cysteines separated by one amino acid and are primarily chemotactic for polymorphonuclear leukocytes such as neutrophils (50). The most widely studied CXC chemokine is CXC ligand-8, also known as IL-8, which is a chemoattractant for neutrophils (3) and T lymphocytes (23). It has been associated with inflammatory cell influx in many disease states. The human alveolar epithelial cell line A549 (25), human monocyte and macrophage cell lines THP-1 and Mono Mac (10), human polymorphonuclear granulocytes (37), human blood neutrophils (18), primary human monocytes from active TB patients (19), as well as primary alveolar macrophages harvested from TB patients by bronchoalveolar lavage (BAL) (55) can all be stimulated by mycobacteria to produce message for IL-8. BAL fluids from human TB patients show elevated levels of immunoreactive IL-8 protein compared to those of healthy controls (55).
The CC chemokine subfamily, in which the first two cysteines are adjacent, have general chemotactic activity for mononuclear cells such as monocytes, T lymphocytes, and NK cells. CC ligand-2 (CCL2), also known as monocyte chemoattractant protein 1 (MCP-1), is the prototypic CC chemokine and is a chemoattractant for monocytes and T lymphocytes (4) as well as other cell types (48). MCP-1 is produced by human monocyte/macrophage (11) and alveolar epithelial (25) cell lines, primary cultures of human blood monocytes taken from TB patients (19, 24), and murine bone marrow-derived macrophages (36) when stimulated by mycobacteria. It has been detected in the BAL fluids of both low-dose aerosol-infected mice (36) and active TB patients (21).
In this paper we demonstrate for the first time that primary alveolar macrophages harvested from BCG-vaccinated guinea pigs by BAL and stimulated in vitro with a variety of stimuli including infection with virulent mycobacteria produce significantly higher levels of mRNA and protein for IL-8 than do alveolar macrophages harvested from nonvaccinated guinea pigs. Infection with live attenuated M. tuberculosis (H37Ra) stimulated alveolar macrophages isolated from BCG-vaccinated guinea pigs to produce significantly more IL-8 mRNA than that produced by alveolar macrophages infected with a virulent strain (H37Rv). Significant MCP-1 mRNA production was detected in stimulated or infected alveolar macrophages taken from nonvaccinated and BCG-vaccinated guinea pigs; however, prior vaccination did not affect levels of MCP-1 mRNA at the time points analyzed. These observations are discussed in the context of understanding how prior vaccination may alter very early events in the M. tuberculosis-infected lung.
MATERIALS AND METHODS
Bacteria.
M. tuberculosis strain H37Ra (ATCC 25177) and H37Rv (ATCC 27294) were obtained from the American Type Culture Collection (Rockville, Md.) and prepared and stored at −70°C as described in previous reports (14). Heat-killed M. tuberculosis was prepared by incubating H37Ra and H37Rv suspensions at 80°C for 1 h, followed by storage at −70°C. All mycobacteria were thawed quickly and sonicated briefly at low power before use. Mannose-capped lipoarabinomannan (ManLAM) and cell wall (RvCW) isolated from H37Rv were provided by the laboratory of John Belisle (Department of Microbiology, Colorado State University, Fort Collins) through funds from the National Institutes of Health, National Institute of Allergy and Infectious Diseases, contract NO1-AI-75320 (entitled “Tuberculosis Research Materials and Vaccine Testing”).
Experimental animals and vaccination.
Outbred Hartley strain guinea pigs (Charles River Breeding Laboratory, Inc., Wilmington, Mass.) were individually housed in an air-filtered environment, within polycarbonate cages with stainless steel grid floors and feeders. They were given food (Ralston Purina, St. Louis, Mo.) and tap water ad libitum. All procedures were reviewed and approved by the Texas A&M University Laboratory Animal Care Committee. Vaccinated animals received a subcutaneous injection of 0.1 ml of Mycobacterium bovis BCG vaccine (Danish 1331: Statens Seruminstitut, Copenhagen, Denmark) in the left inguinal region as described previously (30). The lyophilized BCG vaccine was prepared by reconstitution in 0.9% sterile saline and delivered approximately 103 viable organisms per animal. Guinea pigs were rested at least 6 weeks postvaccination before their alveolar macrophages were harvested.
Isolation of alveolar macrophages.
Euthanasia was carried out by intraperitoneal injection of 3 ml of sodium pentobarbital (100 mg/ml) (Sleepaway Euthanasia Solution; Fort Dodge Laboratories, Inc., Fort Dodge, Iowa). The abdominal and thoracic cavities of each guinea pig were opened aseptically, and the trachea was separated from the surrounding tissue. BAL was performed by instilling 10 to 15 ml of ice-cold 12 mM lidocaine in phosphate-buffered saline (PBS) with 3% fetal bovine serum into the exposed trachea by an 18-gauge cannula fixed to a 20-ml syringe. Previous studies have not demonstrated a detrimental effect of lidocaine on alveolar macrophage functions (15, 31, 54). The solution was left in the lungs for 1 min to allow the lidocaine to loosen adherent cells, then it was removed by drawing the fluid back into the syringe. BAL fluid was collected in sterile 50-ml centrifuge tubes (Becton Dickinson Labware, Franklin Lakes, N.J.). Three lavages (10 to 15 ml of fluid) were performed on each animal. Lavage cells were washed one time in PBS, and cell viability was determined by Trypan blue exclusion (Gibco Life Technologies, Grand Island, N.Y.). The number of cells was adjusted to 5 × 105 cells/ml in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 10 μM 2-mercaptoethanol, and 2 μM l-glutamine (RPMI-1640 complete medium).
Cell stimulation.
Cells from the BAL fluid were allowed to adhere to 12-well, flat-bottom tissue culture plates (Becton Dickinson Labware) for 1 h. Nonadherent cells were poured off, and adherent cells were washed one time with warm PBS. Adherent cells were over 97% alveolar macrophages as determined by Diff-Quik staining (Dade Behring Inc., Newark, Del.). Adherent alveolar macrophages were infected in vitro with live attenuated M. tuberculosis H37Ra or live virulent M. tuberculosis H37Rv (MOI 1:1) or cultured with or without lipopolysaccharide (LPS), 10 μg/ml, from Escherichia coli serotype 0111:B4 (Sigma Chemical Co., St. Louis, Mo.) for 3, 12, and 24 h. Adherent alveolar macrophages were also cultured with heat-killed H37Ra (HKRa), heat-killed H37Rv (HKRv) (multiplicity of infection [MOI], 1:1), total cell wall (RvCW, 10 μg/ml), or ManLAM (10 μg/ml) isolated from H37Rv, for 24 h. Total RNA from the macrophages was collected by using the RNeasy kit (QIAGEN, Valencia, Calif.) with the addition of RNase-free DNase according to the manufacturer's instructions. Supernatants were collected and stored at −70°C until analyzed by enzyme-linked immunosorbent assay (ELISA).
Real-time PCR.
Approximately 1 to 5 μg of total RNA from each treatment group was reverse transcribed by using MultiScribe reverse transcriptase, Random Hexamers, and reverse transcriptase reagents (Applied Biosystems, Branchburg, N.J.). Real-time primers for guinea pig IL-8, MCP-1, and 18s rRNA were designed by Primer Express software (Applied Biosystems) using the sequences from these cloned genes (52, 53). Primers were constructed as follows: IL-8 (forward primer, GGCAGCCTTCCTGCTCTCT; reverse primer, CAGCTCCGAGACCAACTTTGT), MCP-1 (forward primer, TGCCAAACTG GACCAGAGAA; reverse primer, CGAATGTTCAAAGGCTTTGAAGT), and 18s (forward primer, TGCATGGCCGTTCTTAGTTG; reverse primer, AGTTAGCATGCCAGAGTCTCGTT). Reverse-transcribed cDNA was amplified with primer sets for guinea pig IL-8, MCP-1, and 18s as indicated above using SYBR Green PCR core reagents and an Applied Biosystems prism 7700 sequence detector (Applied Biosystems) following the manufacturer's instructions. Fold induction of mRNA was determined from the threshold cycle (Ct) values normalized for 18s expression and then normalized to the value derived from nonvaccinated controls.
ELISA for IL-8 protein.
Guinea pig IL-8 protein was measured in experimental supernatants by using the human IL-8 ELISA, which cross-reacts significantly with guinea pig IL-8, as shown previously (7). Briefly, ELISA plates were made with the DuoSet ELISA Development System for human IL-8 (R&D Systems, Minneapolis, Minn.). Supernatants from stimulated alveolar macrophage cultures were added to the plates according to the manufacturer's instructions. Plates were read on a Dynatech MR5000 automated plate reader and analyzed with Biolinx Software, version 2.10 (Dynatech Laboratories, Inc., Chantilly, Va.).
Statistical analysis.
Differences between groups were compared by Student's one-tailed t test, assuming equal variances. P values of <0.05 were considered significant.
RESULTS
Vaccination status and IL-8 mRNA production by alveolar macrophages.
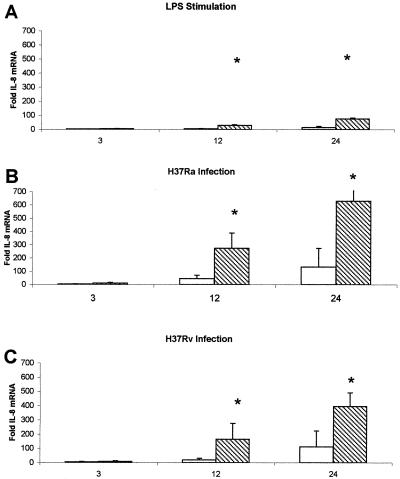
Alveolar macrophages taken from nonvaccinated and BCG-vaccinated guinea pigs were harvested and cultured for 24 h as indicated. Trypan blue exclusion showed no significant decrease in viability among cells taken from either group of guinea pigs incubated with the stimuli listed below (data not shown). Alveolar macrophages from nonvaccinated and BCG-vaccinated guinea pigs were infected with live M. tuberculosis H37Ra or H37Rv (MOI 1:1) or cultured with or without LPS (10 μg/ml) for 3, 12, and 24 h. Total RNA from the macrophages was collected, reverse transcribed to cDNA, and detected by real-time PCR by using probes for guinea pig IL-8, MCP-1, and 18s rRNA. Figure 1 presents the effects of vaccination status, type of stimulus, and duration of stimulus on IL-8 mRNA levels. Alveolar macrophages isolated from BCG-vaccinated guinea pigs expressed significantly more mRNA for IL-8 when stimulated with LPS (Fig. 1A) at 12 and 24 h poststimulation than did alveolar macrophages isolated from nonvaccinated guinea pigs. Alveolar macrophages isolated from BCG-vaccinated animals expressed significantly more mRNA for IL-8 when infected in vitro with live M. tuberculosis H37Ra (Fig. 1B) and H37Rv (Fig. 1C) at 12 and 24 h poststimulation than did alveolar macrophages isolated from nonvaccinated guinea pigs. Staining of infected guinea pig alveolar macrophages showed intracellular acid-fast bacilli, suggesting that the effects observed were generated by guinea pig alveolar macrophages that had taken up M. tuberculosis in vitro (data not shown). These data demonstrate that BCG vaccination status has a significant impact upon the IL-8 response of guinea pig alveolar macrophages to infection with both attenuated and virulent mycobacteria, as well as stimulation with LPS.
FIG. 1.
Vaccination status affects IL-8 mRNA production by alveolar macrophages. In vitro-cultured alveolar macrophages from nonvaccinated (open bars) and vaccinated (hatched bars) guinea pigs were infected with live M. tuberculosis H37Ra (B) or H37Rv (MOI, 1:1) (C) or cultured with or without LPS (10 μg/ml) (A) for 3, 12, and 24 h. Total RNA from the macrophages was collected, reverse transcribed to cDNA, and detected by real-time PCR by using probes for IL-8 and 18s rRNA. Fold induction was determined from Ct values normalized for 18s expression and then normalized to the value derived from nonvaccinated controls. Alveolar macrophages isolated from vaccinated animals produced significantly more RNA for IL-8 when stimulated with LPS (A) or infected with M. tuberculosis H37Ra (B) and H37Rv (C) at 12 and 24 h poststimulation than did alveolar macrophages isolated from nonvaccinated guinea pigs. Data are means ± standard errors of the means of four animals. P values of <0.05 (*) were considered significant.
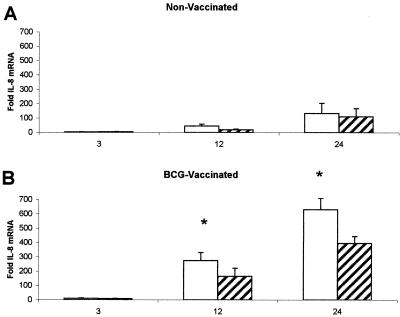
Effect of mycobacterial virulence on levels of IL-8 mRNA in infected macrophages.
The attenuated laboratory strain of M. tuberculosis, H37Ra, replicates within guinea pig lungs and other tissues but is controlled and eliminated much more effectively than the virulent H37Rv strain (1). To characterize the differences between these two common laboratory strains in terms of their ability to stimulate IL-8 expression, alveolar macrophages from nonvaccinated and BCG-vaccinated guinea pigs were infected with live M. tuberculosis H37Ra and H37Rv (MOI, 1:1), for 3, 12, and 24 h. Figure 2 illustrates the differential effects that each mycobacterial strain exhibits over time on IL-8 mRNA levels. Alveolar macrophages isolated from nonvaccinated guinea pigs infected with the attenuated H37Ra strain showed no significant difference in expression of IL-8 mRNA detected by real-time PCR when compared to alveolar macrophages infected with virulent H37Rv at any time point (Fig. 2A). H37Ra-infected alveolar macrophages isolated from BCG-vaccinated guinea pigs, however, exhibited significantly more IL-8 mRNA expression than did alveolar macrophages infected with H37Rv at both 12 and 24 h postinfection (Fig. 2B).
FIG. 2.
Effects of vaccination on differential IL-8 mRNA expression between M. tuberculosis H37Ra- and H37Rv-infected alveolar macrophages. In vitro-cultured alveolar macrophages from nonvaccinated (A) and BCG-vaccinated (B) guinea pigs were infected with live M. tuberculosis H37Ra (open bars), or H37Rv (MOI, 1:1) (hatched bars) for 3, 12, and 24 h. Total RNA from the macrophages was collected, reverse transcribed to cDNA, and detected by real-time PCR by using probes for guinea pig IL-8 and 18s rRNA. Fold induction was determined from Ct values normalized for 18s expression and then normalized to the value derived from nonvaccinated controls. Alveolar macrophages isolated from nonvaccinated guinea pigs infected with H37Ra showed no significant difference in expression of IL-8 RNA when compared to alveolar macrophages infected with H37Rv at any time point (A). H37Ra-infected alveolar macrophages isolated from vaccinated guinea pigs showed significantly more IL-8 RNA expression than did alveolar macrophages infected with H37Rv at 12 and 24 h (B). Data are means ± standard errors of the means of four animals. P values of <0.05 (*) were considered significant.
Vaccination status and MCP-1 RNA expression by alveolar macrophages.
Alveolar macrophages harvested from nonvaccinated and BCG-vaccinated guinea pigs were assayed for the expression of MCP-1 mRNA. Table 1 illustrates the effects of vaccination status, type of stimulus, and duration of stimulus on MCP-1 mRNA levels. Significant MCP-1 mRNA production was detected in LPS-stimulated and mycobacterium-infected alveolar macrophages taken from both nonvaccinated and BCG-vaccinated guinea pigs compared to unstimulated controls. Despite the obvious increases in MCP-1 message detected, there was no statistically significant difference in MCP-1 mRNA expression between alveolar macrophages isolated from nonvaccinated or BCG-vaccinated guinea pigs at any time point when the cells were stimulated with LPS or infected with M. tuberculosis H37Ra and H37Rv (Table 1).
TABLE 1.
Effect of vaccination and in vitro stimulation on MCP-1 mRNA in guinea plg alveolar macrophages
| Vaccination statusa | Treatment of cells in vitrob | Mean ± SEM of fold induction at indicated time poststimulationc
|
||
|---|---|---|---|---|
| 3 h | 12 h | 24 h | ||
| Nonvaccinated | Control | 1.0 ± 0 | 1.0 ± 0 | 1.0 ± 0 |
| LPS | 2.2 ± 0.2 | 2.1 ± 0.4 | 3.2 ± 1.3 | |
| H37Ra | 2.2 ± 0.8 | 2.9 ± 0.4 | 2.6 ± 1.3 | |
| H37Rv | 2.8 ± 0.8 | 2.4 ± 1.5 | 0.9 ± 0.7 | |
| BCG vaccinated | Control | 2.1 ± 1.3 | 2.7 ± 0.8 | 1.3 ± 1.0 |
| LPS | 4.5 ± 2.8 | 4.1 ± 2.2 | 2.8 ± 3.2 | |
| H37Ra | 4.2 ± 2.8 | 4.1 ± 2.1 | 9.9 ± 10.6 | |
| H37Rv | 2.6 ± 1.8 | 3.1 ± 1.5 | 3.3 ± 3.1 | |
Vaccinated animals received a subcutaneous injection of 0.1 ml of M. bovis BCG vaccine (Danish 1331: Statens Seruminstitut, Copenhagen, Denmark) in the left inguinal region as described in Materials and Methods.
Alveolar macrophages isolated from nonvaccinated and BCG-vaccinated guinea pigs were infected with two laboratory strains of M. tuberculosis, H37Ra and H37Rv (MOI, 1:1), or were cultured with lipopolysaccharide (LPS, 10 μg/ml.) from E. coli. Control alveolar macrophages were incubated in complete media alone.
In vitro cultured alveolar macrophages from nonvaccinated and BCG-vaccinated guinea pigs (four animals per treatment group) were infected with live M. tuberculosis H37Ra or H37Rv or cultured with or without LPS for 3, 12, and 24 h. Total RNA from the macrophages was collected, reverse transcribed to cDNA, and detected by real-time PCR. Differences between groups were compared by Student's one-tailed t test, assuming equal variances. P values of <0.05 were considered significant.
Effects of mycobacterial virulence on MCP-1 mRNA expression in infected macrophages.
Since BCG-vaccinated guinea pig alveolar macrophages infected with the more virulent laboratory strain H37Rv had lower levels of IL-8 mRNA production than those infected with the attenuated strain H37Ra, we wanted to see if this was a generalized phenomenon that may act on a signal whose targets include cells known to appear later in the immune response, such as the monocyte/T-lymphocyte-attracting chemokine MCP-1. Alveolar macrophages from nonvaccinated and BCG-vaccinated guinea pigs were infected with live M. tuberculosis H37Ra and H37Rv (MOI, 1:1), for 3, 12, and 24 h. Alveolar macrophages isolated from nonvaccinated and BCG-vaccinated guinea pigs showed no significant differences in expression of MCP-1 mRNA when infected with either M. tuberculosis H37Ra or H37Rv at any time point (Table 1).
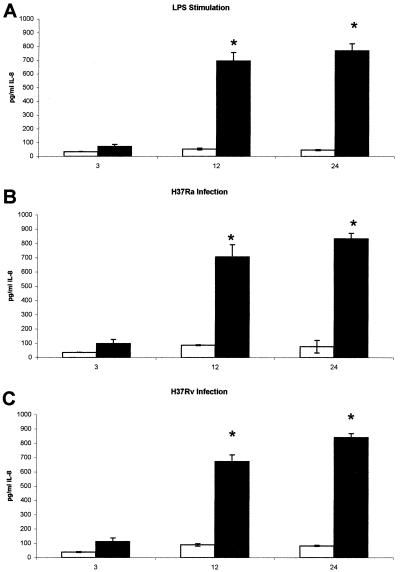
Levels of IL-8 protein in alveolar macrophage supernatants.
The amount of mRNA detected for a given protein may not correspond to the amount of subsequent protein produced due to posttranslational regulation. Therefore, levels of IL-8 protein were measured in the supernatants recovered from control, LPS-stimulated, H37Ra-, and H37Rv-infected alveolar macrophages isolated from nonvaccinated and BCG-vaccinated guinea pigs by using the human IL-8 ELISA, which has been demonstrated to detect guinea pig IL-8 (7). Figure 3 illustrates the effects of vaccination status, type of stimulus, and duration of stimulus on IL-8 protein levels recovered from cell supernatants. Consistent with the mRNA data, supernatants recovered from alveolar macrophages isolated from BCG-vaccinated guinea pigs showed significantly more immunodetectable IL-8 protein in LPS-stimulated (Fig. 3A), H37Ra-infected (Fig. 3B), and H37Rv-infected (Fig. 3C) alveolar macrophages at 12 and 24 h than did supernatants recovered from alveolar macrophages isolated from nonvaccinated guinea pigs. Despite the difference in IL-8 mRNA levels seen in alveolar macrophages isolated from BCG-vaccinated guinea pigs infected with H37Ra and H37Rv (Fig. 2B), the amounts of immunodetectable protein in the infected cell supernatants at 12 h (706.6 ± 84.8 and 672.5 ± 46.95 pg/ml) and 24 h (833.0 ± 38.6 and 841.5 ± 27.3 pg/ml) showed no significant differences.
FIG. 3.
Effects of vaccination and in vitro stimulus on IL-8 production. Alveolar macrophages isolated from nonvaccinated and BCG-vaccinated guinea pigs were infected with two laboratory strains of M. tuberculosis, H37Ra and H37Rv (MOI 1:1) or were cultured with LPS (10 μg/ml) from E. coli. Control alveolar macrophages were incubated in complete media alone. Levels of guinea pig IL-8 protein were measured in the supernatants recovered from control, LPS-stimulated (A), H37Ra-infected (B) , and H37Rv-infected (C) alveolar macrophages isolated from nonvaccinated (open bars) and BCG-vaccinated (solid bars) guinea pigs by using the human IL-8 ELISA. Data are reported as means ± standard errors of the means. There were four animals per treatment group. Differences between groups were compared by Student's one-tailed t test, assuming equal variances. P values of <0.05 (*) were considered significant.
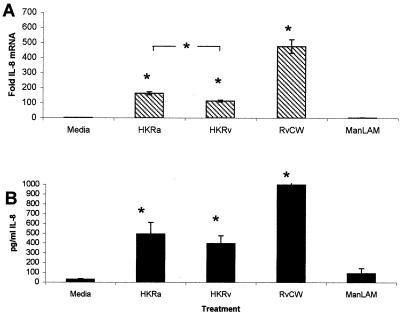
Effects of heat-killed M. tuberculosis and ManLAM on IL-8 mRNA and protein production by BCG-vaccinated guinea pig alveolar macrophages.
IL-8 mRNA production from BCG-vaccinated guinea pig alveolar macrophages infected with the virulent M. tuberculosis strain H37Rv was lower than the production of alveolar macrophages infected with the attenuated H37Ra. This observation was further analyzed to determine if living bacilli were required or whether the same observation could be mimicked by stimulating cells with RvCW or the principal bioactive cell wall constituent, ManLAM. Alveolar macrophages harvested from BCG-vaccinated guinea pigs were incubated with HKRa, HKRv, RvCW, or ManLAM taken from H37Rv. Figure 4 shows that HKRa, HKRv, and RvCW can stimulate significant IL-8 mRNA expression and protein production from alveolar macrophages isolated from BCG-vaccinated guinea pigs when compared with media alone (Fig. 4A and B). Incubation of this same cell type with ManLAM did not stimulate IL-8 mRNA or protein at levels significantly above stimulation with media alone (Fig. 4A and B). As was seen following infections with living mycobacteria, alveolar macrophages isolated from BCG-vaccinated guinea pigs stimulated with HKRa exhibited significantly more IL-8 mRNA expression than alveolar macrophages stimulated with HKRv (Fig. 4A). Despite this difference in IL-8 mRNA levels, no significant effect of mycobacterial virulence on the amount of immunodetectable protein in the infected cell supernatants was seen (Fig 4B).
FIG. 4.
Ability of dead bacilli or their constituents to stimulate IL-8 production. (A) In vitro-cultured alveolar macrophages from BCG-vaccinated guinea pigs were stimulated with media alone, HKRa, HKRv, 10-μg/ml total cell wall harvested from H37Rv (RvCW), or 10-μg/ml ManLAM harvested from H37Rv (ManLAM) for 24 h. Total RNA from the macrophages was collected, reverse transcribed to cDNA, and detected by real-time PCR by using probes for IL-8 and 18s rRNA. Fold induction was determined from Ct values normalized for 18s expression and then normalized to the value derived from controls. Alveolar macrophages stimulated with heat-killed H37Ra, H37Rv, and total H37Rv cell wall showed significantly more IL-8 mRNA expression than did alveolar macrophages stimulated with media alone or H37Rv ManLAM. (B) Levels of guinea pig IL-8 protein were measured in the supernatants recovered from cultures of alveolar macrophage harvested from BCG-vaccinated guinea pigs stimulated with media alone, HKRa, HKRv, 10-μg/ml RvCW, or 10-μg/ml ManLAM harvested from H37Rv for 24 h by using the human IL-8 ELISA. Alveolar macrophages stimulated with HKRa, HKRv, and total H37Rv cell wall produced significantly more IL-8 protein than did alveolar macrophages stimulated with media alone or H37Rv ManLAM. All data are reported as means ± standard errors of the means. There were four animals per treatment group. Differences between groups were compared by Student's one-tailed t test, assuming equal variances. P values of <0.05 (*) were considered significant.
DISCUSSION
Studies with murine models of TB and with TB patients have shown that many cell types can be stimulated by mycobacteria to express mRNA and produce protein for the chemokine IL-8 or the murine homolog neutrophil-activating protein 1 (NAP-1) (10, 19, 25, 36, 37). Human alveolar macrophages have been stimulated by mycobacteria to express message for IL-8, and BAL fluids from human TB patients were shown to contain elevated levels of immunodetectable IL-8 protein compared to healthy controls (55).
In this paper we demonstrate for the first time that primary alveolar macrophages harvested from BCG-vaccinated guinea pigs and stimulated in vitro with a variety of stimuli, including infection with attenuated or virulent mycobacteria, produce levels of IL-8 mRNA detected by real-time PCR significantly higher than the levels observed with alveolar macrophages harvested from nonvaccinated guinea pigs (Fig. 1). Prior work in our laboratory has shown that the differences in IL-8 mRNA levels from nonvaccinated and BCG-vaccinated guinea pig alveolar macrophages can be detected by Northern blot analysis (reference 26 and data not shown). Our laboratory has also reported that whole splenocytes and adherence-purified peritoneal exudate cells taken from BCG-vaccinated guinea pigs express mRNA for other cytokines (e.g., IL-1β) and chemokines (e.g., Regulated upon Activation, Normally T cell Expressed and Secreted, RANTES) at a significantly increased level when compared to cells taken from nonvaccinated guinea pigs (16).
This paper demonstrates that primary alveolar macrophages harvested from BCG-vaccinated guinea pigs also produce significantly higher levels of protein for IL-8 than do alveolar macrophages harvested from nonvaccinated guinea pigs when stimulated or infected in vitro (Fig. 3). The immune status of the host has been previously shown to be important in the production of chemokines. Human TB patients in both the active and the convalescent phases of disease showed protein levels of the chemokines IL-8, MCP-1, and RANTES in their BAL fluids that were significantly elevated compared to those of healthy subjects (21).
Since M. bovis BCG is a live, attenuated vaccine known to reach the lungs of vaccinated hosts (44), it is likely that animals that had received BCG prior to BAL had alveolar macrophages that were in a higher state of responsiveness. This allowed the cells to produce more message and protein for IL-8 than those quiescent cells isolated from nonvaccinated guinea pigs (Fig. 1 and 3). It is also formally possible that our adherent alveolar macrophage cell cultures contained a very small population of contaminating alveolar lymphocytes, which could have boosted the macrophage response in the presence of mycobacterial antigens only in vaccinated guinea pigs. However, the fact that LPS-stimulated alveolar macrophage cultures from BCG-vaccinated guinea pigs also produced significantly more IL-8 mRNA suggests that the effect was due to some vaccine-induced alteration in intrinsic macrophage function.
The early influx of neutrophils that mycobacterially induced alveolar macrophage-derived IL-8 likely generates may be crucial for a protective immune response to develop. The presence of neutrophils, while not very helpful in reducing bacterial numbers by phagocytosis and intracellular killing (18, 41), may contribute to the accumulation of critical soluble mediators of the successful transition to an antigen-specific, adaptive immune response in the lung, such as IL-12, TNF-α, IL-1β, MCP-1, and gamma interferon (6, 20, 34, 40, 46). These mediators could work in autocrine and/or paracrine fashion to affect the cytokine and chemokine balance of the whole infected region. Prior work has shown that the depletion of neutrophils results in an increased susceptibility to infections with mycobacteria (2, 33, 43) and a diminished capacity for granuloma formation (12, 20).
But it must be noted that at present, the full contribution of the neutrophil to resistance to infections by M. tuberculosis is still unknown. Since IL-8 is a pleotrophic cytokine, it is quite possible that IL-8 production by infected alveolar macrophages is for T-cell recruitment, which is an early requirement for granuloma formation, and the early influx of neutrophils is an unintended side effect. Furthermore, the presence of the neutrophil could be responsible for local tissue destruction, causing more unintended damage instead of aiding in protection. Lastly, all of these experiments were performed in vitro. Chemokine expression, by its very nature, is very complicated. In order to see how IL-8 functions during a more rational infection, these experiments must be repeated in vivo. Those studies are under way and will be completed in the near future.
Significant MCP-1 mRNA production also was detected in stimulated or infected alveolar macrophages taken from nonvaccinated and BCG-vaccinated guinea pigs; however, prior vaccination did not appear to affect levels of MCP-1 mRNA at the time points analyzed (Table 1). MCP-1 is suspected to be an important chemokine in the recruitment of monocytes and T lymphocytes (4) in response to infection with mycobacteria (11, 21, 36). The production of MCP-1 by infected alveolar macrophages may be responsible for the replacement of early responding neutrophils with monocytes and antigen-reactive T lymphocytes that characterize the later-stage granulomatous immune response to tuberculosis (9).
While mRNA for MCP-1 was detected as early as 3 h poststimulation, prior vaccination did not appear to significantly affect the level of MCP-1 mRNA expressed up to 24 h in culture. Some increase in MCP-1 mRNA levels was seen at that interval in the alveolar macrophages taken from BCG-vaccinated guinea pigs and infected with the attenuated H37Ra strain (Table 1). The virulence of the M. tuberculosis strain used did not appear to affect MCP-1 mRNA production significantly, unlike IL-8 mRNA levels, although the mean values for H37Ra-infected cells were somewhat higher than H37Rv-infected cells at 24 h (Table 1). It could be that MCP-1 is more important later in the macrophage response to infection, since the cellular targets for MCP-1, i.e., monocytes and T lymphocytes, do not appear until after the first wave of neutrophils has arrived (9). It is also possible that alveolar macrophage-derived MCP-1 may not account for the majority of inflammatory cell accumulation observed in the lungs of mycobacterially infected animals. Examining MCP-1 mRNA production by guinea pig alveolar macrophages at time points later than 24 h may allow us to test this hypothesis, and those experiments are under way.
H37Ra-infected alveolar macrophages isolated from BCG-vaccinated guinea pigs exhibited significantly more IL-8 mRNA expression than did alveolar macrophages infected with H37Rv at both 12 and 24 h postinfection (Fig. 2B). Prior work in our lab has shown that the infection of guinea pig macrophages with the virulent strain of M. tuberculosis H37Rv, compared to the attenuated strain H37Ra, results in reduced mRNA expression of the cytokine IL-1β and the chemokine RANTES (16). These data suggest that the virulent H37Rv strain modulates the cytokine response of guinea pig alveolar macrophages by unknown mechanisms to dampen the early inflammatory response in the lung. This would delay an effective host response so that this slow-growing pathogen would have time to replicate and establish an infection. This lowering of cytokine production by alveolar macrophages incubated with virulent mycobacteria is not associated with any detectable loss of alveolar macrophage viability, likely due to the relatively low infection level (MOI, 1:1) and short time (24 h) in culture.
A review of the literature has shown that differences in the virulence of mycobacterial species can affect the amount of cytokine or chemokine produced by many different cell types. Peripheral blood mononuclear cells harvested from healthy individuals produced significantly more TNF-α, IL-1α, IL-β, and IL-8 protein when infected with an avirulent strain of Mycobacterium avium complex than did infection with a virulent variant (17, 32). Human peripheral blood mononuclear cells infected with M. tuberculosis H37Ra made more nitric oxide than those infected with H37Rv (22). Sadek et al. (39) detected more IL-8 and MCP-1 protein in the supernatant of H37Ra-infected primary human alveolar macrophages than in the supernatant of those infected with H37Rv.
Supernatants recovered from LPS-stimulated, H37Ra-infected, and H37Rv-infected alveolar macrophages isolated from BCG-vaccinated guinea pigs contained significantly more immunodetectable IL-8 protein at 12 and 24 h than supernatants recovered from similarly treated alveolar macrophages isolated from nonvaccinated guinea pigs (Fig. 3). Protein concentrations correlate well with the mRNA levels detected by real-time PCR (Fig. 1) and Northern analysis (data not shown), suggesting that the general effects of cell stimulation on IL-8 mRNA were reflected in the amount of immunoreactive IL-8 actually secreted by the cells.
However, despite the significant difference in IL-8 mRNA levels seen in alveolar macrophages isolated from BCG-vaccinated guinea pigs infected with H37Ra versus H37Rv (Fig. 2B), no significant effect of mycobacterial virulence on the amount of immunoreactive IL-8 protein in the infected cell supernatants was seen at these time points (Fig. 3). This discrepancy between IL-8 mRNA and protein levels in the same cultures could be due to the short time (24 h) in culture. Later time points may allow for a difference in protein accumulation between H37Ra- and H37Rv-infected cell supernatants to develop. These experiments are currently under way.
The observed lower IL-8 mRNA expression from BCG-vaccinated guinea pig alveolar macrophages infected with the virulent M. tuberculosis strain H37Rv compared to the attenuated H37Ra strain was further analyzed. This effect did not appear to require living bacilli since alveolar macrophages isolated from BCG-vaccinated guinea pigs stimulated with HKRv had significantly lower expression of IL-8 mRNA than those stimulated with HKRa (Fig. 4A). Despite the lower levels of IL-8 mRNA expression observed by stimulation with HKRv, total cell wall isolated from H37Rv was a potent stimulator for both IL-8 mRNA and protein (Fig. 4A and B). This discrepancy may be due to the processing differences between HKRv and the H37Rv cell wall. Since both heat-killed bacillus treatments produced lower levels of IL-8 message than infection with live bacteria, the heat killing of the mycobacteria may have altered crucial immunostimulatory cell wall constituents that the total cell wall processing technique left intact.
It is important to note that stimulation with HKRa, HKRv, and RvCW induced levels of IL-8 message and protein by alveolar macrophages harvested from BCG-vaccinated guinea pigs that were significant when compared to the levels observed with alveolar macrophages that were stimulated with media alone. Prior research has implicated ManLAM in the suppression of cytokine production (38, 51) but lipoarabinomannans isolated from other mycobacterial strains have been shown to be stimulatory in nature (35, 55). Alveolar macrophages stimulated with purified ManLAM did not produce significant levels of IL-8 mRNA or protein (Fig. 4). Since ManLAM is presumably present in live and HKRv, which appeared to stimulate lower levels of IL-8 message from alveolar macrophages than did H37Ra, and its total cell wall isolate, which appeared to stimulate the macrophage to produce IL-8 mRNA and protein at high levels, it is unknown if the lack of IL-8 mRNA and protein observed from BCG-vaccinated guinea pig alveolar macrophages stimulated with purified ManLAM was due to active down-regulation of the alveolar macrophages or whether this preparation of ManLAM was unable to activate this cell type sufficiently on its own. This phenomenon will be analyzed further by stimulating alveolar macrophages with different fractions of H37Rv cell wall which include and exclude ManLAM or by adding ManLAM to cultures stimulated by LPS or H37Ra. This would allow for the participation of this and other cell wall components to be studied.
In summary, it appears that the immune status of the host has a strong influence on the production of chemokines by guinea pig alveolar macrophages. Vaccination of the host with M. bovis BCG may cause a significant up-regulation of crucial chemotactic factors that may aid in the recruitment of inflammatory cells into the lung, leading to the expression of protective immunity. Increased production of IL-8 may suggest an important role of early infiltrating neutrophils in antimycobacterial immunity. The virulence of the mycobacterial strain used to infect guinea pig cells may also strongly influence the amount of chemokines produced. Further elucidation of the cellular and molecular mechanisms underlying these observations may help with the development and testing of new tuberculosis vaccine candidates in the guinea pig model.
Acknowledgments
This work was supported by National Institutes of Health grant RO1-AI-15495.
We are indebted to Shannon Sedberry Allen for her helpful suggestions and aid with the real-time PCR.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Alsaadi, A., and D. W. Smith. 1973. The fate of virulent and attenuated mycobacteria in guinea pigs infected by the respiratory route. Am. Rev. Respir. Dis. 107:1041-1046. [DOI] [PubMed] [Google Scholar]
- 2.Appleberg, R., A. G. Castro, S. Gomes, J. Pedrosa, and M. T. Silva. 1995. Susceptibility of beige mice to Mycobacterium avium: role of neutrophils. Infect. Immun. 63:3381-3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baggiolini, M., A. Walz, and S. Kunkel. 1989. Neutrophil-activating peptide-1/interleukin-8, a novel cytokine that activates neutrophils. J. Clin. Investig. 84:1045-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baggiolini, M., B. Dewald, and B. Moser. 1994. Interleukin-8 and related chemotactic cytokines-CXC and CC chemokines. Adv. Immunol. 55:97-102. [PubMed] [Google Scholar]
- 5.Baldwin, R. A., C. D'Souza, A. D. Roberts, B. P. Kelly, A. A. Frank, M. A. Lui, J. B. Ulmer, K. Huygen, D. N. McMurray, and I. M. Orme. 1998. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infect. Immun. 66:2951-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bliss, S. K., Y. Zhang, and E. Y. Denkers. 1999. Murine neutrophil stimulation by Toxoplasma gondii antigen drives high level production of IFN-gamma-independent IL-12. J. Immunol. 163:2081-2088. [PubMed] [Google Scholar]
- 7.Danahay, H., K. J. Broadley, P. J. McCabe, A. T. Nials, and S. Sanjar. 1999. Temporal relationships between leukocytes, IL-5 and IL-8 in guinea pig lungs, plasma cortisol and airway function after antigen challenge. Inflamm. Res. 48:41-47. [DOI] [PubMed] [Google Scholar]
- 8.Ehrt, S., D. Schnappinger, S. Bekiranov, J. Drenkow, S. Shi, T. R. Gingeras, T. Gaasterland, G. Schoolnik, and C. Nathan. 2001. Reprogramming of the macrophage transcriptome in response to interferon-γ and Mycobacterium tuberculosis: signaling roles of nitric oxide synthase-2 and phagocyte oxidase. J. Exp. Med. 194:1123-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenton, M., and M. W. Vermeulen. 1996. Immunopathology of tuberculosis: roles of macrophages and monocytes. Infect. Immun. 64:683-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedland, J. S., D. G. Remick, R. Shattock, and G. E. Griffin. 1992. Secretion of interleukin-8 following phagocytosis of Mycobacterium tuberculosis by human monocyte cell lines. Eur. J. Immunol. 22:1373-1378. [DOI] [PubMed] [Google Scholar]
- 11.Friedland, J. S., R. J. Shattock, and G. E. Griffin. 1993. Phagocytosis of Mycobacterium tuberculosis particulate stimuli by human monocytic cells induces equivalent monocyte chemotactic protein-1 gene expression. Cytokine 5:150-156. [DOI] [PubMed] [Google Scholar]
- 12.Gao, J., T. A. Wynn, Y. Chang, E. J. Lee, H. E. Broxmeyer, S. Cooper, H. L. Tiffany, H. Westphal, J. Kwon-Chung, and P. M. Murphy. 1997. Impaired host defense hematopoiesis granulomatous inflammation and type 1-type 2 cytokine balance in mice lacking CC Chemokine Receptor 1. J. Exp. Med. 185:1959-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Global Tuberculosis Programme. 1998. Global Tuberculosis Control, W.H.O. Report 1998. World Health Organization, Geneva, Switzerland.
- 14.Grover, A. A., H. K. Kim, E. H. Wiegeshaus, and D. W. Smith. 1967. Host-parasite relationship in experimental airborne tuberculosis. II. Reproducible infection by means of an inoculum preserved at −70°C. J. Bacteriol. 95:832-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holian, A., J. H. Dauber, M. S. Diamond, and R. P. Daniele. 1983. Separation of bronchoalveolar cells from the guinea pig on continuous gradients of Percoll: functional properties of fractionated lung macrophages. J. Reticuloendothel. Soc. 33:157-164. [PubMed] [Google Scholar]
- 16.Jeevan, A., T. Yoshimura, G. Foster, and D. N. McMurray. 2002. Effect of Mycobacterium bovis BCG vaccination on interleukin-1b and RANTES mRNA expression in guinea pig cells exposed to attenuated and virulent mycobacteria. Infect. Immun. 70:1245-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson, J. L., H. Shiratsuchi, Z. Toosi, and J. J. Ellner. 1997. Altered IL-1 expression and compartmentalization in monocytes from patients with AIDS stimulated with Mycobacterium avium complex. J. Clin. Immunol. 17:387-395. [DOI] [PubMed] [Google Scholar]
- 18.Kasahara, K., I. Sato, K. Ogura, H. Yakeuchi, K. Kobayashi, and M. Adachi. 1998. Expression of chemokines and induction of rapid cell death in human blood neutrophils by Mycobacterium tuberculosis. J. Infect. Dis. 178:127-137. [DOI] [PubMed] [Google Scholar]
- 19.Kasahara, K., T. Tobe, M. Tomita, N. Mukaida, S. Shao-Bo, K. Matsushima, T. Yoshida, S. Sugihara, and K. Kobayashi. 1994. Selective expression of monocyte chemotactic and activation factor/monocyte chemoattractant protein 1 in human blood monocytes by Mycobacterium tuberculosis. J. Infect. Dis. 170:1238-1247. [DOI] [PubMed] [Google Scholar]
- 20.Kilgore, K. S., M. M. Imlay, J. P. Szaflarski, F. S. Silverstein, A. N. Malani, V. M. Evans, and J. S. Warren. 1997. Neutrophils and reactive oxygen intermediates mediate glucan-induced pulmonary granuloma formation through the local induction of Monocyte Chemoattractant Protein-1. Lab. Investig. 76:191-201. [PubMed] [Google Scholar]
- 21.Kurashima, K., N. Mukaida, M. Fujimura, M. Yasui, Y. Nakazumi, T. Matsuda, and K. Matsushima. 1997. Elevated chemokine levels in bronchoalveolar lavage fluid of tuberculosis patients. Am. J. Respir. Crit. Care Med. 155:1474-1477. [DOI] [PubMed] [Google Scholar]
- 22.Kwon, O. J. 1997. The role of nitric oxide in the immune response of tuberculosis. J. Korean Med. Sci. 12:481-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsen, C. G., A. O. Anderson, E. Appella, J. J. Oppenheim, and K. Matsushima. 1989. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science 243:1464-1466. [DOI] [PubMed] [Google Scholar]
- 24.Lin, Y., J. Gong, M. Zhang, W. Xue, and P. Barnes. 1998. Production of monocyte chemoattractant protein 1 in tuberculosis patients. Infect. Immun. 66:2319-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin, Y., M. Zhang, and P. Barnes. 1998. Chemokine production by human alveolar epithelial cell line in response to Mycobacterium tuberculosis. Infect. Immun. 66:1121-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyons, M. J., T. Yoshimura, and D. N. McMurray. 2000. Interleukin (IL)-8 expression by guinea pig alveolar macrophages following stimulation by Mycobacterium tuberculosis. Tuber. Lung Dis. 80:101-102. [Google Scholar]
- 27.McMurray, D. N. 2001. Determinants of vaccine-induced resistance in animal models of pulmonary tuberculosis. Scand. J. Infect. Dis. 33:175-178. [DOI] [PubMed] [Google Scholar]
- 28.McMurray, D. N. 2001. Disease model: animal models of pulmonary tuberculosis. Trends Mol. Med. 7:135-137. [DOI] [PubMed] [Google Scholar]
- 29.McMurray, D. N., G. Dai, and S. Phalen. 1999. Mechanisms of vaccine-induced resistance in a guinea pig model of pulmonary tuberculosis. Tuber. Lung Dis. 79:261-266. [DOI] [PubMed] [Google Scholar]
- 30.McMurray, D. N., M. A. Carlomagno, C. L. Mintzer, and C. L. Tetzlaff. 1985. Mycobacterium bovis BCG vaccine fails to protect protein-deficient guinea pigs against respiratory challenge with virulent Mycobacterium tuberculosis. Infect. Immun. 50:555-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMurray, D. N., M. S. Kimball, C. L. Tetzlaff, and C. L. Mintzer. 1986. Effects of protein deprivation and BCG vaccination on alveolar macrophage function in pulmonary tuberculosis. Am. Rev. Respir. Dis. 133:1081-1085. [DOI] [PubMed] [Google Scholar]
- 32.Michelini-Norris, M. B., D. K. Blanchard, C. A. Pearson, and J. Y. Djeu. 1992. Differential release of interleukin (IL)-1 alpha, IL-1 beta, and IL-6 from normal human monocytes stimulated with a virulent and an avirulent isogenic variant of Mycobacterium avium-intracellulare complex. J. Infect. Dis. 165:702-709. [DOI] [PubMed] [Google Scholar]
- 33.Pedrosa, J., B. M. Saunders, R. Appelberg, I. M. Orme, M. T. Silva, and A. M. Cooper. 2000. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection in mice. Infect. Immun. 68:577-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petrofsky, M., and L. E. Bermudez. 1999. Neutrophils from Mycobacterium avium-infected mice produce TNF-alpha, IL-12, and IL-1-beta and have a putative role in early host response. Clin. Immunol. 91:354-358. [DOI] [PubMed] [Google Scholar]
- 35.Prinzis, S., D. Chatterjee, and P. J. Brennan. 1993. Structure and antigenicity of lipoarabinomannan from Mycobacterium bovis BCG. J. Gen. Microbiol. 139:2649-2658. [DOI] [PubMed] [Google Scholar]
- 36.Rhoades, E. R., A. M. Cooper, and I. M. Orme. 1995. Chemokine response in mice infected with Mycobacterium tuberculosis. Infect. Immun. 63:3871-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riedel, D. D., and S. H. Kaufmann. 1997. Chemokine secretion by human polymorphonuclear granulocytes after stimulation with Mycobacterium tuberculosis and lipoarabinomannan. Infect. Immun. 65:4620-4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roach, T. I. A., C. H. Barton, D. Chatterjee, and J. M. Blackwell. 1993. Macrophage activation. Lipoarabinomannan from avirulent and virulent strains of Mycobacterium tuberculosis differentially induces the early genes c-fos, KC, JE, and tumor necrosis factor-α. J. Immunol. 150:1886.. [PubMed] [Google Scholar]
- 39.Sadek, M. I., E. Sada, Z. Toossi, S. K. Schwander, and E. Rich. 1998. Chemokines induced by infection of mononuclear phagocytes with mycobacteria and present in lung alveoli during active pulmonary tuberculosis. Am. J. Respir. Cell Mol. Biol. 19:513-521. [DOI] [PubMed] [Google Scholar]
- 40.Scapini, P., J. A. Lapinet-Vera, S. Gasperini, F. Calzetti, F. Bazzoni, and M. A. Cassatella. 2000. The neutrophil as a cellular source of chemokines. Immunol. Rev. 177:195-203. [DOI] [PubMed] [Google Scholar]
- 41.Seiler, P., P. Aichele, B. Raupach, B. Odermatt, U. Steinhoff, and S. H. E. Kaufmann. 2000. Rapid neutrophil response controls fast-replicating intracellular bacteria but not slow-replicating Mycobacterium tuberculosis. J. Infect. Dis. 181:671-680. [DOI] [PubMed] [Google Scholar]
- 42.Sibille, Y., and H. Y. Reynolds. 1990. Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am. Rev. Respir. Dis. 141:471-501. [DOI] [PubMed] [Google Scholar]
- 43.Silva, M. T., M. N. T. Silva, and R. Appelberg. 1989. Neutrophil-macrophage cooperation in the host defence against mycobacterial infections. Microb. Pathog. 6:369-380. [DOI] [PubMed] [Google Scholar]
- 44.Smith, D. W., and G. E. Harding. 1978. Influence of BCG vaccination on bacillemic phase of experimental airborne tuberculosis in guinea pigs, p. 85-90. In R. J. Montali (ed.), Mycobacterial infections of zoo animals. Smithsonian Institution Press, Washington, D.C.
- 45.Standiford, T. J., S. L. Kunkel, S. H. Phan, B. J. Rollins, and R. M. Strieter. 1991. Alveolar macrophage-derived cytokines induce Monocyte Chemoattractant Protein-1 expression from human pulmonary type II-like epithelial cells. J. Biol. Chem. 266:9912-9918. [PubMed] [Google Scholar]
- 46.Tateda, K., T. A. Moore, J. C. Deng, M. W. Newstead, X. Zeng, A. Matsukawa, M. S. Swanson, K. Yamaguchi, and T. J. Standiford. 2001. Early recruitment of neutrophils determines subsequent T1/T2 host responses in a murine model of Legionella pneumophila pneumonia. J. Immunol. 166:3355-3361. [DOI] [PubMed] [Google Scholar]
- 47.Turner, O. C., A. D. Roberts, A. A. Frank, S. W. Phalen, D. N. McMurray, J. Content, O. Denis, S. D'Souza, A. Tanghe, K. Huygen, and I. M. Orme. 2000. Lack of protection in mice and necrotizing bronchointerstitial pneumonia with bronchiolitis in guinea pigs immunized with vaccines directed against the hsp60 molecule of Mycobacterium tuberculosis. Infect. Immun. 68:3674-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vaddi, K., M. Keller, and R. C. Newton. 1997. The chemokine factsbook. Academic Press, San Diego, Calif.
- 49.Wallis, R. S., and J. Ellner. 1994. Cytokines and tuberculosis. J. Leukoc. Biol. 55:676-681. [DOI] [PubMed] [Google Scholar]
- 50.Wuyts, A., P. Proost, and J. Van Damme. 1998. Interleukin-8 and other CXC chemokines, p. 271-311. In A. Thomson (ed.), The cytokine handbook, 3rd ed. Academic Press, San Diego, Calif.
- 51.Yoshida, A., and Y. Koide. 1997. Arabinofuranosyl-terminated and mannosylated lipoarabinomannans from Mycobacterium tuberculosis induce different levels of interleukin-12 expression in murine macrophages. Infect. Immun. 65:1953-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoshimura, T. 1993. cDNA cloning of guinea pig monocyte chemoattractant protein-1 and expression of the recombinant protein. J. Immunol. 150:5025-5032. [PubMed] [Google Scholar]
- 53.Yoshimura, T., and D. G. Johnson. 1993. cDNA cloning and expression of guinea pig neutrophil attractant protein-1 (NAP-1). J. Immunol. 151:6225-6236. [PubMed] [Google Scholar]
- 54.Zhang, X., and D. N. McMurray. 1998. Suppression of lymphoproliferation by alveolar macrophages in the guinea pig. Tuber. Lung Dis. 79:119-126. [DOI] [PubMed] [Google Scholar]
- 55.Zhang, Y., M. Broser, H. Cohen, M. Bodkin, K. Law, J. Reibman, and W. N. Rom. 1995. Enhanced interleukin-8 release and gene expression in macrophages after exposure to Mycobacterium tuberculosis and its components. J. Clin. Investig. 95:586-592. [DOI] [PMC free article] [PubMed] [Google Scholar]